
Effect of Different Type Catalyst on Biodiesel Production from
Jatropha Curcas Oil via Transesterification using Ultrasonic Assisted
Muh. Irwan
1,2
, Ramli
1,2
, Mardhiyah Nadir
1,2
,
Marlinda
1,2
and Arief Adhiksana
1,2
1
Politeknik Negeri Samarinda, Jalan Dr. Ciptomangunkusumo Kampus Gunung Lipan, Samarinda, Indonesia
2
Department of Chemical Engineering, Politeknik Negeri Samarinda, Samarinda, Indonesia
Keywords: Biodiesel, Catalyst, Jatropha, Oil, Transesterification, Ultrasonic.
Abstract: Biodiesel as an alternative energy can be produced through transesterification reaction from different
vegetable oils such as palm oil, coconut oil, jatropha oil and other sources. Jatropha oil has 4.61% free fatty
acids (FFA<5%) so the biodiesel production can be done directly by the transesterification reaction. The
purpose of this study is to determine the effect of transesterification time and the type of catalyst on the yield
of biodiesel obtained. The catalysts used are sodium hydroxide (NaOH), potassium hydroxide (KOH), and
sodium methoxide (CH
3
ONa) with a concentration of 1% mass of oil dissolved into methanol using of 1:6
molar ratio. Transesterification reaction assisted by ultrasound 35 kHz with different time of reaction (10, 15,
20, 25, and 30 minutes). The highest biodiesel yield was obtained at 30 minutes with 89,531% using KOH as
catalyst. The results of physical properties such as viscosity of 5.71 cSt, density of 860.17%, and moisture
content of 0.0136% have met SNI 7182-2015 standard.
1 INTRODUCTION
The depletion of fossil fuels in the near future makes
the world community look for alternative energy
sources (Mohandass. et al, 2016). Indonesia is one of
the world's petroleum-producing countries, but to
date is still importing fuel oil to meet fuel needs in the
transport and energy sectors. Thus, the government is
trying to reduce the amount of imported fuel oil by
increasing the use of biodiesel to be mixed in fossil
oils as diesel fuel. One of them is biodiesel used as a
substitute of petroleum fuel (Kementerian ESDM,
2013). The Government of Republic Indonesia issued
a Minister of Energy and Mineral Resources
Regulation No. 25 of 2013 on supply, utilization and
trading biofuel, which requires increased use of
biodiesel in the transportation, industrial, commercial
and power sectors. It is targeted that in 2013 it saved
1.3 million kilo liter of diesel fuel and 4.4 million kilo
liter in 2014, in the next year there will be a decrease
in diesel fuel imports by 5.6 million kilo liter
(Kementerian ESDM, 2013). Biodiesel, is a fuel that
is potentially used as a substitute for petroleum fuel.
This is because of being from renewable, non toxic
and biodegradable like vegetable oil . (Ji. et al, 2006;
Deng et al, 2010). In addition, biodiesel / biofuel is
also environmentally friendly fuels, it does not
contain sulfur to reduce the environmental damage
caused by acid rain (Azis, 2010). Biodiesel can be
produced by the transesterification process or
alcoholysis of vegetable oil or animal fats. The cost
of biodiesel production is mainly influenced by the
system used and the cost of raw materials. Currently,
waste oil or fats are used in biodiesel production
because 70 to 90 % of production costs are due to raw
materials. Alternatively, overall production costs can
also be reduced by optimizing the efficiency and the
catalyst used (Talha and Sulaiman, 2016). The aim
this process to reduce the viscosity of vegetable oil
or animal fats can be applied in regular combustion
engine (Prabaningrum et al, 2014). The alcoholysis
process is the reaction between short-chain alcohol
with triglyceride from vegetable oil to produce fatty
acid alkyl ester (biodiesel) and glycerol as by-product
(Hoque and Gee, 2013). The presence of catalysts in
this process is very necessary to enhance the reaction
rate and ultimately the biodiesel yield. The
alcoholysis reaction is reversible and excess alcohol
shifts are equilibrium to the product side (Rahmah et
al, 2013). Many different type of alcohol can be used
in this reaction, including methanol, ethanol,
propanol and butanol. The general equation of
412
Irwan, M., Ramli, ., Nadir, M., Marlinda, . and Adhiksana, A.
Effect of Different Type Catalyst on Biodiesel Production from Jatropha Curcas Oil via Transesterification using Ultrasonic Assisted.
DOI: 10.5220/0010946700003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 412-415
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
transesterification reaction can be shown in Figure 1
below :
Figure 1: General equation of transesterification reaction.
Figure 1 above shown the comparison of molar
ratio between triglyceride and alcohol to produce of
ester (biodiesel) and also glycerol as by product.
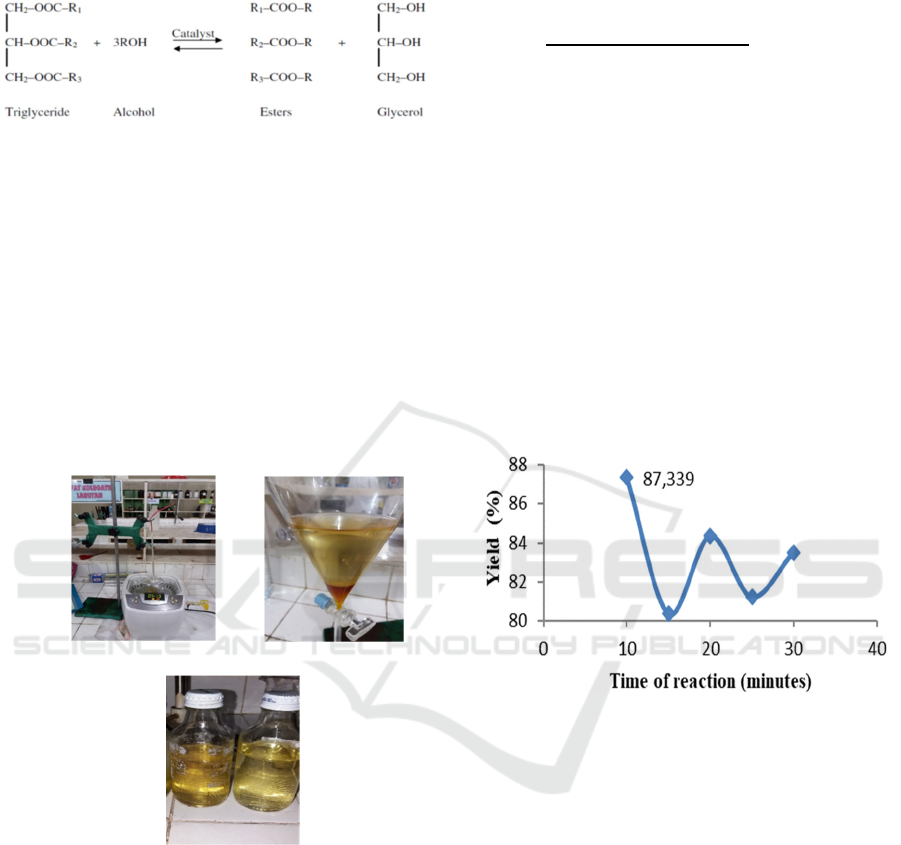
2 METHOD
The ultrasonic apparatus as source of energy was
performed to production of biodiesel in a batch
reactor (Rahmah et al, 2013; Rahmaniah et al, 2013;
Suryanto et al, 2015). The design of equipment used
in this work shown in Figure 2a below :
(a) (b)
(c)
Figure 2: (a). Ultrasonic equipment; (b). two phase layer
product; (c). product of biodiesel.
Figure 2a shown the jatropha curcas oil as raw
materials were transesterified using ultrasonic reactor
assisted. In this work, KOH, NaOH and CH
3
ONa was
dissolved each with methanol. Jatropha curcas oil as
feedstocks was taken at following reaction time 10,
15, 20, 25 and 30 minutes. Once the reaction is
complete, the samples is immediately quenched in
water to stop the reaction until two phases are formed.
The next process, sample is washed using distilled
water 3-4 times until the water layer became clear
(figure 2b). The last, is the heating process to remove
a lot of water in the ester as product. Finally, biodiesel
can be stored (figure 2c). To calculate the percentage
of yield can use the following formula:
Yield =
x 100 %
(1)
3 RESULT AND DISCUSSION
The most commonly used alkaline or base catalyzed
transesterification are NaOH, KOH and CH3ONa.
Alkaline catalyzed transesterification is more rapid
than acid catalyzed and is used in the commercial
production of biodiesel. Event at ambient
temperature, the alkaline catalyzed reaction proceeds
faster usually reaching more than 80 % a few minutes
using ultrasonic assisted (Rahmaniah et al, 2013).
This is the yield comparison of the three types of
catalyst used in this work. When using NaOH as
catalyst, the yield can be shown in Figure 3 as
follows:
Figure 3: The yield of biodiesel using the NaOH as catalyst.
Figure 3 shown the high biodiesel yield using
NaOH as catalyst, namely 87.339 % at 1 : 6 molar
ratio of jatropha curcas oil to methanol and 1 %
concentration of NaOH to raw materials. In this
experiment, only three types of catalyst were used
they were sodium hydroxide (NaOH), potassium
hydroxide (KOH), and sodium methoxide (CH
3
ONa).
For the biodiesel production using KOH as
catalyst, the yield can be shown in Figure 4 as
follows:
Effect of Different Type Catalyst on Biodiesel Production from Jatropha Curcas Oil via Transesterification using Ultrasonic Assisted
413
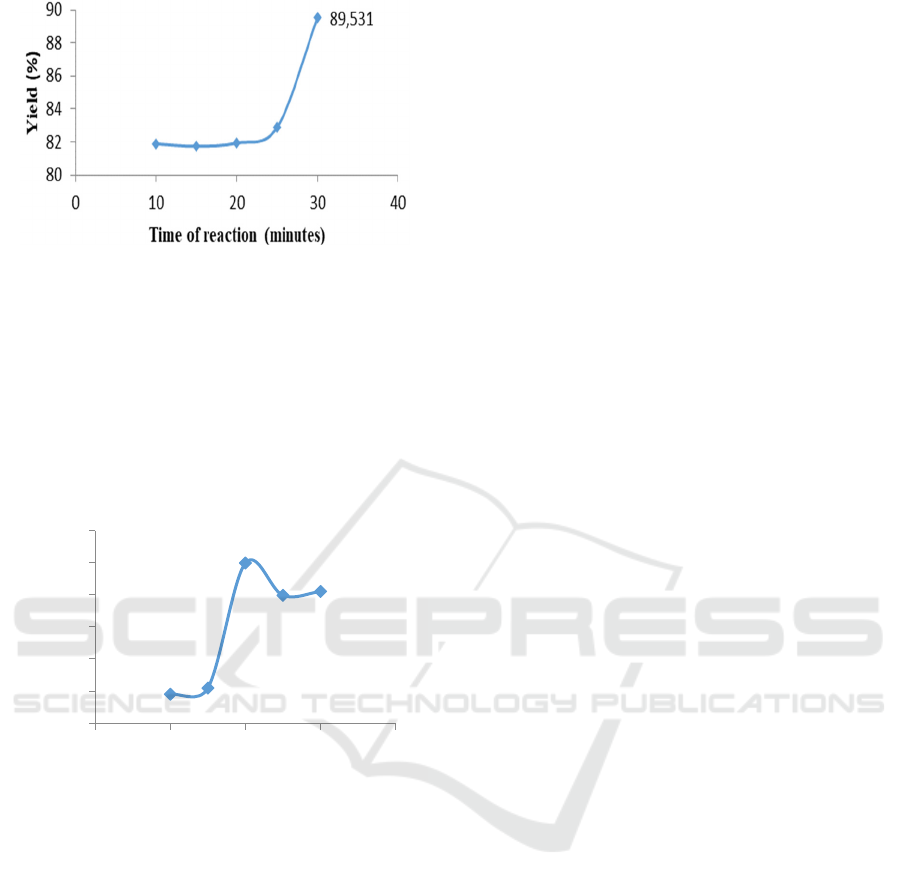
Figure 4: The yield of biodiesel using the KOH as catalyst.
Figure 4 shown the high biodiesel yield using
KOH as catalyst, namely 89.531 % at 1 : 6 molar ratio
of jatropha curcas oil to methanol and 1 %
concentration of KOH to raw materials. The results
showed that KOH gave the better yield, compared
with NaOH.
For the biodiesel production using CH
3
ONa as the
third catalyst in this work, the yield can be shown in
Figure 5 as follows:
Figure 5: The yield of biodiesel using the CH
3
ONa as
catalyst.
Figure 5 shown the high biodiesel yield using
CH
3
ONa as catalyst, namely 85.986 % at 1 : 6 molar
ratio of jatropha curcas oil to methanol and 1 %
concentration of CH
3
ONa to raw materials.
There are no significant different between these
three catalyst in term of biodiesel yield. There are also
no clearly reason to explain why KOH is better than
other catalyst. But, there are many researchers found
that types of catalyst performance are strongly
dependent on raw materials used. Besides, catalysts
performances were also affected by the reaction
conditions.
4 CONCLUSIONS
From the results of this study, it was found that of the
three types of catalyst used, KOH produced the
highest yield of 89.53 %, while the use of ultrasonic
assisted in the transesterification reaction give the
impact of reaction time reduction compared of
conventional process which can take between 2-4
hours. Results of the physical biodiesel properties
namely of the 5.71 cSt viscosity, density of 860.17 %
and moisture content of 0.0136 % has met SNI 7182-
2015 standard.
ACKNOWLEDGEMENTS
This study was financially supported by the Centre of
Research and Community Service, Politeknik Negeri
Samarinda with the scheme grand Hibah Penelitian
Doktor 2021.
REFERENCES
Azis, (2010). Uji Performance Mesin Diesel Menggunakan
Biodiesel Dari Minyak Goreng Bekas.
Deng X, Fang Z, Liu Yh, (2010). “Ultrasonic
transesterification of jatropha curcas L. oil to biodiesel
by a two-step process”, Journal of Energy Conversion
and Management., Vol.51, pp 2802-2807.
Hoque, M.E, Gee, L.P, (2013). “Biodiesel from Plant
Resources-Sustainable Solution to Ever Increasing
Fuel Oil Demands”, Journal of Sustainable Bioenergy
System., Vol.5, pp.163- 170.
Ji J, Wang J, Li Y, Yu Y, Xu Z, (2006). “Preparation of
biodiesel with the help of ultrasonic and hydodynamic
cavitation”, Journal of Ultrasonic., Vol.44, pp. 411-414.
Kementerian ESDM. (2013). Program Percepatan
Pemanfaatan Bahan Bakar Nabati (BBN).
Mohandass, R, Ashok, K, Selvaraju, A. Rajagopan, S
(2016). “Homogeneous Catalysts used in Biodiesel
Production: A Review”, International Journal of
Engineering Research & Technology (IJERT)., Vol.5,
Issue.5, pp. 264-268.
Prabaningrum, N, Ismail, L.B, Subbarao, (2014). “In-Situ
Methanolysis of Jatropha curcas Seeds in Soxhlet
Extractor”, Journal of Advanced Materials Rexearch.,
Vol.917, pp. 72-79.
Rahmah, N, Sulaiman, H, Hazwani, N. (2013). “Process to
Produce Biodiesel Using JatrophaCurcas Oil (JCO)”,
International Journal of Materials Science and
Engineering., vol.1, no.2, pp. 100-103.
Rahmaniah, O, Ju, Y.H, Vali, S.R, Tjondronegoro, I,
Musfil, A.S. (2015). “A Study on Acid-Catalyzed
Transesterification of Crude Rice Bran Oil for
Biodiesel Production”,
85,986
81
82
83
84
85
86
87
0 10203040
Yield (%)
Time of reaction (minutes)
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
414

Suryanto, A, Suprapto, Mahfud. (2015). “The production of
Biofuels from Coconut Oil Using Microwave”, Journal
of Modern Applied Science., Vol.9, no.7, pp. 93-98.
Talha, N.S, Sulaiman, S. (2016). “Overview of Catalysts in
Biodiesel Production”, ARPN Journal of Engineering
and Applied Science., Vol.11, No.1, pp. 439-448.
Effect of Different Type Catalyst on Biodiesel Production from Jatropha Curcas Oil via Transesterification using Ultrasonic Assisted
415
