
Experimental Study of the Effect of Reactor Temperature
Reconstruction on Fuel Consumption and Distillate Quantity
Ida Bagus Puspa Indra
a
, I Made Anom Adiaksa
b
and Gusti Ngurah Ardana
c
Mechanical Enginering Departement, Bali State Polytechnic, Mangupura, Bali, Indonesia
Keywords: Temperature, Transfer, Heat, Energy, Distillate.
Abstract: Temperature is a measure or degree of hotness or coldness of an object or system, temperature is defined as a
physical quantity that is shared between two or more objects that are in thermal equilibrium. The second law
of thermodynamics is based on the fact that there is no reversible process in which heat flows naturally from
a high-temperature object to a low-temperature object, and not vice versa. Heat is energy that is transferred
due to a temperature difference. The concept of this law of thermodynamics will always occur and the process
will stop until the concept of thermal equilibrium occurs. It can be concluded that an object or fluid will have
a temperature and naturally that temperature can flow from a high temperature to a lower temperature until
the concept of thermal equilibrium is formed. Heat treatment through certain media can increase the
temperature or the temperature of the existing fluid. In the distillation column the high temperature distillate
fluid will tend to be at the top and the bottom will tend to be cooler even though heat treatment is carried out
at the bottom. Equilibrium temperature will be achieved in a relatively long time because the system used for
distillation is open. Temperature reconstruction by providing additional tools aims to achieve thermal
equilibrium more quickly. The process is carried out for 60 minutes using 25 liters of raw materials of the
same quality and the temperature is set at 90 degrees Celsius. The results obtained are the average temperature
difference in the reactor is 86.11%, the decrease in fuel consumption is 30.3%, and the distillation quantity
increases by 16.67% between the reactor without a pump compared to the reactor with a pump.
1 INTRODUCTION
Distillation is a process of separating two or more
components of a liquid based on the boiling point. In
simple terms, distillation is done by
heating/evaporating the liquid and then the steam is
cooled back to become liquid with the help of a
condenser. The reactor is tightly closed so that no
steam comes out of the lid gap or connection pipe.
The temperature increases gradually until the
maximum can evaporate water and other dissolved
materials which then flow through the connecting
pipe and undergo a process of condensation/phase
change from vapor to liquid.
Temperature is a measure or degree of hotness or
coldness of an object or system, temperature is
defined as a physical quantity that is shared between
two or more objects that are in thermal equilibrium
(Putra, 2007). If heat is transferred at the temperature
of the object, then the temperature of the object will
decrease if the object in question loses heat. The
relationship between the heat unit and the temperature
unit is not a constant, because the magnitude of the
increase in temperature due to receiving a certain
amount of heat will be influenced by the heat capacity
of the receiving object (Lakitan, 2002). Heat is energy
that is transferred due to a temperature difference.
Another definition of heat / heat is something that
moves between the system and its environment due to
changes in temperature (Zemansky, 1986). The
second law of thermodynamics is based on that there
is no reversible process. This law is a statement about
the processes that occur in nature. One of the
statements expressed by R.J.E Clausius is that heat
flows naturally from objects at high temperature to
objects at low temperature, not the other way around.
The development of the second law of
thermodynamics is based on the study of heat
engines, namely devices that can convert thermal
energy into mechanical work, such as steam engines
(Giancoli, 2005). Heat is energy that is transferred
due to a temperature difference. Another definition of
heat / heat is something that moves between the
system and its environment due to changes in
Indra, I., Adiaksa, I. and Ardana, G.
Experimental Study of the Effect of Reactor Temperature Reconstruction on Fuel Consumption and Distillate Quantity.
DOI: 10.5220/0010949600003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 595-599
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
595

temperature (Zemansky, 1986). This concept of the
law of thermodynamics will always occur and the
process will stop until the concept of thermal
equilibrium occurs (Ninik, 2019). It can be concluded
that an object or fluid will have a temperature and
naturally that temperature can flow from a high
temperature to a lower temperature until the concept
of thermal equilibrium is formed. This flow of
temperature is often referred to as heat transfer.
Heat transfer is the science of predicting the
transfer of energy that occurs due to temperature
differences between objects or materials. Where the
energy transferred is called heat. Heat is known to be
able to move from a higher place to a lower
temperature (Evalina, 2019). Heat transfer is the
process of transferring energy from one place to
another due to differences in temperature in these
place, heat transfer can also take place in several ways
by convection, conduction and radiation (Indriatma,
2016). Conduction is the transfer of energy from a
particle with a high concentration of an adjacent
substance to a particle with a lower concentration as
a result of the interaction between the particles.
Convection is a model of energy transfer between a
solid surface and an adjacent gas or liquid motion,
and it involves a combination of the effects of
conduction and fluid motion. Radiation is the
emission of energy from matter in the form of
electromagnetic waves (photons) as a result of
changes in the electronic form of molecules or atoms
(J.P Holman, 6th Edition). Theoretically, the heat
transfer can occur through solid, liquid or gas due to
the treatment.
The existence of a distillation column as one of
the vital tools in the separation place, is an almost
always part of the complete process design. A special
understanding of this tool is considered very
important (Komariah, 2009), so in planning a
distillation reactor, it is very important to pay
attention to the manufacture of the reactor. Heat
treatment through certain media can increase the
temperature or the temperature of the existing fluid.
Looking at the second law of thermodynamics, the
heat that occurs will tend to move or flow to a place
that has a lower temperature. This process will occur
continuously until thermal equilibrium is established.
In the distillation column (reactor) the high
temperature distillate fluid will tend to be at the top
and the bottom will tend to be cooler even though heat
treatment is carried out at the bottom. Equilibrium
temperature will be achieved in a relatively long time
because the system used for distillation is open. This
time will greatly affect energy consumption which in
turn will increase the cost of distillate production.
This research will be carried out on the treatment
of the temperature that occurs in the reactor tube.
Temperature reconstruction by providing additional
tools with the aim of thermal equilibrium can be
achieved more quickly. The hope is that as soon as
this equilibrium is reached, it will affect the energy
consumption in the production process. The fixed
variables in this study are temperature and fluid flow
rate, while the independent variables are energy
consumption and distillate quantity.
Based on the background of the problem, the
problem is how to design a distillation reactor with
the addition of a temperature reconstruction tool and
whether the temperature reconstruction treatment can
affect energy consumption and the quantity of
distillate produced.
The problem will be limited by applying existing
applied science to design an appropriate
technological tool that can speed up production time
to reduce production costs that affect energy
consumption. The problem discussed in this study is
the design of a distillation reactor using a 1.5 mm
thick stainless steel plate with dimensions of 40 cm
base and 60 cm height wrapped with heat cover (glass
wool and burlap sacks). The heater uses an LPG stove
with a temperature setting to adjust the flame to suit
the needs of the reactor tube temperature. Steam
outlet using stainless steel pipe. Temperature data is
recorded between 15 menit. LPG weight is measured
in a certain time to get the amount of energy use. The
distillate quantity will be measured after the
distillation process runs at a predetermined time.
2 RESEARCH METHODS
2.1 Research Design
The application of traditional distillation equipment
is to make alcoholic beverages with coconut tree sap
or palm tree sap as raw materials. To obtain 1 liter of
alcoholic beverage it takes at least 16 liters of palm
juice, while the time required to heat the raw
materials to become steam is about 3 to 4 hours, this
takes a relatively long time to reach equilibrium.
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
596
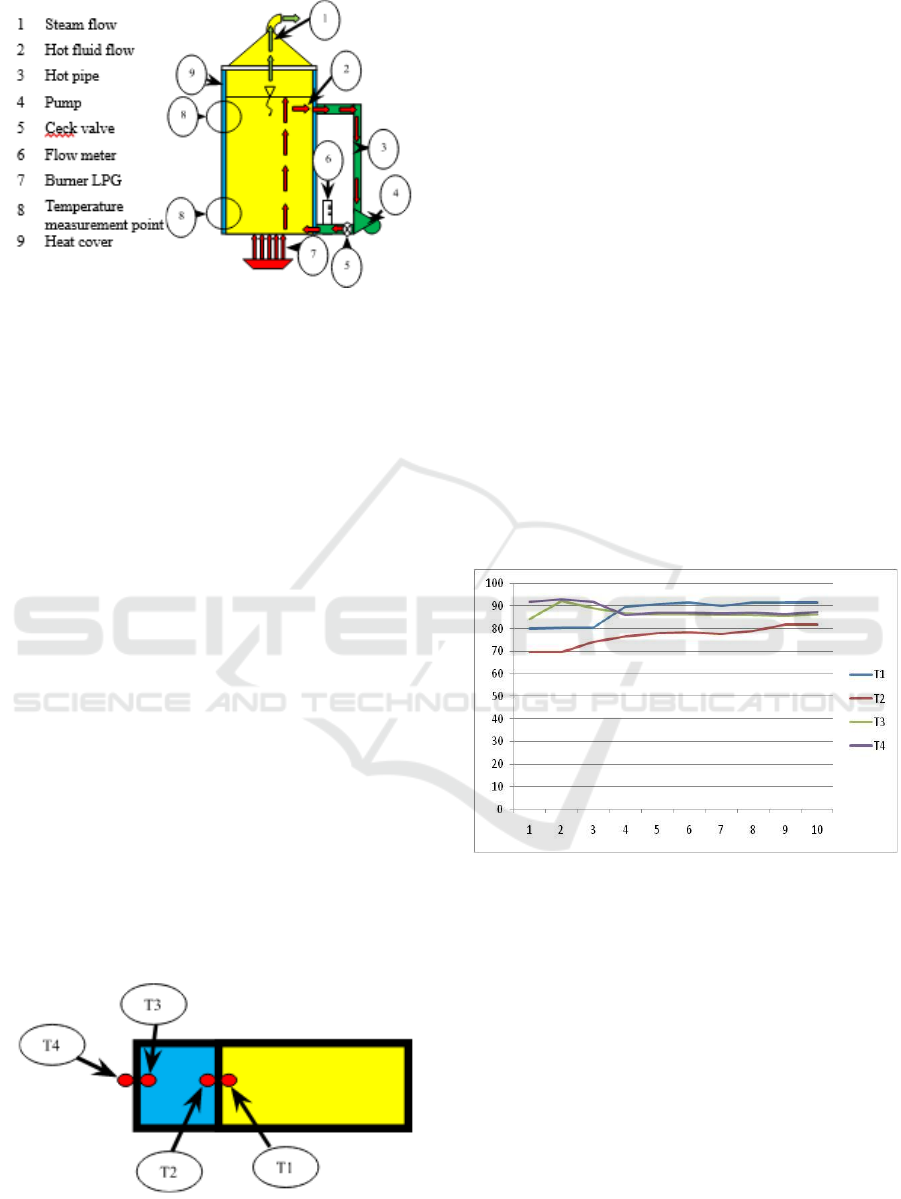
Figure 1: Distillation reactor design with temperature
reconstruction.
The addition of a pump in a device designed to flow
hot fluid that tends to be on the surface back to the
bottom of the reactor. The hope is that with this
circulation, the heat balance will occur more quickly.
The faster the increase in temperature is achieved, the
faster the evaporation of alcohol that occurs and thus
the energy consumption will decrease.
Previous research has been carried out with the
addition of heat cover to prevent heat losses causing
energy requirements to be relatively high. The spread
of heat in the reactor tube still adopts the natural law
where the highest heat will be on the surface of the
fluid.
The addition of a fluid pump in this study is to
obtain thermal equilibrium so that the evaporation
process will be faster and affect energy consumption.
2.2 Research Instruments
The instrument used in the research with the title of
experimental study is the effect of temperature
reconstruction on fuel consumption and distillate
quantity. Collecting data in the test using a
thermocouple by measuring the temperature at
several locations on the distillation tube as shown in
Figure 2.
Figure 2: Location of data retrieval.
The test is carried out based on the data that has
been taken and the variables that have been set. Fixed
variables are heating temperature and fluid flow rate.
The independent variables are fuel consumption and
distillate quantity. The test will be limited to only 5
trials and then find the average.
3 RESULT
The test was carried out 5 times with raw materials of
the same quality for each treatment. The volume
of raw materials is 25 liters. Preheating is done to
reach a temperature of 90 degrees Celsius according
to the temperature setting. Data collection on the
internal temperature and weight of the fuel begins
when the distillation reactor temperature has reached
90 degrees Celsius and the circulation pump is
started. Data were taken every 15 minutes for 60
minutes of heating. The distillate quantity and final
fuel weight were measured after 60 minutes of
processing. The data is displayed according to graph
1.
Figure 3: Test result data.
Figure 1 shows a very significant difference
between a reactor using a pump and a reactor without
a pump. T1 and T2 are the temperatures in the reactor
without a pump. T3 and T4 are the temperatures in
the reactor using a circulation pump. Understanding
the pump in general is a tool used to move fluid from
one place to another (Hirt, 1986). In principle, the
pump converts the mechanical energy of the motor
into fluid flow energy, the energy received by the
fluid will be used to increase the pressure and
overcome the losses that occur in the line.
In this reactor are use a centifugal pump. They use
a rotating impeller to increase the pressure of a fluid.
Centrifugal pumps are commonly used to move
liquids through a piping system. The fluid enters the
Experimental Study of the Effect of Reactor Temperature Reconstruction on Fuel Consumption and Distillate Quantity
597

pump impeller along or near to the rotating axis and
is accelerated by the impeller, flowing radially
outward into a diffuser or volute chamber (casing),
from where it exits into the downstream piping
system. Centrifugal pumps are used for large
discharge through smaller heads. The principle work
of the pump is driven by a motor. Power from the
motor is given to the pump shaft to rotate the impeller
attached to the shaft. The liquid in the impeller will
also rotate due to the impetus of the blades. Because
the centrifugal force arises, the liquid flows from the
center of the impeller out through the channel
between the blades and leaves the impeller at high
speed. The liquid that comes out of the impeller at
high speed will then come out through a channel
whose cross section is getting bigger (volute/diffuse)
so that there is a change from the velocity head to the
pressure head. Suction occurs because after the liquid
is thrown by the impeller, the space between the
blades becomes lower in pressure so that the liquid
will be sucked in. The pump in the reactor is useful
for draining the fluid on the surface to the bottom. The
hot fluid on the surface is returned to the bottom of
the reactor. Pumps used with a capacity of 10 liters
per minute.
The temperature of the reactor without a pump
(T1 and T2) shows a very significant difference
where there is an average temperature difference of
11.09 degrees Celsius. This temperature
difference affects the fuel consumption used. The
initial fuel weight is on average 6.980 kg and after the
process becomes an average of 6.585 kg there is a
decrease in weight of 0.395 kg. That’s an average of
5 times off/on burner with a very short time span from
off burner into on burner to maintain a stable
temperature. The quantity of distillation during the
process obtained an average of 1,250 ml after one
hour of process.
The reactor temperature with the pump (T3 and
T4) looks almost the same where there is only an
average temperature difference of 1.54 degrees
Celsius. This almost small difference indicates that
the temperature in the reactor becomes more even
with the addition of a pump for fluid circulation. This
affects the fuel consumption used only 0.275 kg from
an average initial weight of 6.950 kg to an average of
6.675 kg. That’s average 2 times off/on burner with a
long time span from off to on. The quantity of
distillation results during the process obtained an
average of 1,500 ml after one hour of process.
4 CONCLUSIONS
The conclusions of this study are:
1. The difference in temperature is 86.11%
between the reactor without a pump compared
to the reactor with a pump.
2. Decrease in fuel consumption by 30.3%
between reactors without pumps compared to
reactors with pumps.
3. An increase in the quantity of distillation results
by 16.67% between reactors without pumps
compared to reactors with pumps.
ACKNOWLEDGEMENTS
Thank you to the Bali State Polytechnic Research and
Community Service Center for providing full support
for the implementation of this research.
REFERENCES
Intang, A., & Darmansyah, D. (2018). Analisa
termodinamika laju perpindahan panas dan
pengeringan pada mesin pengering berbahan bakar gas
dengan variabel temperatur lingkungan. FLYWHEEL:
Jurnal Teknik Mesin Untirta, 1(1), 34- 38.
Chen, Y., & Athienitis, A. K. (1998). A three-dimensional
numerical investigation of the effect of cover materials
on heat transfer in floor heating systems. Ashrae
Transactions, 104, 1350.
Krasnoshlykov, A. S. (2016). Analysis of Influence of Heat
Transfer Conditions on the Upper Cover to Heat
Transfer in Thermosyphon. In MATEC Web of
Conferences. Vol. 72: Heat and Mass Transfer in the
System of Thermal Modes of Energy–Technical and
Technological Equipment (HMTTSC-2016).— Les
Ulis, 2016. (Vol. 722016, p. 1052). [sn].
Budiman, A. (2009). Penghematan energi pada menara
distilasi. Reaktor, 12(3), 146-153.
Budiyanto, A., Fadiawati, N., Tania, L., & Syamsuri, M.
M. F. (2015). Alat Distilasi Sederhana Berbasis Barang
Bekas. Jurnal Pendidikan dan Pembelajaran Kimia,
4(3), 1137-1150.
Bergman, T. L., Bergman, T. L., Incropera, F. P., Dewitt,
D. P., & Lavine, A. S. (2011). Fundamentals of heat and
mass transfer. John Wiley & Sons.
Adams, E. E., & Sato, A. (1993). Model for effective
thermal conductivity of a dry snow cover composed of
uniform ice spheres. Annals of Glaciology, 18, 300-304.
Ferahta, F. Z., Bougoul, S., Médale, M., & Abid, C. (2012).
Influence of the air gap layer thickness on heat transfer
between the glass cover and the absorber of a solar
collector. Fluid Dynamics & Materials Processing,
8(3), 339-351.
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
598

Kros, S. J. (1986). Elemen Mesin, edisi kedua. Erlangga,
Jakarta.
Holman, J. P. (1986). Heat transfer, 1986. Mc Gran–Hill
Book Company, Soythern Methodist University.
Supu, S. I., Usman, U. B., & Basri, B. S. (2016). Sunarmi,”.
Pengaruh Suhu Terhadap Perpindahan Panas Pada
Material Yang Berbeda” Pogram Studi Fisika,
Fakultas Sains Universitas Cokroaminoto Palopo.
Indriatma. (2016). “Heat Analisis at Rotary Kiln Unit Iii Pt.
Antam, Tbk ( Persero ),” Ubpn Sultra, Enthalpy Jurnal
Mechanical Enginering. e-ISSN:2502-8944, Vol. 2,
No.2.
Syaichurrozi, I., Karina, A. M., & Imanuddin, A. (2014).
Study of Plate and Frame Heat Exchanger Performance:
The Effects of Mass Flow Rate, Inlet Temperature and
Type of Flow Againts The Overall Heat Transfer
Coefficient. Eksergi, 11(2), 11-18.
Mursito, J. A., Sukadana, I. G. K., & Tenaya, I. G. N. P.
(2017). Perancangan Dan Pengujian Alat Distilasi
Minyak Dari Limbah Sampah Plastik. Jurnal Ilmiah
Teknik Desain Mekanika, 6(4), 311-17.
Walangare, K. B. A. (2016). Design of a Seawater
Conversion Tool into Drinking Water Using a
Distillation Process. Journal of Electrical and
Computer Engineering, Department of Electrical
Engineering-Ft. Unsrat Manado.
Lakitan, B. (2002). Dasar-dasar klimatologi. PT. Raja
Grafindo Persada. Jakarta.
Komariah, L. N., Ramdja, A. F., & Leonard, N. (2009).
Tinjauan Teoritis Perancangan Kolom Distilasi untuk
Pra-Rencana Pabrik Skala Industri. Jurnal Teknik
Kimia, 16(4).
Noufal, M., Kusuma, I. G. B. W., & Suarnadwipa, I. N.
(2017). Analisa Perpindahan Panas Pada Heater Tank
FASSIP-01. Jurnal Mettek: Jurnal Ilmiah Nasional
dalam Bidang Ilmu Teknik Mesin, 3(1), 1- 10.
Evalina, N., Riza, M. K., Arfis, A., & Rimbawaty, R. (2019,
May). Pemanfaatkan Bahan Bakar Sampah Plastik
Dengan Menggunakan Pembangkit Listrik Hot Air
Stirling Engine. In Seminar Nasional Teknik
(SEMNASTEK) UISU (Vol. 2, No. 1, pp. 71-76).
Akhyar, O., & Mashuri, M. T. (2016). Perancangan Dan Uji
Kualitas Alat Destilasi Sederhana Sebagai Langkah
Kreatif Mewujudkan Kemandirian Laboratorium. AL
ULUM JURNAL SAINS DAN TEKNOLOGI, 1(2).
Putra, S., & Kelana, M. (2007). Rancangan Bangunan dan
Analisa Perpindahan Panas pada Ketel Uap Bertenaga
Listrik. Medan: USU.
Khurmi, R. S., & Gupta, J. K.(2008). Text Book of Machine
Design Eurasia. Ram Nagar ltd, New Dehli, 68.
Zemansky, M. F. (1986). Social Support and Burden In
Adult Child Caregivers of Physically Ill Elderly.
Giancoli, D. C. (2005). Physics: Principles with
applications (Vol. 1). Pearson Educación.
Ninik, T. S., Zaenal, A., Dyah, I., & Nurhidayat, N. (2019).
Sensitive and stable ethanol biosensor development
based on Acetobacter aceti biofilm for halal detection
of food and beverages.
Hirt, P. I. E. R. R. E., Hiller, G., & Wittek, R. (1986).
Localization and fine structure of a vaccinia virus gene
encoding an envelope antigen. Journal of Virology,
58(3), 757-764.
Experimental Study of the Effect of Reactor Temperature Reconstruction on Fuel Consumption and Distillate Quantity
599
