
Development of Hydrogen Gas Generator Prototype Model for
Vehicle Fuel with Electrolysis Method
I Putu Darmawa
a
, Ikeh Wiryanta
b
and I Nyoman Sutarna
c
Department of Mechanical Engineering, Politeknik Negeri Bali Badung, Bali, Indonesia
Keywords: Generator, Hydrogen, Electrolysis, Emission.
Abstract: The problem of energy needs in Indonesia is a serious problem in human life. The increase in consumption
of fossil fuels is not matched by the availability of natural resources, therefore alternative energy is needed to
reduce dependence on fossil fuels. One alternative energy that has been found is the electrolysis of water to
produce HHO gas. In this study, the model of a prototype hydrogen gas generator was developed using the
electrolysis method. The HHO generator aims to reduce emissions in vehicle exhaust gases. From the results
of the hydrogen gas generator development, it is found that the dimensions of the generator are length x width
x height = 210 mm x 130 mm x 300 mm, using aluminium plate and polycarbonate mica as the cover. The
electrolyte used is KOH with a percentage of 4%, the test results on the performance of the HHO generator
show that exhaust gas emissions in vehicles will decrease compared to the use of fossil fuels with octane 90,
a decrease in CO emission gas levels of 67% from 1.35% has decreased to 0.47% then the reduction in HC
emission gas levels was 34% from 645.33 ppm, decreased to 422.66 ppm.
1 INTRODUCTION
The problem of energy needs in Indonesia is a serious
problem in human life. Energy is an important
component for human survival because almost all
activities of human life are highly dependent on the
availability of sufficient energy, national energy
needs are still met by petroleum. Indonesia's oil
reserves are predicted to remain at around 3.6 billion
barrels. The reserves are estimated to be exhausted in
the next 12 years (viva.co.id, September 2013). The
cause of this problem is because petroleum is a non-
renewable natural resource, so it takes hundreds of
millions of years to get it back. In terms of controlling
the sustainability of national energy, the Indonesian
government also helps the use of energy composition
by issuing Presidential Regulation no. 5 of 2006
concerning the Goals and Targets of the National
Energy Policy states that the composition of energy
types in Indonesia in 2025 is coal 33%, natural gas
30%, petroleum 20%, and renewable energy 17%.
Included in this 17% are 5% biofuels, 5% geothermal,
a
https://orcid.org/0000-0001-7621-6420
b
https://orcid.org/0000-0002-1501-569X
c
https://orcid.org/0000-0003-2615-1854
biomass, nuclear, hydro, solar and wind 5%, and 2%
liquefied coal."
Hydrogen research and development continues to
be developed very intensively to welcome the era of
hydrogen-based energy which is predicted to be
achieved in the decade of 2050. Research and
development of energy in developed countries agrees
that future energy must have the following
characteristics (Crosbie, L. M., 2003):
1. Future energy technology must be developed,
because energy demand will continue to
increase
2. Future energy sources must be environmentally
Friendly.
3. An effective distribution system must be able to
be created, so that energy can really be widely
affordable and improve people's living
standards.
4. Must be safe both in terms of production,
transportation, storage, and use.
5. Energy technology must be economical.
Darmawa, I., Wiryanta, I. and Sutarna, I.
Development of Hydrogen Gas Generator Prototype Model for Vehicle Fuel with Electrolysis Method.
DOI: 10.5220/0010949400003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 581-586
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
581
6. As an energy carrier, hydrogen is quite
promising and fulfills the characteristics of
future energy.
Hydrogen is a chemical element on the periodic
table that has the symbol H and atomic number 1. At
standard temperature and pressure, Hydrogen is a
colorless, odorless, non-metallic, single-valent, and a
highly flammable diatomic gas. Hydrogen is the
lightest element in the world. Most hydrogen exists in
a compound state with other elements such as
hydrocarbons and water. One way to produce
Hydrogen is through the process of electrolysis with
the help of electrical energy. Hydrogen gas is also
known as HHO gas (Brown Gas).
Several previous studies on the development of
hydrogen gas as an alternative fuel have been carried
out. Study on hydrogen gas production using the PEM
Water Electrolysis method has been conducted. The
review discusses recent developments in PEM water
electrolysis including the high performances low cost
HER and OER electrocatalysts and the challenges
associated with electrocatalysts and PEM cell
components (Kumar, S. Shiva, 2019). Study on the
effect of using a hydrogen gas mixture in internal
combustion engines, both gasoline and diesel
engines. Research shows that hydrogen gas mixtures
can be easily used in internal combustion engines
without the expense of modifying existing engine
configurations (SR. Premkartikkumar, 2015). One
form of innovation to improve energy efficiency in
vehicles is to add or inject HHO (Brown gas) gas into
the internal combustion engine. This HHO gas is
produced from the electrolysis of water with the
addition of a KOH catalyst. The addition of HHO gas
in an internal combustion engine can improve the
quality of combustion because this gas has a high
calorific value and octane, hydrogen fuel is able to
reduce NOx and HC gas emissions (R.F. Horng,
2008). The hydrogen-injected engine eliminates
knock and backfiring. The effect of using hydrogen in
a spark ignition (SI) engine can increase thermal
efficiency by 14% and NOx emissions can be reduced
by up to 95% (T. Suzuki, 2006). From previous
research, it has been compared hydrogen injection in
a spark ignition engine with a carburetor and an
engine with an injection system. The result is that the
fuel injection engine with the addition of hydrogen
has greater power and less backfiring risk (S.
Verhelst, 2005). Another research about hydrogen
gas as a fuelfor combustion also done by Mujebuu,
MA, 2016 and Chi, Jun, 2018. Both of them made a
gas hydrogen by electrolyze water.
From this description, on this research will design
and apply an electrolyser to produce sufficient HHO
(Brown Gas) gas for motorcycles engines by making
a prototype hydrogen gas generator which will later
be used for environmentally friendly vehicle fuel.
2 RESEARCH METHODOLOGY
The development of hydrogen gas generator prototype
model for vehicle fuel were divided into two sections.
The first was creates a design of the HHO generator.
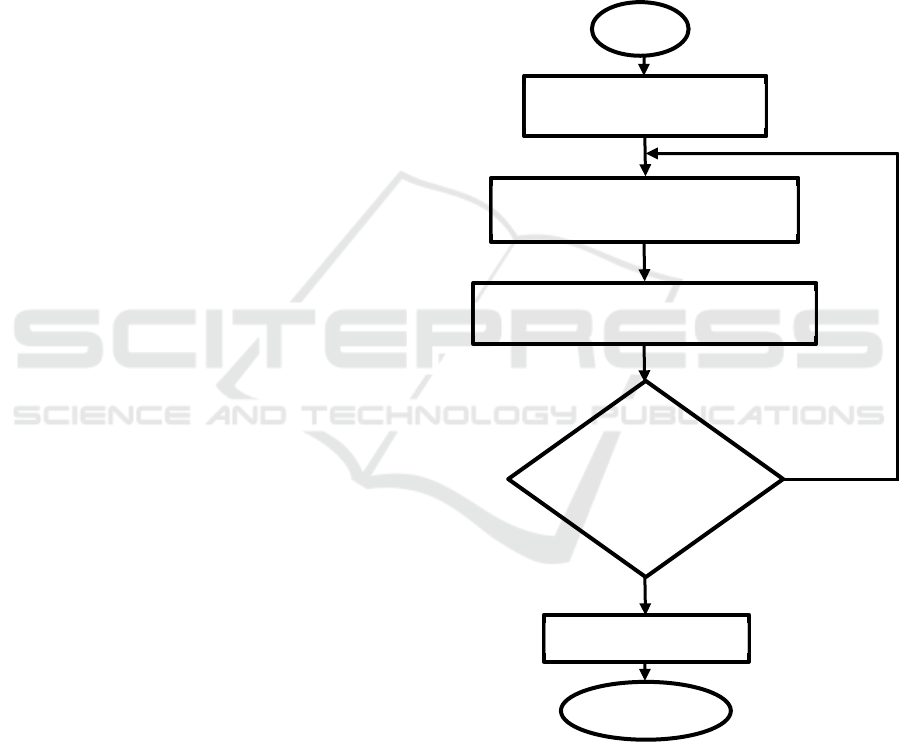
The flowchart of the research are shown in Figure 1.
Figure 1: Flowchart of the research.
By using CAD the design of the micro hydro
power screw Archimedes turbines were shown in
Figure 2.
Yes
N
o
Start
Design prototype of HHO
g
enerator model
Material selection for the generator
and the catalyst solution
Build and fabrication of HHO generator
Experimental report
Testing of
HHO
generator
Finish
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
582

Figure 2: Design of HHO generator.
3 RESULT AND DISCUSSION
From the geometry design using CAD in Fig.2, then
the prototype of HHO generator were built and
developed. The hydrogen generator made has the
following sizes, length x width x height = 210 mm x
130 mm x 300 mm, number of plates = 13 sheets,
number of cells = 12 cells, plate material =
aluminium. The prototype of HHO generator were
shown in Figure 3 below:
Figure 3: Prototype HHO generator engine.
In this HHO generator engine, there are several
types of components, including:
1. Aluminium plate with a thickness of 2 mm. The
generator made is divided into 3 plates, namely:
a. Positive plate, positive plate is a plate that
carries positive current. The positive plate has
the following sizes, the negative plate size is
270 mm high, 160 mm wide and 2 mm thick,
2 pieces.
b. Negative plate, negative plate is a plate that
carries negative current. The negative plate
size is 270 mm high, 160 mm wide, and 2mm
thick, 1 piece.
c. Neutral plate, neutral plate is a plate that limits
the negative plate and positive plate. The
neutral plate has the following dimensions,
250 mm high, 160 mm wide and 2 mm thick
with a total of 10 plates.
2. Polycarbonate mica, using polycarbonate mica
with a length of 210 mm x a width of 297 mm with
a thickness of 5 mm.
3. The rubber gasket used has a height of 270 mm, a
width of 160 mm and a thickness of 5mm.
The HHO generator that has been developed then
tested in a 100cc four stroke gasoline engine. The test
was conducted to determine the flow rate and
efficiency of the dry cell type HHO generator, with
the main ingredient in the form of water and
additional KOH catalyst with a mass percentage of
4% of the water volume of 2000 ml.
Figure 4: Performance test of HHO generator.
The result from the test shown in table 1 below:
Table 1: Test Result.
No Catalyst Voltage
(V)
Curren
t (A)
Time
(s)
𝒙
𝟎
∆𝒙
1 KOH 14.8 20 3.56
5.30
4.58
2.05
3.36
3.772.72
Development of Hydrogen Gas Generator Prototype Model for Vehicle Fuel with Electrolysis Method
583

3.1 P HHO Generator
From the result above it can be seen that the KOH
catalyst requires a voltage of 14.8 volts with a current
of 20 amperes, which has an average time of
3.77±2.72 seconds to produce 10 ml of HHO gas.
The power used in HHO generator then can be
known:
P = V.I
P = 14.8 V. 20 A
P = 296 watt
3.2 HHO Gas Flow Rate
From the result in table 1, the flow rate of HHO gas
can be known:
𝑉
=
𝑣𝑜𝑙𝑢𝑚𝑒
𝑡
V
=
10 ml
3.77 s
= 2.56 ml/s
3.3 HHO Gas Flow Rate
Based on the results of the calculation of the HHO
generator power consumption and HHO gas flow rate,
it can be calculated to find the efficiency of the HHO
generator tool that uses a KOH catalyst with a
percentage of 4%, with an additional value of the
density of HHO gas of 0.491167 gr/l and a value of
0.491167 gr/l. from the LHV of HHO gas of 13.25
kJ/gr.
Ƞ
=
×
×
× 100%
Ƞ
=
2.56 × 0.491167 × 13.25
296
× 100%
Ƞ
=5.6 %
The ratio of hydrogen gas and oxygen gas in
Brown's gas is the same, so the density of HHO and
LHV HHO does not affect the cause of the increase
or decrease in the efficiency of the generator. While
the amount of power greatly affects the increase and
decrease in efficiency in this study. The higher the
power used, the lower the efficiency, it can be seen
that the greater the current, the higher the
productivity, but not proportional to the greater the
energy used so that the efficiency will decrease. A lot
of the energy used is turned into heat and not used to
break the bonds of water, so a lot of energy is wasted
and the efficiency will decrease.
3.4 Exhaust Emission Testing
The exhaust testing was conducted in a single
cylinder 4 stroke engine, 110 cc. The experimental set
up was shown in Figure 5 below:
Figure 5: Engine set-up.
The results from the emission test (CO and HC)
without using a hydrogen generator shown in table 2
below. The results from the CO emission test are in
percentage (%) and the results from the HC test are
parts per million (ppm)
Table 2: Emission test without hydrogen generator.
No CO (%) HC (ppm)
1 0.93 677
2 1.44 629
3 1.79 630
Average 1.35 645.33
The results from the emission test (CO and HC) using
dry cell type hydrogen generator, and water – KOH
as the catalyst shown in table 3 below. The results
from the CO emission test are in percentage (%) and
the results from the HC test are parts per million
(ppm).
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
584
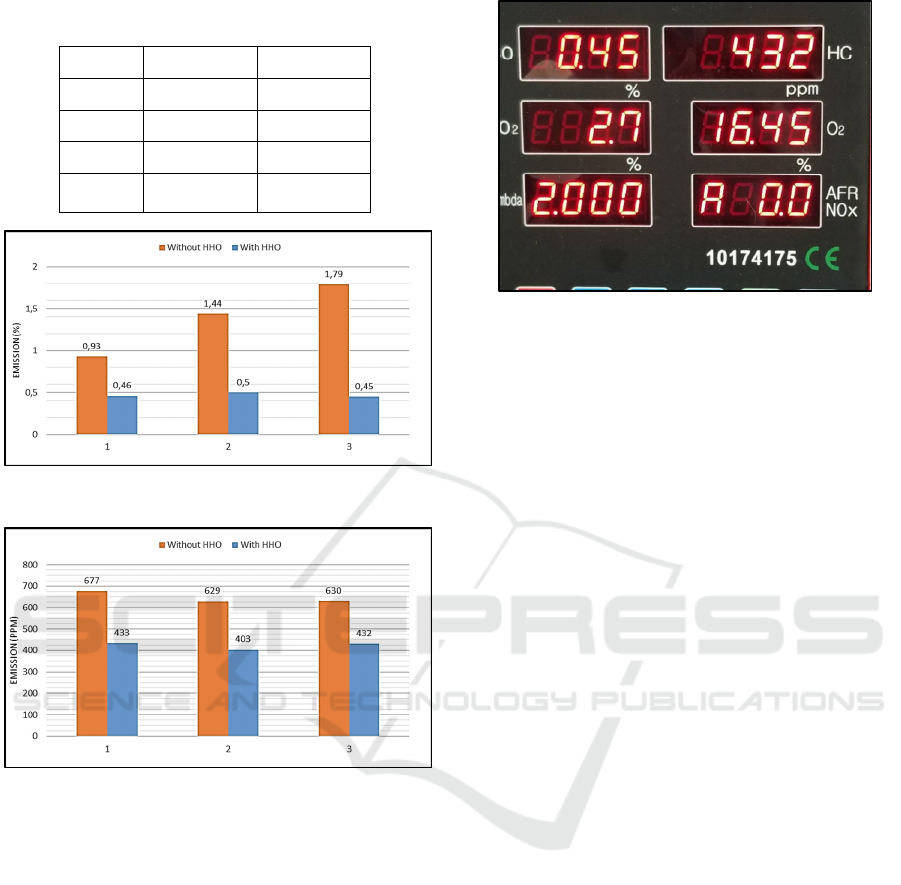
Table 3: Emission test with hydrogen generator (water-
KOH catalyst).
No CO (%) HC (ppm)
1 0.46 433
2 0.50 403
3 0.45 432
Average 0.47 422.66
Figure 6: Emission test of CO (%).
Figure 7: Emission test of HC (ppm).
From Figure 6 it can be seen that the ratio of CO
gas when not using an HHO generator is much greater
than using an HHO generator. The use of a hydrogen
generator causes differences in exhaust gas emissions
in CO gas (carbon monoxide) which is quite far,
compared to the standard which uses RON 90 fuel,
the average concentration of CO gas without HHO
generator was 1.35% then in using HHO generator,
the average concentration decreasing into 0.47%.
The emission test of HC gas with and without
using HHO generator were shown in Figure 7. The
result showed that the use of HHO generator also can
decreasing the emission of HC gas compared to the
standard which uses RON 90 fuel. The average
emission of HC gas were 645.33 ppm and the average
emission of HC using HHO generator were 422.6
ppm, which the decreasing was about 34.5%.
Figure 8: Emission test using gas analyser.
4 CONCLUSIONS
From the discussion above, it can be concluded that
the HHO generator that has been made can work
properly. The generator can produce enough HHO
gas that can be use in motorcycle engine. The test
result of uses HHO gas to the engine showed that the
use of HHO generator can reduce the emission of
exhaust gas (CO and HC) compared to the standard
which uses RON 90 fuel. The average concentration
of CO gas decreasing about 0.47%, and the average
emission of HC decreasing about 34.5%.
ACKNOWLEDGEMENTS
The authors would like to thank the Director and the
Head of P3M Politeknik Negeri Bali for funding this
research. Authors also like to thanks to all the staff of
mechanical engineering department of PNB for all
their hands.
REFERENCES
Chi, Jun, Hongmei Yu, (2018). Water electrolysis based on
renewable energy for hydrogen production. Chinese
Journal of Catalysis. 39, p. 390–394.
Crosbie, L. M., Chapin, Douglas, (2003). Hydrogen
Production by Nuclear Heat. GENES4/ANP2003. Sep.
15-19, 2003, Kyoto, JAPAN.
Kumar, S. Shiva, V. Himabindu, (2019). Hydrogen
production by PEM water electrolysis – A review.
Materials Science for Energy Technologies. Vol. 2, p
442–454.
Mujebuu, MA., (2016). Hydrogen and syngas production
by superadiabatic combustion – A review. Applied
Energy. 273, p. 210-224.
Development of Hydrogen Gas Generator Prototype Model for Vehicle Fuel with Electrolysis Method
585

R.F. Horng, et al, (2008). Driving characteristics of a
motorcycle fuelled with hydrogen-rich gas produced by
an onboard plasma reformer. Int. Journal of hydrogen
energy. 33, p. 7619 – 7629.
SR. Premkartikkumar, AR. Pradeepkumar, (2015). Effect
of using hydrogen mixed gases as a fuel in internal
Combustion engines – A Review. International Journal
of Innovative Research in Advanced Engineering
(IJIRAE). Vol. 2, p. 29 – 31.
T. Suzuki, Yoshihito Sakurai, (2006). Effect of Hydrogen
Rich Gas and Gasoline Mixed Combustion on Spark
Ignition Engine. Powertrain & Fluid Systems
Conference & Exhibition, Toronto, Canada, October
16-19.
Verhelst, S., R. Sierens, (2001), Aspects concerning the
optimisation of a hydrogen fueled engine. Int. Journal
of hydrogen energy. 26, p. 981 – 985.
viva.co.id, September 2013.
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
586
