
Development and Characterization of Jatropha Oil as Innovative
Bio-sourced Phase Change Material for Thermal Energy Storage
Made Rasta
1a
, Nyoman Suamir
1b
, Wayan Adi Subagia
1c
, Adi Winarta
1
,
Ketut Bangse
1d
and Gusti Ketut Puja
2e
1
Mechanical Engineering Department, Bali State Polytechnic, Bali-80364, Indonesia
2
Mechanical Engineering Department, Sanata Dharma University, Yogyakarta-55002, Indonesia
Keywords: Jatropha Oil, Bio-phase Change Material, Thermal Energy Storage.
Abstract: Thermal energy storage (TES) using phase change materials (PCM) is very promising to meet future energy
needs because of its high storage capacity and low cost. This study aims to develop Jatropha oil as a new
candidate for solid-liquid latent heat energy storage for cold storage applications. The characteristics of
Jatropha oil as a candidate for PCM were tested experimentally using T-history methods. The test results
show that Jatropha oil as a PCM candidate does not undergo super-cooling or super-cooling is very small
compared to tap water and mineral water of 10 K and 15 K. The phase transition temperature of Jatropha oil
ranges from -14 ºC to -16 ºC, lower than with tap freezing point and mineral water at 0 ºC. Thus, the candidate
PCM has good thermal properties that can meet the thermal energy storage requirements for cold storage
applications.
1 INTRODUCTION
Along with the increase in the economy and
population development, the need for energy is
increasing. Energy is a vital need in development.
Energy is still sourced from fossil fuels which can
cause climate change and environmental degradation.
Therefore, it is important to save energy and use new
and renewable energy sources that are
environmentally friendly for sustainable
development. Energy storage (ES) systems play an
important role in supporting energy security. Energy
can be stored in electrical, mechanical and thermal
energy.
One of the important ES systems for storing and
retrieving energy for energy conversion systems is
thermal energy storage (TES). The use of a TES
system based on latent heat technology can save
energy use from fossil fuels and conserve energy.
TES helps rational use of thermal energy and has the
a
https://orcid.org/0000-0002-9610-3738
b
https://orcid.org/0000-0003-0594-7511
c
https://orcid.org/0000-0001-9261-3549
d
https://orcid.org/0000-0003-0220-056X
e
https://orcid.org/0000-0002-6025-3865
advantage that it allows the transfer of peak loads
beyond peak loads, is significant in energy savings
and mitigation of CO
2
that causes pollution to the
environment.
Latent heat thermal energy storage (LHTES) is an
attractive technique because it can provide a higher
energy storage density than conventional TES
systems. Has the ability to store the heat of fusion at
a constant (relatively constant) temperature
corresponding to the phase transition temperature of
the phase change material (PCM).
One of the typical applications of PCM is for the
storage of solar thermal energy which is considered
the most abundant renewable energy. Utilization of
PCM for TES can overcome the intermittency of solar
energy, thus providing a better solution to rationalize
the utilization of solar thermal energy, compared to
the sensible heat TES system.
ES is a promising power management method to
obtain sustainable energy utilization. ES using
716
Rasta, M., Suamir, N., Subagia, W., Winarta, A., Bangse, K. and Puja, G.
Development and Characterization of Jatropha Oil as Innovative Bio-sourced Phase Change Material for Thermal Energy Storage.
DOI: 10.5220/0010952000003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 716-720
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

LHTES is currently receiving a lot of attention to
reduce grid energy requirements.
TES schemes using PCM are categorized into
organic, inorganic and organic-inorganic mixtures.
Organic PCMs, such as paraffin and fatty acids, have
little super-cooling properties (no super-cooling
occurs) because they have self-nucleation but lower
thermal conductivity and higher cost. Inorganic
PCMs, such as hydrated salts, are readily available
and less expensive, but occurs phase separation, and
corrosive (Rasta and Suamir, 2019). Therefore, it is
important to select the optimal PCM in a given
application to meet operating and cost requirements.
The main problem of TES technology is
developing effective PCM for energy storage (Wang
& Lu, 2013). The main criteria in determining the
selection of PCM material types for TES applications
are to have a phase change temperature in the
practical application range, have high latent heat and
good thermal conductivity, as they involve the energy
storage and thermal stability characteristics of the
PCM.
By far the most famous PCM is water because it
has good thermal properties, but has the disadvantage
of high super-cooling (Rasta and Suamir, 2018). In
this study, Jatropha oil was developed as a PCM
material. Oil is a complex fatty acid/fatty acid ester.
Oils or fatty acids are a new class of organic PCM
materials and the thermal data available in the
literature are still limited (Aydin & Okutan, 2011).
Oils or fatty acids are derivatives of materials easily
found in nature and are labelled as bio-based
ingredients (Cellata, et al., 2015; Sharma, et al.,
2015). Another advantage is that vegetable oils are
available continuously (Sharma, et al., 2014; Fauzi, et
al., 2015; Suamir, et al., 2019).
Given the complexities of LHTES problems,
developing an effective PCM for energy storage in a
suitable form is still a big challenge and many
methods have been sought (Yingbo, et al., 2014). The
aim of this paper is to develop new bio-materials for
low temperature thermal energy storage below 0 ºC
for refrigeration system applications.
2 MATERIAL AND METHOD
The material used as Latent Heat Thermal Energy
Storage in this research is Jatropha oil. Jatropha oil
was chosen because of its sufficient availability in the
field and cultivated by farmers to increase their
income. The Jatropha oil tested here has undergone a
hydrolysis process. Jatropha oil contains various fatty
acids, both saturated and unsaturated fatty acids
(PUFA).
The thermal properties of Jatropha oil will be
compared with the thermal properties of tap water and
mineral water. Water is an excellent PCM material,
but has the disadvantage of high super-cooling and a
freezing point of 0 ºC. The results of the Jatropha oil
test will be compared with water, to explain the
characteristics of Jatropha oil.
Figure 1: Schematic diagram of the experimental test
equipment T-history method (Rasta, et al., 2016a).
Figure 1 shows that the prospective PCM Jatropha
oil in various volumes is put into a glass tube and
immersed in a water bath as a cooling medium. The
cooling medium is a mixture of 40% (volume)
polypropylene glycol with tap water. The cooling
medium is circulated by pump through the evaporator
of the system. The temperature of the cooling medium
can be as low as -25 ºC. However, for testing, the
temperature of the cooling medium was maintained
stable at -20 ºC by using a digital thermostat with an
accuracy of ±0.2 ºC.
The data recording system shown in Figure 1 is
equipped with a data acquisition module and a
computer for recording or display systems. The data
acquisition module utilizes the Data scan 7000 series
from MSL (Measuring Systems Ltd) which includes
the Data scan 7320 measurement processor and the
7020 expansion module. Type T thermocouples are
used to measure the temperature of the PCM
candidate and cooling medium. The thermocouple
has a temperature measurement range of -250 ºC to
350 ºC with an error of ± 0.5 ºC. The thermocouple
was calibrated using a water bath calibration and the
uncertainty of the thermometer precision was ± 0.04
ºC. In the test temperature range is -25 ºC to 50 ºC
(Rasta, et al., 2016b).
3 RESULT AND DISCUSSION
Super-cooling degree is an important parameter in
LHTES technology using PCM. Figures 2 and 3 show
Development and Characterization of Jatropha Oil as Innovative Bio-sourced Phase Change Material for Thermal Energy Storage
717
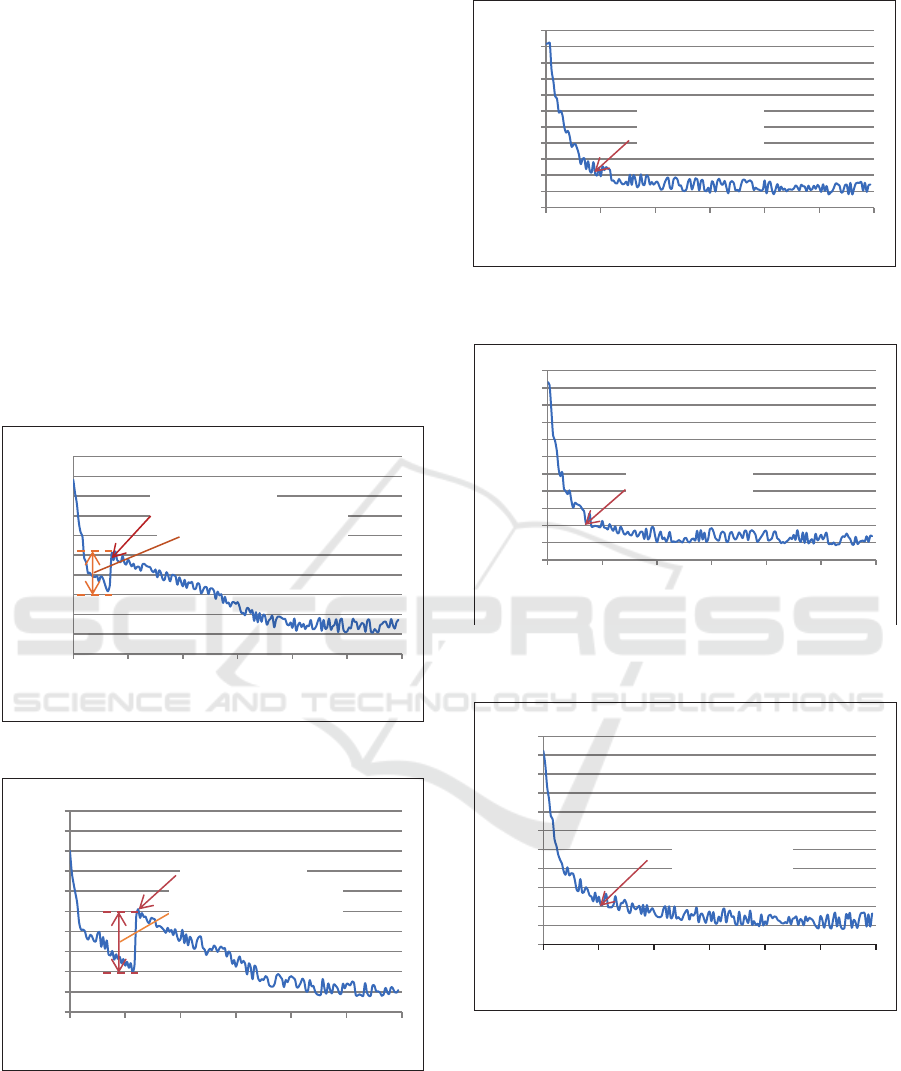
that tap water and mineral water have high super-
cooling, namely 10 K and 15 K. Super-cooling is a
condition that indicates the presence of tap water and
mineral water still in liquid form below the freezing
point of 0 ºC. A LHTES technology with high super-
cooling, will result in a much lower evaporation
temperature required when applied in the cooling
system. Thus will reduce the performance of the
system. In addition, before tap water and mineral
water freeze, the energy stored is only in the form of
sensible heat, causing a very small energy storage
capacity. In order for tap water and minerals to store
energy in the form of latent heat, more energy is
needed to change the state from liquid to solid. The
greater energy requirements in PCM technology are
in direct conflict with thermal energy storage
technology. So high super-cooling is a disadvantage
in LHTES technology
Figure 2: Cooling process and super-cooling tap water.
Figure 3: Cooling process and super-cooling mineral water.
Figure 4: Cooling process and Jatropha oil freezing point
for sample 4 ml.
Figure 5: Cooling process and Jatropha oil freezing point
for sample 8 ml.
Figure 6: Cooling process and Jatropha oil freezing point
for sample 12 ml.
-25
-20
-15
-10
-5
0
5
10
15
20
25
0 5 10 15 20 25 30
Temperature (
o
C)
Time (minute)
Tap water
-25
-20
-15
-10
-5
0
5
10
15
20
25
0 5 10 15 20 25 30
Temperature (
o
C)
Time (minute)
Mineral water
-25
-20
-15
-10
-5
0
5
10
15
20
25
30
0 5 10 15 20 25 30
Temperature (
o
C)
Time (minute)
Volume = 4 ml
-25
-20
-15
-10
-5
0
5
10
15
20
25
30
0 5 10 15 20 25 30
Temperature (
o
C)
Time (minute)
Volume = 8 ml
‐25
‐20
‐15
‐10
‐5
0
5
10
15
20
25
30
0 5 10 15 20 25 30
Temperature (
o
C)
Time (minute)
Volume = 12 ml
Freezing point
Freezing point
Freezing point
Su
p
e
r
-coolin
g
de
g
ree
Su
p
e
r
-coolin
g
de
g
ree
Freezing point
Freezin
g
p
oint
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
718
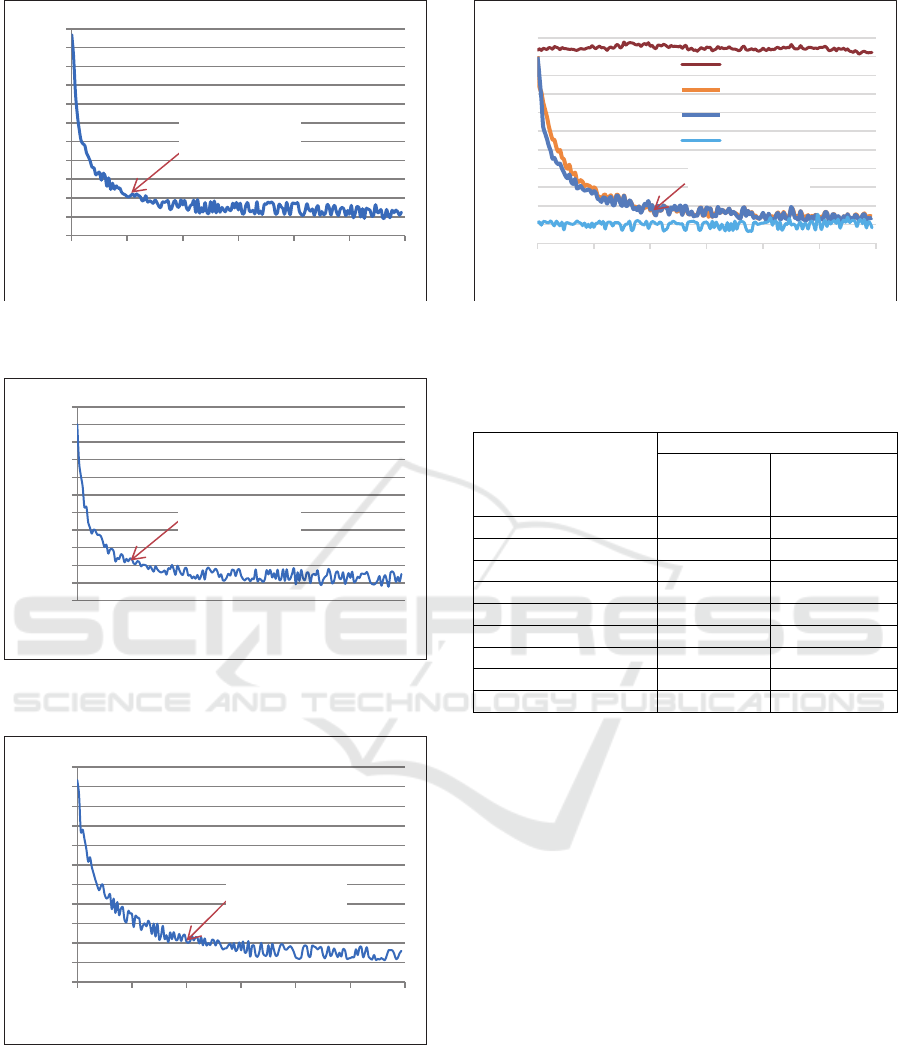
Figure 7: Cooling process and Jatropha oil freezing point
for sample 16 ml.
Figure 8: Cooling process and Jatropha oil freezing point
for sample 20 ml.
Figure 9: Cooling process and Jatropha oil freezing point
for sample 30 ml.
Figure 10: Cooling process and Jatropha oil freezing point
for sample 40 ml.
Table 1: Thermal energy storages properties of tap water,
mineral water and Jatropha oil.
Samples
(Volume, ml)
Cooling process
Freezing
temperature
(
ºC
)
Super-cooling
degree
(
K
)
Ta
p
water, 20 ml 0 10
Mineral water, 20 ml 0 15
Jatropha oil, 4 ml 14 0
Jatropha oil, 8 ml 14 0
Jatro
p
ha oil, 12 ml 15 0
Jatro
p
ha oil, 16 ml 15 0
Jatro
p
ha oil, 20 ml 15 0
Jatropha oil, 30 ml 16 0
Jatropha oil, 40 ml 16 0
Figures 4-10 shows the cooling process of each
volume variation of the Jatropha oil sample
developed as PCM. The test results of the T-history
method show that in the early stages of the cooling
process, there is a very rapid decrease in temperature
to the initial limit of the transition phase temperature
process. At the beginning of the phase transition
process until it ends there is no significant or constant
temperature change. When a phase transition occurs
from the beginning to the end, this is where the
storage of latent heat energy occurs. After that the
freezing process takes place, the temperature
decreases towards the final test temperature setting.
Overall, all samples developed with different
volume fractions of Jatropha oil are summarized in
Table 1. The freezing point of the PCM candidate
developed is lower than that of tap water and mineral
water. The freezing point of Jatropha oil in various
volumes of 4 ml, 8 ml, 12 ml, 16 ml, 20 ml, 30 ml and
40 ml, ranged from -14 ºC to -16 ºC. The results
showed that increasing the volume of the sample
tested did not significantly affect the change in
freezing point. Thus, the advantages of Jatropha oil
-25
-20
-15
-10
-5
0
5
10
15
20
25
30
0 5 10 15 20 25 30
Temperature (
o
C)
Time (minute)
Volume = 16 ml
-25
-20
-15
-10
-5
0
5
10
15
20
25
30
0 5 10 15 20 25 30
Temperature (
o
C)
Time (minute)
Volume = 20 ml
-25
-20
-15
-10
-5
0
5
10
15
20
25
30
0 5 10 15 20 25 30
Temperature (ºC)
Time (minute)
Volume = 30 ml
-25
-20
-15
-10
-5
0
5
10
15
20
25
30
0 5 10 15 20 25 30
Temperature (
o
C)
Time (minute)
Volume = 40 ml
Ambient temperature
Sample-1
Sample-2
Water bath
Freezing point
Freezing point
Freezing point
Freezing point
Development and Characterization of Jatropha Oil as Innovative Bio-sourced Phase Change Material for Thermal Energy Storage
719

do not occur super-cooling, have stable properties, are
not corrosive and environmentally friendly. These
properties make Jatropha oil a potential PCM material
for energy storage for latent heat thermal energy
storage applications in low temperature refrigeration
systems below 0 ºC, compared to water (tap water and
mineral water).
4 CONCLUSIONS
An investigation of Jatropha oil as a candidate for the
development of a new phase change material (PCM)
has been carried out. T-history thermal analysis was
applied in the investigation and it was found that
Jatropha oil has a phase transition temperature or
freezing point ranging from -14 ºC to -16 ºC. The
results of the investigation also found that the
candidate PCM under test conditions had a minimum
or even none (negligible) super-cooling level. In
addition, Jatropha oil has a continuous supply, is non-
corrosive and non-toxic. Meanwhile, tap water and
mineral water have high super-cooling, which are 10
K and 15 K, respectively, with a freezing point or
phase transition temperature of 0 ºC. This makes
Jatropha oil applicable as a new PCM for cold storage
applications below 0 ºC.
ACKNOWLEDGEMENTS
The authors would like to thank the financial support
received from the Ministry of Education, Culture,
Research, and Technology of the Republic of
Indonesia. This fee is charged to the Bali State
Polytechnic DIPA Number: SP DIPA-
023.18.2.677608/2021 dated November 23, 2020.
which has been submitted to the head of the research
as stated in the DIPA Fund Research Implementation
Agreement Letter No.: 887/PL8/PG/2021, April 6,
2021.
REFERENCES
Aydın, A.A., Okutan, H. (2011). High-chain fatty acid
esters of myristyl alcohol with even carbon number:
Novel organic phase change materials for thermal
energy storage-1. Solar Energy Materials and Solar
Cells 95, 2752-2762.
Aydın, A.A., Okutan, H. (2011). High-chain fatty acid
esters of myristyl alcohol with odd carbon number:
Novel organic phase change materials for thermal
energy storage-2. Solar Energy Materials and Solar
Cells 95, 2417-2423.
Cellata, K., Beyhana, B., Güngörb, C., Konukluc, Y.,
Karahand, O., Dündare, C., Paksoya, H. (2015).
Thermal enhancement of concrete by adding bio-based
fatty acids as phase change materials. Energy and
Building 106, 156-163.
Fabiani, C., Pisello, A.L., Barbaneraa, M., Cabeza, L.F.
(2020). Palm oil-based bio-PCM for energy efficient
building applications: Multipurpose thermal
investigation and life cycle assessment. Journal of
Energy Storage 28 (2020) 101129
Fauzi, H., Hendrik, S.C., Metselaar, Mahlia, T.M.I,
Silakhori, M., Chyuan-Ong, H. (2015). Thermal
characteristic reliability of fatty acid binary mixtures as
phase change materials (PCMs) for thermal energy
storage applications. Applied Thermal Engineering 80,
127-131.
Rasta, I.M., Suamir, I.N. (2018). The role of vegetable oil
in water based phase change materials for medium
temperature refrigeration. Journal of Energy Storage
15, 368-378.
Rasta, I.M., Suamir, I.N. (2019). Study on Thermal
Properties of Bio-PCM Candidates in Comparison with
Propylene Glycol and Salt Based PCM for sub-Zero
Energy Storage Applications. IOP Conference Series:
Materials Science and Engineering 494, 012024.
Rasta, I.M., Wardana, I.N.G., Hamidi, N., Sasongko, M.N.
(2016a). The role of heterogeneous nucleation in water
based Phase change material for medium temperature
Refrigeration. ARPN journal of engineering and
applied sciences
11(2), 978-985.
Rasta, I.M.,
Wardana I.N.G.,
Hamidi, N.,
Sasongko M.N.
(2016b). The Role of Soya Oil Ester in Water-Based
PCM for Low Temperature Cool Energy Storage.
Journal of Thermodynamics, Volume 2016, Article ID
5384640, 8 pages.
Suamir,
I.N., Rasta, I.M., Sudirman, Tsamos, K.M. (2019).
Development of Corn-Oil Ester and Water Mixture
Phase Change Materials for Food Refrigeration
Applications. Energy Procedia 161, 198-206.
Sharma, A., Shukla, A., Chen, C.R., Wu, T.N. (2014).
Development of phase change materials (PCMs) for
low temperature energy storage applications.
Sustainable Energy Technology Assessment 7, 17-21.
Sharma, R.K., Ganesan, P., Tyagi, V.V., Metselaar, H.S.C.,
Sandaran, S.C. (2015). Developments in organic solid-
liquid phase change materials and their applications in
thermal energy storage. Energy Conversion and
Management 95, 193-228.
Wang, H.Y., Lu, S.S. (2013). Study on thermal properties
of phase change material by an optical DSC system.
Applied Thermal Engineering 60, 132-136.
Yingbo, C., Shifeng, Z., Qi, Z., Yusheng, C., Yufeng, Z.
(2014). Composite phase change materials prepared by
encapsuling paraffinin PVC macrocapsules.
Thermochimica Acta 578, 10-14.
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
720
