
Preparation of Kerosene from Lubricating Oil Waste using
Microwave-assisted and Activated Carbon Pyrolysis from Lignite
Marinda Rahim
a
, Mardhiyah Nadir, Fitriyana
b
and Wanda Syahira
Department of Chemical Engineering, Politeknik Negeri Samarinda, Ciptomangunkusumo Street, Samarinda, Indonesia
Keywords: Kerosene, Lignite, Lubricating Oil Waste, Microwave, Pyrolysis.
Abstract: This study discusses one method that offers a simultaneous solution to reduce lubricating oil waste while
providing fuel. Both the treatment of lubricating oil waste and supply of fuel are classic problems in
developing countries such as Indonesia, but require serious handling because they will become crucial
problems in the future. Lubricating oil waste is classified as hazardous and toxic material, and particularly the
amount of motorcycle lubricating oil waste reaches 1.2 GL. which will continue to increase from year to year.
The goal of this study is to convert lubricating oil waste, specifically from motorcycles, into kerosene through
microwave-assisted pyrolysis and using activated carbon from lignite sized 12 mesh as microwave absorbent.
The experiment was conducted in various absorbent mass i.e. 80, 90, 100, 120, and 140 g, then each was
added in 200 mL of lubricating oil waste. The mixture then took place in glass reactor and was heated in 800
W powered of microwave at constant temperature of 400 ⁰C for 3 hours. The vapour product of pyrolysis was
cooled in a series of condenser to obtain fuel. Fraction similarity of kerosene was analysed with GC-FID,
meanwhile its classification of the carbon chain length compound was identified by GC-MS. Properties were
measured for its density (15 ⁰C) using ASTM D-1298 method and specific energy using bomb calorimeter.
140 g mass of absorbent produced the most similar chromatogram to kerosene standard and was able to obtain
the composition of C
9
-C
15
fraction in amount of 90.61%. This product has density of 817.363 kg/m
3
and
specific energy of 45.49 MJ/kg. It is important to develop this research to increase the kerosene fraction by
examining the effect of temperature to control the endothermic thermal cracking reaction.
1 INTRODUCTION
Because people still rely heavily on private
transportation, Indonesia becomes a country with
relatively high motorcycle users reaching
112,771,136 units in 2019 (Badan Pusat Statistik
Indonesia, 2020) and will grow to about 6% per year.
This circumstance effects on the increasing amount of
both of lubricating oil waste and also fuel needs. The
data above then could be used to predict Indonesia’s
potential of lubricating oil waste in that year i.e. 1.2
GL
Lubricating oil waste is categorized into
hazardous and toxic material so that it requires the
proper handling method. One of the methods that can
be developed is to process lubricating oil waste into
fuel as well as kerosene through cracking technique.
This method is capable of cutting long hydrocarbon
a
https://orcid.org/0000-0002-5546-6209
b
https://orcid.org/0000-0002-9707-0050
chain (C
31
- C
40
), which is the main compound of
lubricating oil waste, to be hydrocarbon compound of
kerosene which has shorter hydrocarbon chain (C
9
-
C
15
). Kerosene that is processed in this way can be an
alternative source of fuel supply that complements the
source of fossil fuel which is non-renewable energy.
Kerosene has a variety of utility such as being a
precursor for aviation turbine gasoline, for
illumination, as well as for tractor vaporizing oil
(Jones & Pujado, 2008).
Cracking techniques as previously described have
been investigated through the catalytic cracking
process using sulfated zirconia catalyst and can
produce kerosene fraction as much as 9,04%
(Permsubscul, Vitidsant, & Damronglerd, 2007).
Moreover, other researchers has been using
Fe/SiO
2
-Al
2
O
3
for catalytic cracking process and
able to produce kerosene fraction of 15.71%
794
Rahim, M., Nadir, M., Fitriyana, . and Syahira, W.
Preparation of Kerosene from Lubricating Oil Waste using Microwave-assisted and Activated Carbon Pyrolysis from Lignite.
DOI: 10.5220/0010953800003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 794-799
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

(Makvisai, Promdee, Tanatavikorn, & Vitidsan,
2016). Even though these experiments do not produce
kerosene in particular, but both have succeeded to
show the potential of cracking lubricating oil waste
into fuel including kerosene.
Cracking process of lubricating oil waste into
kerosene can be done through microwave-assisted
pyrolysis method. This technique is one of the most
promising methods of enhancing and accelerating
chemical reactions (Motasemi & Afzal, 2013).
Microwaves can heat materials at specific targets
because heat is generated from within the material
itself through the mechanism of polar molecular
agitation under the effect of an oscillating electricity
or magnetic field, so that heating can take place
effectively (Taylor, Singh, & Minhas, 2005).
Therefore the using microwave heating required less
energy (del Mundo, Cavarlez, Pe, & Roces, 2018).
However, this method can be potently carried out
using microwave absorbent to overcome the low
dielectric property of lubricating oil waste.
Microwave absorbents can be made from a variety of
raw materials that are cheap and easy to obtain, like
coconut shells, coal,
or banana peels, if treated further
into activated carbon. The capability of activated
carbon as a microwave absorbent is very reliable
because it is supported by relatively high dielectric
characteristics (Menéndez et al., 2010).
The success of the microwave-assisted pyrolysis
process using activated carbon absorbents has been
proven by the following researchers. Bu et al., (2013)
has developed the microwave pyrolysis process of
Douglas fir sawdust pellets using activated carbon
from lignite coal. Meanwhile Lam, Russell, Lee, &
Chase, (2012) has developed pyrolysis process to
crack high density polyethylene into liquid product
that is matching with petrol and diesel using
microwave-assisted process and commercial
activated carbon (Aquacarb 207EA, Chemviron). In
addition, Rahim, (2017) has also developed
microwave-assisted pyrolysis to convert waste from
lubricating oil from motorcycles to gasoline using
activated carbon absorbent from lignite. On the other
hand, activated carbon from coconut husk was used
and succeed to transform waste shipping oil into a
diesel-like fuel via microwave-assisted pyrolysis
(Mahari et al., 2017). The term pyrolysis refers to the
process by which material decomposes thermally in
the absence of oxygen (Rabiu, Auta, & Kovo, 2018).
In particular, this study aims to treat lubricating
oil waste into product that has a rich kerosene fraction
through microwave-assisted pyrolysis techniques by
observing the effect of the microwave absorbent mass
made from lignite. Cracking process of heavy fraction
hydrocarbon is the complex reaction and since the
specific target of the hydrocarbon structure to be
generated is substances type with the C
9
-C
15
chain
length, this research becomes important to carry out.
The amount of absorbent utilized can influence the
amount of microwaves absorbed and then released as
heat that can lead to the desired reaction.
In this research lignite was chosen as activated
carbon raw material for absorbing microwave due to
lignite is one of the feedstock with potential amount
in Indonesia but less beneficial if use as fuel with
combustion directly. British Petroleum, (2020) has
released the data that Indonesia’s total proved
reserves of low rank coal, including lignite, reach
29.4% at end 2019. Indonesian lignite, especially at
East Kalimantan Province, has relatively high enough
fixed carbon that is 31.55% (Patmawati, Alwathan, &
Ramadani, 2020).
2 MATERIALS AND METHODS
The main materials, namely lubricating oil waste and
lignite, were obtained from motorcycle garage and
coal mining areas located in Samarinda City, East
Kalimantan Province. The study began by first
preparing activated carbon from lignite, as a
microwave absorbent, using the method in detail
described by Rahim and Fitriyana, (2018).
Furthermore 200 mL of lubricating oil waste
from motorcycle was mixed with various absorbent
mass of activated carbon sizing 12 mesh in the reactor
flask. Mass variations used were 80, 90, 100, 120 and
140 g. The material mixture was then placed in 800
W microwave and then the reactor flask was
connected to a series of condensers. The pyrolysis
process was carried out for three hours and the
temperature was maintained at 400
o
C with a
temperature controller. Vapour product from
pyrolysis was then passed through a series of
condensers to get liquid fuel. Nitrogen flow of 200
mL/min was used to support flowing process of
vapour to the condenser. The kerosene result fraction
of each variation was analysed using a gas
chromatography flame ionization detector (GC-FID)
and gas chromatography mass spectrometry (GC-
MS), while the characteristics of the resulting
kerosene product were measured through the density
(15
o
C) also calorific value respectively using the
ASTM D-1298 and bomb calorimeter methods.
Preparation of Kerosene from Lubricating Oil Waste using Microwave-assisted and Activated Carbon Pyrolysis from Lignite
795
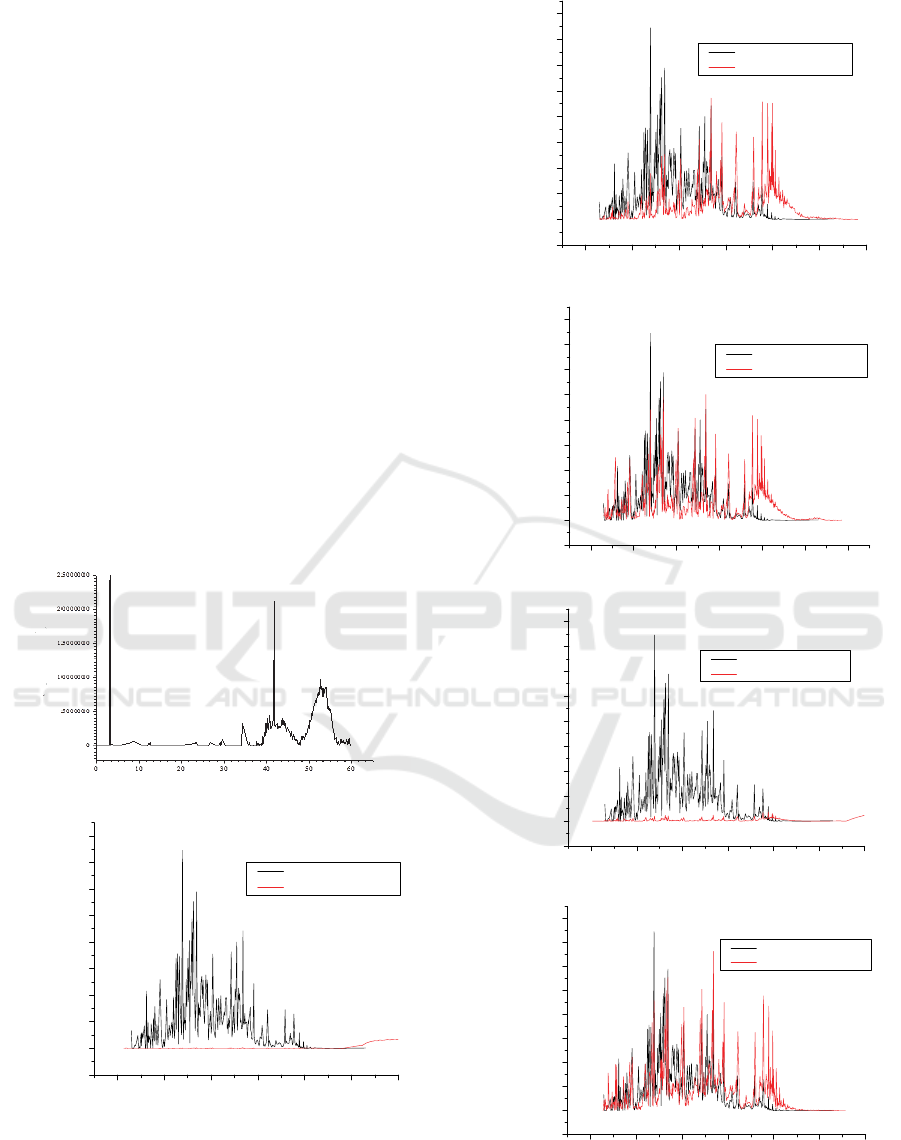
3 RESULT AND DISCUSSION
The results of GC FID chromatogram from the fuel
products obtained were compared to similarity with
the standard chromatogram of lubricating oil waste
and kerosene. Chromatograms data were processed
using Origin 7 software. The illustration of the
chromatogram peaks is shown in figure 1.
When viewed from the characteristics of the
retention time and the peak formed, it is seen the
difference on the chromatogram of the product with
the lubricating oil waste chromatogram. The output
of the gas chromatography analysis in figure 1 (a)
shows a significant peak for lubricating oil waste
occurring at a retention time of 40 - 60 minutes. This
shows lubricating oil waste containing heavy
fractions with long carbon chain hydrocarbons.
While the chromatogram for the product resulting
from the process of pyrolysis of lubricating oil waste,
shows those significant peaks in figure 1 (b) to 1 (f)
occur at shorter retention times, which are on average
of 3 - 50 minutes. This indicates that there has been
cracking process of long chain hydrocarbons to
hydrocarbons with shorter chains.
(a)
(b)
(c)
(d)
(e)
(f)
Figure 1: Chromatograms of (a) Lubricating oil waste;
product compared with standard kerosene for absorbent
mass (b) 80 g; (c) 90 g; (d); 100 g; (e) 120 g; (f) 140 g.
0 102030405060
-200000
0
200000
400000
600000
800000
1000000
1200000
1400000
1600000
Height (
V)
Retention Time (min)
Standard of kerosene
Product 1
0 102030405060
-200000
0
200000
400000
600000
800000
1000000
1200000
1400000
1600000
Height (
V)
Retention Time (min)
Standard of kerosene
Product 2
0 102030405060
-200000
0
200000
400000
600000
800000
1000000
1200000
1400000
1600000
Retention Time (min)
Height (
V)
Standard of kerosene
Product 3
0 102030405060
-200000
0
200000
400000
600000
800000
1000000
1200000
1400000
1600000
Height (V)
Retention Time (min)
Standard of kerosene
Product 4
0 102030405060
-200000
0
200000
400000
600000
800000
1000000
1200000
1400000
1600000
Height (
V)
Retention Time (min)
Standard of kerosene
Product 5
Height (mV)
Retention Time (minute)
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
796
The results of the product chromatogram which
are considered to represent the kerosene fraction are
those that show significant peaks at the retention time
of 3 - 40 minutes as shown by the standard kerosene
chromatogram. While chromatograms that appear at
retention times lower than 3 minutes are expressed as
lighter fractions of kerosene and vice versa that
appear at retention times higher than 40 minutes are
considered to be heavier fractions of kerosene.
In the use of a relatively low absorbent mass that
is 80 g, the expected pyrolysis has not been occurred
yet completely which is evident from none emergence
of significant peaks of the product chromatogram at
3-40 minutes retention time as showed in figure 1 (b).
This exhibits that the absorbent mass utilized is not
sufficient to produce heat that can crack the long
hydrocarbon chain.
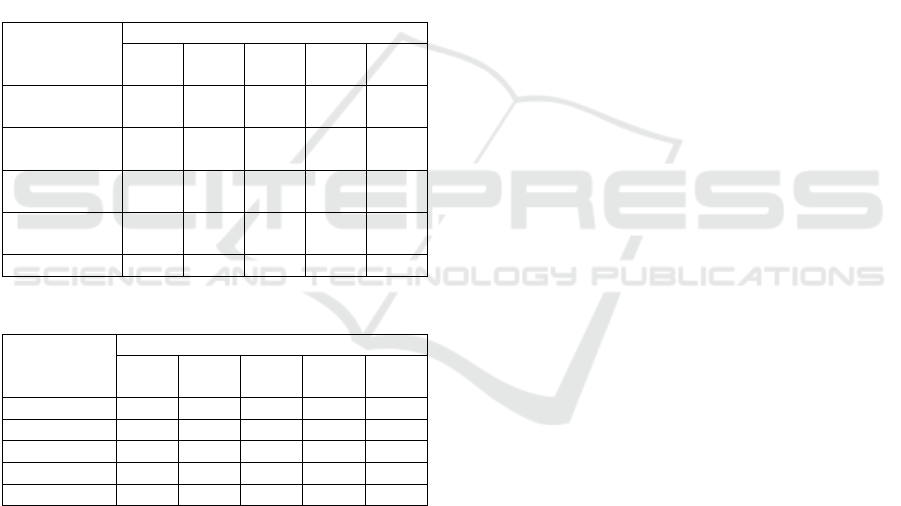
Table 1: Composition of hydrocarbon compound.
Hydrocarbon
compound
(
%
)
Mass of absorbent (g)
80 90 100 120 140
Paraffin 60.5
4
55.4
8
44.5
3
44.8
1
38.7
0
Iso
p
araffin
17.3
7
17.0
1
16.7
9
19.5
6
23.1
1
Olefin 19.5
6
23.1
9
32.1
2
28.9
7
32.5
1
Cycloparaffi
n 0.00 1.64 0.86 0.93 3.38
Aromatic 1.98 1.96 4.97 5.03 1.61
Table 2: Composition of carbon chain length.
Carbon
chain length
(
%
)
Mass of absorbent
(g)
80 90 100 120 140
C
5
- C
8
3.54 3.17 9.79 8.20 7.25
C
9
- C
12
73.59 74.53 84.60 84.30 88.32
C
13
-C
15
17.13 16.14 2.58 4.29 2.29
C
16
-C
20
4.19 5.44 2.30 2.51 1.45
C
26
1.00 - - - -
Although the cumulative composition of C
9
-C
15
could
reach 90.72% but product still contain heavy fraction (C
13
-
C
15
), in range kerosene fraction, quite much namely 17.13%,
and lighter fraction (C
9
-C
12
) only reach 73.59%. Meantime
the heaviest fraction (C
16
-C
26
) reach 5.19%. Although the
C
16
-C
26
fraction appeared at retention time of 3-40 minutes,
the carbon chain length produced was not a kerosene
fraction,
as can be looked in the distribution of
composition of carbon chain length in table 2. Based on data
in table 1, the hydrocarbon compound is still dominated by
paraffin species as much as 60.54%.
On the use of more absorbent mass i.e. 90 to 140
g, significant chromatogram peaks appear at 3-40
minutes retention times and it has indicated the
formation of compounds with hydrocarbon chain of
kerosene fraction. This also further tends increasing
the composition of the C
9
-C
12
fraction up to 88.32%
on the utilization of 140 g mass of absorbent as well
as the amount of C
13
-C
15
and C
16
-C
20
could be
reduced till 2.294% and 1.45% respectively. The
escalation in absorbent mass, that listed in table 1,
also causes trend of raising the composition of
hydrocarbon in olefin and isoparaffin species which
exhibits that the stages of primary cracking reaction
lead to olefin formation an also drive isomerization as
secondary reaction. As it is known the secondary
reactions, such as reforming, isomerization,
alkylation, and polymerization, occur in thermal
cracking (Speight, 2015). This phenomenon is a
nature of endothermic thermal cracking reactions.
Where the utilization of greater absorbent mass will
further increase the absorption of microwaves and
then released as greater heat into lubricating oil waste.
As a consequence, primary and secondary reactions
can take place more quickly.
An interesting result is shown in the utilization of
120 g absorbent mass. Previously, it was expected
that the chromatogram resulted would be closer to the
standard kerosene chromatogram along with the
increasing mass of the absorbent used. Although the
cumulative result of products in the kerosene fraction
range is quite high at 88.59%, the elevation of the
product chromatogram peaks tends to be very low
compared to the standard kerosene peaks so as to
form a flat chromatogram as shown in figure 1 (e).
The product obtained shows the tendency of the
formation of compounds in the range of kerosene
fractions with more diverse types of compounds, as
consequence there are no compounds with high
composition. The results of GC MS analysis have
identified 98 types of compounds that appear in this
product.
The product chromatogram that is most similar to
the kerosene standard chromatogram is the product
chromatogram that used the most absorbent mass of
140 g as shown in figure 1 (f). In the use of this
absorbent mass can be absorbed microwaves which
then produce sufficient heat to break the chain of
hydrocarbons so as to result a product that mostly has
a carbon chain length in the range of hydrocarbon
chains for kerosene.
Preparation of Kerosene from Lubricating Oil Waste using Microwave-assisted and Activated Carbon Pyrolysis from Lignite
797
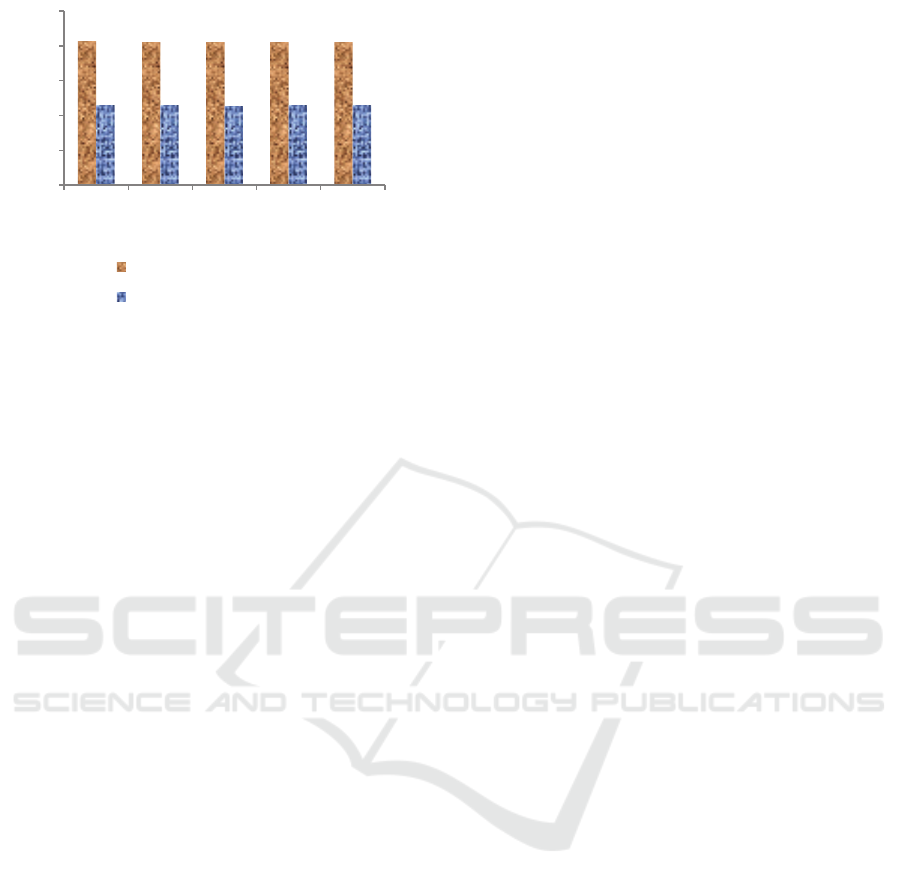
Figure 2: Properties value of product.
Refer to the data that showed in figure 2, it is seen
that the mass of activated carbon as a microwave
absorbent influences the density value of pyrolysis
products. The higher mass of activated carbon causes
the pyrolysis product density inclines to decrease
which indicates that the pyrolysis product formed has
the smaller molecular weight. All of products density
has value lower than raw material which has a density
of 871.114 kg/m
3
.
Specific energy is one of important characteristic
of kerosene as fuel. The specific energy of products
was not influenced significantly by the elevation of
mass absorbent and those values are in the range
45.42 – 45.60 MJ/kg. However, it seems that this
property is influenced by the quantity of paraffin and
isoparafin compounds formed. Overall the specific
energy of the products are convenient of kerosene
value based on Defence Standard 91-091 which
requires a minimum value of 42.80 MJ/kg (Ministry
of Defence, 2019).
This research can be developed to further improve
the kerosene fraction (C
9
-C
15
), and reduce the lightest
fraction (C
5
-C
8
) and the heaviest fraction (C
16
-C
26
).
This can be conducted by examining the effect of
temperature, because the thermal cracking reaction is
endothermic.
4 CONCLUSIONS
This research has provided some key information
related to pyrolysis of lubricating oil waste into
kerosene with assisted microwaves, namely:
1. Active carbon from lignite is potential as a
microwave absorber to generate heat to crack long
chain hydrocarbon of lubricating oil waste.
2. The mass of activated carbon as much as 140 g
can generate adequate heat to result a stable
product in the kerosene fraction hydrocarbon
range (C
9
-C
15
) as much as 90.61%.
3. This product has properties of density and specific
energy values are 817.363 kg/m
3
and 45.49 MJ/kg
consecutively.
4. In general, the experimental results obtained in
this research indicate the potential for conversion
of lubricating oil waste into kerosene.
ACKNOWLEDGEMENTS
Our highest gratitude and appreciation are extended
to the Research and Community Service Center of
Politeknik Negeri Samarinda for funding this
research.
REFERENCES
Badan Pusat Statistik Indonesia. (2020). Perkembangan
jumlah kendaraan bermotor menurut jenis, 1949-2018.
Retrieved January 5, 2021, from https://www.bps.go.id/
linkTableDinamis/view/id/1133
British Petroleum. (2020). Statistical review of world
energy. Retrieved from https://www.bp.com/content/
dam/bp/business-sites/en/global/corporate/pdfs/
energy-economics/statistical-review/bp-stats-review-
2020-full-report.pdf
Bu, Q., Lei, H., Wang, L., Wei, Y., Zhu, L., Liu, Y., …
Tang, J. (2013). Renewable phenols production by
catalytic microwave pyrolysis of Douglas fir sawdust
pellets with activated carbon catalysts. Bioresource
Technology, 142, 546–552. https://doi.org/10.1016/
j.biortech.2013.05.073
del Mundo, I. C., Cavarlez, J. M., Pe, A. M., & Roces, S.
(2018). Microwave assisted glycerolysis of neem oil.
ASEAN Journal of Chemical Engineering, 18(1), 17–
23.
Jones, D. S. J., & Pujado, P. R. (2008). Handbook of
petroleum processing. Netherland: Springer.
Lam, S. S., Russell, A. D., Lee, C. L., & Chase, H. A.
(2012). Microwave-heated pyrolysis of waste
automotive engine oil: Influence of operation
parameters on the yield, composition, and fuel
properties of pyrolysis oil. Fuel, 92(1), 327–339.
https://doi.org/10.1016/j.fuel.2011.07.027
Mahari, W. A. W., Zainuddin, N. F., Chong, C. T., Lee, C.
L., Lam, W. H., Poh, S. C., & Lam, S. S. (2017).
Conversion of waste shipping oil into diesel-like oil via
microwave-assisted pyrolysis. Journal of
Environmental Chemical Engineering, 5(6), 5836–
5842. https://doi.org/10.1016/j.jece.2017.11.005
Makvisai, W., Promdee, K., Tanatavikorn, H., & Vitidsan,
T. (2016). Petroleum and coal. Petroleum and Coal, 58,
83–94.
82,646
82,256 82,226
82,016
81,736
45,6 45,59
45,42
45,47
45,49
0
20
40
60
80
100
80 90 100 120 140
Value
MassofAbsorbent(g)
Density(15°C)(kg/m³)x10
SpecificEnergy(MJ/kg)
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
798

Menéndez, J. A., Arenillas, A., Fidalgo, B., Fernández, Y.,
Zubizarreta, L., Calvo, E. G., & Bermúdez, J. M.
(2010). Microwave heating processes involving carbon
materials. Fuel Processing Technology, 91(1), 1–8.
https://doi.org/10.1016/j.fuproc.2009.08.021
Ministry of Defence. (2019). Defence Standard 91-091
Turbine Fuel , Kerosene Type , Jet A- 1; NATO Code:
F-35; Joint Service Designation: AVTUR. Retrieved
from http://inaca.or.id/wp-content/uploads/2019/11/
Def-Stan-91-091-Issue-11-Oct-2019-Turbine-Fuel-
Kerosene-Type-Jet-A-1-NATO-CodeF-35-Joint-
Service-Designation-AVTUR.pdf.
Motasemi, F., & Afzal, M. T. (2013). A review on the
microwave-assisted pyrolysis technique. Renewable
and Sustainable Energy Reviews, 28, 317–330.
https://doi.org/10.1016/j.rser.2013.08.008
Patmawati, Y., Alwathan, ., & Ramadani, N. H. (2020).
Characterization of Activated Carbon Prepare from
Low-rank Coal of East Kalimantan by using Acid and
Base Activation. Proceedings of the 8
th
Annual
Southeast Asian International Seminar, (Asais 2019),
178–181. https://doi.org/10.5220/0010021901780181
Permsubscul, A., Vitidsant, T., & Damronglerd, S. (2007).
Catalytic cracking reaction of used lubricating oil to
liquid fuels catalyzed by sulfated zirconia. Korean
Journal of Chemical Engineering, 24(1), 37–43.
Rabiu, S. D., Auta, M., & Kovo, A. S. (2018). An upgraded
bio-oil produced from sugarcane bagasse via the use of
HZSM-5 zeolite catalyst. Egyptian Journal of
Petroleum, 27(4), 589–594. https://doi.org/10.1016
/j.ejpe.2017.09.001
Rahim, M. (2017). Application of microwave absorbent
from East Kalimantan lignite on microwave pyrolysis
of waste lubricating oil. International Journal of
ChemTech Research, 10(1), 34–38. Retrieved from
http://sphinxsai.com/2017/ch_vol10_no1/ch01.htm
Rahim, M., & Fitriyana. (2018). Upgrading East
Kalimantan lignite into activated carbon as a
microwave absorbent. 2018 International Conference
on Applied Science and Technology (ICAST), 275–278.
https://doi.org/10.1109/iCAST1.2018.8751519
Speight, J. G. (2015). Fouling in Refineries. Gulf
Professional Publlishing.
Taylor, M., Singh, B., & Minhas, S. (2005). Special reports:
Developments in microwave chemistry. In Chemistry
World (Vol. 2). United Kingdom: Evaluerserve.
Preparation of Kerosene from Lubricating Oil Waste using Microwave-assisted and Activated Carbon Pyrolysis from Lignite
799
