
Thermal Performance of Bio-Nano-PCM based Passive Cooling for
Vaccine Carriers
I Nyoman Suamir
a
, I Wayan Adi Subagia
b
, Luh Putu Ike Midiani
c
,
I Dewa Gede Agus Tri Putra
d
and I Made Rasta
e
Mechanical Engineering Department, Politeknik Negeri Bali, Campus Street, Kuta Selatan, Badung, Bali 80364, Indonesia
Keywords: Temperature Performance, Bio-Nano-PCM, Passive Cooling, Vaccine Carrier.
Abstract: Vaccine storage requires special attention because vaccines are biological supplies that are susceptible to
changes in environmental temperature. Vaccines are also biological products that are easily damaged so they
must be stored at temperature of 2 to 8 ºC. The vaccine can be damaged if exposed to direct sunlight. The
vaccine distribution box (vaccine carrier) presented in this paper was developed with considerations the box
is easy to carry, have aesthetics, light weight, practical use and save energy while still having good cooling
capabilities of 2-8 ºC. The vaccine box incorporates 3.2 kg Bio-Nano PCM as cold thermal storage material.
The box has a capacity of 2.8 liters or capable of loading 20 bottles of vaccine. Total weight of vaccine carrier
is approximately 7 kg. Test results showed that Bio-Nano PCM technology integrated in the vaccine carrier
box could cool and kept the vaccine at temperature range of 2-8 ºC for more than 24 hours.
1 INTRODUCTION
In the current situation of the Covid-19 pandemic, the
distribution of vaccines is very important and vital for
the people of Indonesia. The Indonesian government
is currently preparing various types of vaccines for all
Indonesian people. Considering that Indonesia is an
archipelagic country consisting of 34 provinces, the
distribution of vaccines is very important for the
community so that when the vaccine arrives it is still
in good and safe conditions.
In general, vaccine distribution boxes (vaccine
carriers) are indeed needed in the national vaccine
program for eradicating infectious diseases and
infections. More specifically, the national Covid-19
vaccination program is urgently needed to protect and
prevent the spread of the Covid-19 pandemic in
Indonesia (World Health Organization, 2020).
Vaccination or immunization is very important for
both children and adults. Therefore, immunization
can prevent the transmission of various diseases and
infections by increasing the body's immunity. The
a
https://orcid.org/0000-0003-0594-7511
b
https://orcid.org/0000-0001-9261-3549
c
https://orcid.org/0000-0002-2256-6035
d
https://orcid.org/0000-0002-9422-7876
e
https://orcid.org/0000-0002-9610-3738
obstacle encountered in immunization activities in
Indonesia is maintaining the cold chain from the
vaccine manufacturer until the immunization activity
is carried out. Cold chain is a procedure and devices
used in the delivery or storage of vaccines starting
from the manufacturer until the vaccines are given to
the community.
Vaccine storage requires special attention because
vaccines are biological supplies that are susceptible to
change in environmental temperature. According to
the Minister of Health Regulation number 12 (2017)
concerning the implementation of immunization, it is
stated that vaccines are biological products that are
easily damaged so they must be stored at a certain
temperature, namely at a temperature of 2 to 8ºC for
freeze sensitive vaccines (not frozen), and at a
temperature of -15 to -25ºC. for heat sensitive
vaccines. Currently, only polio vaccine still requires
storage at temperatures below 0 °C. A number of
vaccines can potentially be damaged if they exposed
to freezing temperatures. Meanwhile, other vaccines
can potentially be damaged if they exposed to hot
Suamir, I., Subagia, I., Midiani, L., Putra, I. and Rasta, I.
Thermal Performance of Bio-Nano-PCM based Passive Cooling for Vaccine Carriers.
DOI: 10.5220/0010944900003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 329-334
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
329

temperatures. In general, the vaccines, however, can
be damaged if they exposed to direct sunlight
(Primadi, 2017).
The toughest obstacle arises when it comes to
bringing vaccines to remote areas that do not yet have
major infrastructure such as motorized driveways and
electricity networks to run refrigerators. The tools
commonly used in the distribution of vaccines for
long distances are cold boxes that use icepacks as a
cooling medium. The tools have disadvantage of not
being able to maintain the temperature of the vaccine
in the proper range from 2-8 °C, as well as they have
a limited cooling time. Efforts have been made to help
overcome this problem, prototype vaccine carriers
have been developed using a Peltier element (Putra et
al., 2005; Putra et al., 2006; Putra, 2006). The vaccine
boxes developed with Peltier elements is able to
maintain the vaccine temperature from 2-8 °C, but
they still have disadvantages in terms of prototypes
compactness and they also still require other
supporting equipment which makes the products are
less practical.
The concepts of the vaccine carrier have also been
developed in Indonesia. The concepts are based on
thermo-electric refrigeration and are also combined
with ice-packs. The first concept was developed at the
University of Indonesia. This vaccine carrier looks
less practical when carried by health workers as a
carrier. The second concept was developed by ITS
Surabaya. This vaccine carrier concept uses
thermoelectric refrigeration with power supply from
solar panels and is combined with ice-pack or blue-
ice.
The technology concept applied to the vaccine
carrier developed in this study is the concept of
passive cooling (passive refrigeration) using a cold
storage material based on natural materials, namely
Bio-Nano PCM with a phase change temperature in
the range of 0 °C as an application. sustainable
technology. The Bio-Nano PCM is housed in an
encapsulation and integrated with a specially
designed heat transfer wall so that it is able to
maintain the vaccine chamber temperature in the
range of 2-8 °C. The volume of Bio-Nano-PCM was
designed so that the vaccine carrier is able to maintain
a minimum time storage for 24 hours.
For comparison purposes, the paper also presents
the most famous PCM that is water because it has
good thermal properties, but has the disadvantage of
high super-cooling when applied as thermal storage
at below 0 °C. Bio-PCM using ester oil in water
mixture is also presented as an alternative use of the
bio-thermal energy storage. The Bio-PCM has a good
advantage with lower super cooling than pure water
but it can maintain the advantage of high phase
change enthalpy of the water as reported in Rasta and
Suamir (2018), Rasta and Suamir (2019), and Suamir
et al. (2019).
The bio-Nano PCM vaccine carrier developed in
this study was also designed through a concept of
applying appropriate technology which was made to
meet the right needs according to its use. The use of
technology is in accordance with local natural, social,
economic and cultural conditions and can help
improve the economic standard and quality of life of
the community. Therefore, the technology has to have
technical characteristics, such as easy to manufacture,
safe to use, economically inexpensive, low impact to
the environment and energy efficient (Putra et al.,
2006; ITSmis, 2020; Pearce et al., 2014; Zelenika and
Pearce, 2011). The technology is also projected to be
applicable, comfortable, healthful, practical and
efficient (Zelenika and Pearce, 2012; Pearce, 2012;
Shin et al., 2019; Patnaik and Bhowmick, 2019,
Boakye-Ansah, 2020).
2 MATERIALS AND METHODS
2.1 Vaccine Carrier Box
The development of the vaccine carrier with Bio-
Nano PCM involved several stages such as design,
assembly, installation of instrumentation and
performance testing. For the carrier box material, a
cool box that is already on the market is used with an
outer size of 32 cm x 22 cm x 25 cm as shown in
Figure 1.
Figure 1: The cool box used as the base container of the
vaccine carrier.
The Bio-Nano PCM used in this carrier is liquid
at room temperature. Therefore, in its integration into
the vaccine carrier, the Bio-Nano PCM has to be
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
330

contained in an enclosure. In this study acrylic
enclosure are applied.
There are two types of containers used which
include: ice-pack boxes (available in the market) and
acrylic enclosures (designed and made specifically to
fit in the box and the vaccine bottle holder. The ice-
pack bio-Nano PCM enclosures used are 14 with
similar size 11 cm x 9 cm x 3 cm and each enclosure
can contains 195 g of Bio-Nano PCM. So the total
weight of bio-Nano PCM reaches 2.7 kg.
Figure 2: The bio-Nano PCM containers and vaccine bottle
holder.
The acrylic PCM box is made of 5 boxes. With 3
boxes of size 250 mm x 160 mm x 30 mm. Each
acrylic box can contain 0.865 kg. Two other smaller
boxes size 90 mm x 160 mm x 30 mm which can
contain 0.3 kg bio-Nano PCM each. Therefore, total
mass of bio-Nano PCM for acrylic boxes is 3.2 kg.
The acrylic box construction and the vaccine bottles
holder are presented in Figure 2.
Figure 3: The cooler box completed with bio-Nano PCM
containers and vaccine bottle holder.
The bio-Nano PCM vaccine carrier used in this
study can be detailed: (i) Vaccine carrier with ice-
pack PCM enclosure; (ii) Vaccine carrier with acrylic
PCM enclosure. Furthermore, to place the vaccine
safely in the carrier, both carriers are equipped with
vaccine holders as shown in Figure 2 and Figure 3.
Each vaccine carrier can accommodate 2 vaccine
holders arranged in two layers: top and bottom. Each
holder can accommodate 10 bottles of vaccine, so that
each vaccine carrier can accommodate 20 vaccine
bottles.
Between the vaccine holders and the PCM
enclosures, an acrylic wall partition is installed to
avoid direct contact between the vaccine holder and
the PCM enclosure. The complete assembly of the
bio-Nano PCM vaccine carrier is shown in Figure 3.
2.2 Methods
The type of research is an experimental study. This
research was initiated by conducting surveys and
literature studies to obtain secondary data about
vaccines and vaccine carriers including storage
conditions. A survey was also conducted to obtain the
characteristics of the Bio-Nano PCM that can be used
so that it is able to maintain the temperature of the
vaccine storage room.
Simulation methods with inventor and EES
(engineering equation solver) were also applied to
simulate the design of the Bio-Nano PCM vaccine
carrier and components as well as simulation of
vaccine room temperature based on secondary data.
Furthermore, the simulation was developed using the
primary data from the test results. The prototype of
the Bio-Nano PCM vaccine carrier was made and
function tests were carried out as well as testing the
characteristics of the vaccine room conditioning
process with various conditions of control variables.
Testing is carried out at the laboratory level. Primary
data from the test is recorded and processed to obtain
a consistent conditioning process and in accordance
with the target temperature in the range of 2-8 °C.
3 RESULTS AND DISCUSSION
3.1 Bio-Nano PCM Cooling and
Heating Characterization
An effective alternative PCM material can be used as
a passive cooling material. A vaccine carrier has been
tested for the characteristics of charging or cooling
and discharging or heating. The test results for tap
water, bio-Nano PCM and bio-PCM are presented in
Figure 4.
From Figure 4, it can be seen that the 10% bio-
PCM has a phase change temperature, especially
during discharging, which is relatively less stable
than the other two materials, namely tap water and
bio-Nano PCM. In addition, the phase change time is
very short, although the temperature change rate is
relatively slow and the phase change temperature
(PCT) of -5 °C is quite far from the vaccine
Thermal Performance of Bio-Nano-PCM based Passive Cooling for Vaccine Carriers
331
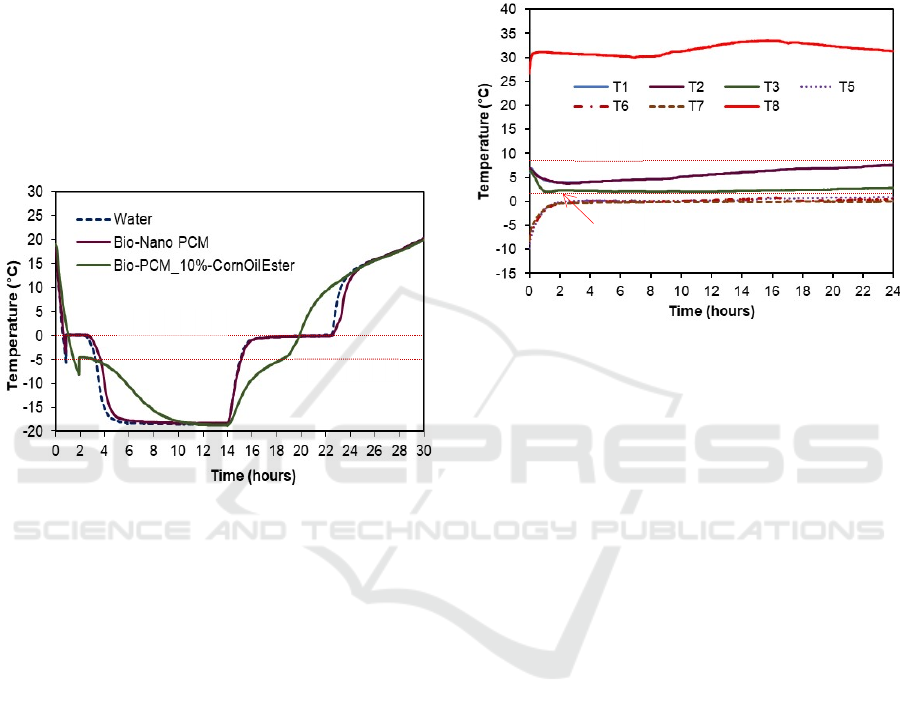
temperature requirement between 0 °C and 10 °C
precisely in the range from 2 °C up to 8 °C. The 10%
bio-PCM is a PCM made from the mixture of 10% of
corn oil ester and 90% tap water by volume as
reported in Rasta and Suamir (2018).
This result shows that if the Bio-PCM 10% is
used, the vaccine holder and the Bio-PCM must be
equipped with a thermal wall capable of maintaining
the temperature difference in such a way that the
temperature of the vaccine is in the storage range.
Otherwise, the temperature of the vaccine can be drop
down below 0 °C then increase sharply to follow the
discharging (heating) characteristic as shown in
Figure 4. This can also make the vaccine temperature
exceed 8 °C in far less than 24 hours.
Figure 4: Charging and discharging test results of PCM
alternatives.
From the figure, it can also be seen that tap water
and bio-Nano PCM are two materials that are very
suitable to be used for vaccine carrier applications.
These two PCM materials have a PCT at 0 °C.
However, the bio-Nano PCM can provide better
higher enthalpy difference of phase change.
Therefore, the vaccine carrier utilizing Bio-Nano
PCM as the passive cooling materials is chosen and
discussed in this paper.
3.2 Temperature Performance of the
Bio-Nano PCM Vaccine Carrier
The test results on the temperature performance of the
Bio-Nano PCM vaccine carrier with different PCM
enclosure or different PCM mass are presented in
Figures 5 and 6. Where T8 is the ambient
temperature, T1-3 is the vaccine temperatures and
T5-7 is the bio-Nano PCM temperatures. In these
tests, water is used to represent the vaccines which are
placed in the vaccine bottles.
The temperature performance tests were carried
out in two stages: (i) the first test was vaccine carrier
utilizing acrylic enclosure bio-Nano PCM with total
mass of 3.2 kg and the test results are shown in Figure
5; (ii) the second test was vaccine carrier utilizing ice-
pack enclosure bio-Nano PCM with total mass of 2.7
kg and the test results are presented in Figure 6.
Figure 5: Vaccine carrier test results utilizing bio-Nano
PCM with total mass of 3.2 kg.
Figure 5 shows the discharging test results of the
vaccine carrier utilizing acrylic enclosure bio-Nano
PCM with total mass of 3.2 kg. From the graph, it can
be clearly seen that the vaccine temperature can be
maintained within a safe range between 2 °C and 8 °C
for more than 24 hours. At the beginning of the test
shows that the vaccines have been store at correct
temperature range and then their temperature
decrease gradually for about two hours until about
2° C when the bio-Nano PCM temperature is still
below its phase change temperature (PCT) of 0°C.
Then, the vaccine temperatures very slowly increase
for more than 24 hours but still far below 8 °C when
bio-Nano PCM temperature reaching its PCT. These
show that vaccine carrier with 3.2 kg bio-Nano PCM
can keep the vaccines safely for more than 24 hours,
precisely 28 hours in total.
While Figure 6 shows the test results of the
vaccine carrier utilizing ice-pack enclosure bio-Nano
PCM with total mass of 2.7 kg. It can be seen that the
vaccine temperatures can be maintained within a safe
range for only 22 hours. It also shows at the beginning
of the test that the vaccines have been store at correct
temperature range and then their temperature
decrease gradually for about two hours until about 2
°C when the bio-Nano PCM temperature is still below
its phase change temperature (PCT) of 0°C. Then, the
vaccine temperatures steadily increase for more than
18 hours but still below 8 °C when bio-Nano PCM
PCT-1 (0°C)
PCT-2 (-5°C)
8°C
2°C
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
332
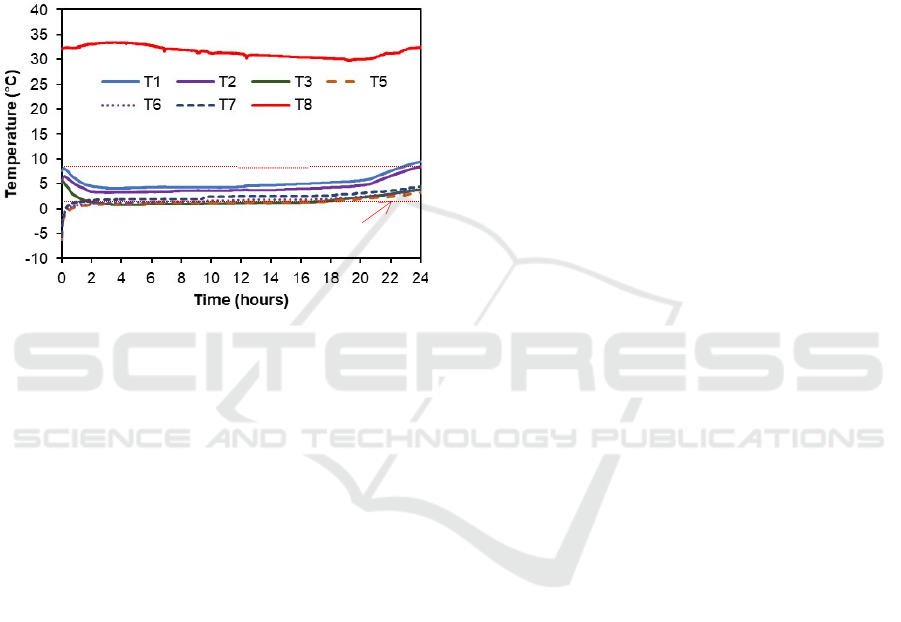
temperature at its PCT. The vaccine temperatures
then gradually increase after the bio-Nano PCM
temperatures leaving its PCT and the vaccine
temperatures exceeding 8 °C after 22 hours.
Results presented in Figures 5 and 6 have shown
that the time difference of safely maintaining
temperature of the vaccines is mainly caused by the
different amounts of bio-Nano PCM mass. For the
Bio-Nano PCM ice-pack vaccine carrier, PCM mass
is 2.7 kg which is much lighter than the acrylic Bio-
Nano PCM vaccine carrier with 3.2 kg PCM mass.
Figure 6: Vaccine box test results using bio-Nano PCM
with total mass of 2.7 kg.
4 CONCLUSIONS
The vaccine carrier with novel bio-Nano PCM
passive cooling has been developed and tested. It is
found that vaccine carrier utilized with 3.2 kg bio-
Nano PCM can safely maintain the vaccine for more
than 24 hours. The vaccine carrier is easy to carry,
have aesthetics, light weight, practical use and energy
efficient while still having good cooling capabilities
of 2-8 ºC. The vaccine carrier has a capacity of 2.8
litres and it is capable of loading 20 bottles of vaccine
with total weight of approximately 7 kg.
ACKNOWLEDGEMENTS
Authors appreciatively acknowledge the financial
support from the Politeknik Negeri Bali through
institutional research with funding scheme: DIPA
Politeknik Negeri Bali number: SP. DIPA-023.18.2.
677608/2021, dated 23 November 2020. The authors
also gratefully thank Centre of Research and
Community Services (P3M) Politeknik Negeri Bali
for the technical and administrative assistances.
REFERENCES
Boakye-Ansah, A.S., Schwartz, K., Zwarteveen, M. (2020).
Aligning stakeholder interests: How ‘appropriate’
technologies have become the accepted water
infrastructure solutions for low-income areas. Utilities
Policy 66, pp. 101081.
ITSmis. (2020). Gagas Kotak Distribusi Vaksin,
Mahasiswa ITS Juarai Ajang Internasional. Dapat
diaksespada: https://www.its.ac.id/news/2020/11/30/
gagas-kotak-distribusi-vaksin-mahasiswa-its-juarai-
ajang-internasional/
Patnaik, J. and Bhowmick, B. (2019). Revisiting
appropriate technology with changing socio-technical
landscape in emerging countries. Technology in Society
57, pp.8-19.
Pearce, J.M. (2012). The case for open source appropriate
technology. Environ Dev Sustain 14, pp. 425–431.
Pearce, J.M., Albritton, S., Grant, G., Steed, G., and
Zelenika, I. (2014). A new model for enabling
innovation in appropriate technology for sustainable
development. Sustainability: Science, Practice, &
Policy 8 (2), pp. 42-53.
Primadi, O. (2017). Pemerintah Serius Untuk Kualitas
Rantai Dingin (Cold Chain) Penyimpanan Vaksin,
Dapat diakses pada: https://sehatnegeriku.kemkes.go.id/
baca/ umum/20170426/2320665/ pemerintah-serius-
kualitas-rantai-dingin-cold-chain-penyimpanan-vaksin/
Putra, N., Tedjo, H., Koestoer, R.A. (2005). Pemanfaatan
Elemen Peltier Bertingkat dua pada aplikasi Kotak
Vaksin. Prosiding Seminar Nasional Tahunan Teknik
Mesin IV. Universitas Udayana, Bali, Indonesia.
Putra, N., Siregar, P.P., Koestoer, R.A. (2006).
Pengembangan “VACCINE CARRIER” dengan
memanfaatkan efek Peltier. Seminar Nasional Tahunan
Teknik Mesin III, 6-7 Desember 2004, ISBN 979-
97158-0-6, Universitas Hasannudin Makasar
Indonesia.
Putra, N. (2006). Uji Unjuk Kerja Kotak Vaksin berbasis
Elemen Peltier Ganda. Seminar Nasional
Perkembangan Riset dan Teknologi di Bidang Industri
Universitas Gajah Mada Yogyakarta.
Putra, N., Veranika, R.M., Danardono, A.S. (2006).
Perancangan dan pengembangan produk kotak vaksin
untuk daerha pedalaman. Seminar Nasional Tahunan
Teknik Mesin (SNTTM) V, Universitas Indonesia, 21-23
November 2006: MI-024/1-7.
Rasta, I.M., Suamir, I.N. (2018). The role of vegetable oil
in water based phase change materials for medium
temperature refrigeration. Journal of Energy Storage
15, 368-378.
Rasta, I.M., Suamir, I.N. (2019). Study on Thermal
Properties of Bio-PCM Candidates in Comparison with
Propylene Glycol and Salt Based PCM for sub-Zero
Energy Storage Applications. IOP Conference Series:
Materials Science and Engineering 494, 012024.
Suamir, I.N., Rasta, I.M., Sudirman, Tsamos, K.M. (2019).
Development of Corn-Oil Ester and Water Mixture
Phase Change Materials for Food Refrigeration
Applications. Energy Procedia 161, 198-206.
8°C
2°C
Thermal Performance of Bio-Nano-PCM based Passive Cooling for Vaccine Carriers
333

The Minister of Health Regulation number 12. (2017). The
Health Minister Decree of the Republic of Indonesia
concerning the implementation of immunization.
World Health Organization. (2020). Program Imunisasi
dan Pengembangan Vaksin, Jakarta, Indonesia.
Zelenika, I., and Pearce, J.M. (2011). Barriers to
Appropriate Technology Growth in Sustainable
Development. Journal of Sustainable Development 4
(6), pp. 12-22.
Zelenika, I., and Pearce, J.M. (2012). Innovation Through
Collaboration: Scaling up Solutions for Sustainable
Development. Environment, Development and
Sustainability 16 (6), pp. 1299-1316.
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
334
