
Synthetic Dye Removal of Methylene Blue on Adsorption Process
using Low-rank Coal of East Kalimantan
Yuli Patmawati and Ibnu Eka Rahayu
Department of Chemical Engineering, Polytechnic State of Samarinda, East Kalimantan, Indonesia
Keywords: Adsorbent, Adsorption, Low-rank Coal, Methylene Blue, Synthetic Dye.
Abstract: Adsorption of synthetic dyes using low-rank coal as an adsorbent needs to be applied considering that
adsorption is the best technology available and low cost to remove some toxic contaminants, both organic and
inorganic contained in wastewater. In addition, the great potential of low rank coal in East Kalimantan is still
abundant and not fully utilized because so far, several studies have focused on medium and high rank coal.
This study aims to determine the dye removal of methylene blue (MB) on adsorption process using low rank
coal as an adsorbent. 100 mg low rank coal adsorbent was added to 100 mL of 100 mg/L methylene blue dye
solution at 30 °C. Initial pH effect adjusted with 0.10 mol/L of HCl or NaOH. Then the synthetic dye solution
of methylene blue was shaker at 150 rpm based on different contact times (10 - 90 min). At the end of the
period, the shaker was stopped, and the supernatants were filtered through a Whatman 40 filter paper to
separate the liquid from the solid phase. The remaining synthetic dye concentrations of methylene blue were
determined using UV-Vis Spectrophotometer at maximum wavelength (λ
max
) of adsorption. The experiment
was repeated for different initial concentrations of synthetic dye methylene blue (50 – 250 mg/L). The best
results were obtained using 100 mg low-rank coal adsorbent of East Kalimantan at optimum pH, initial
concentration, and contact time, respectively as follows 12, 150 mg/L, and 10 minutes with methylene blue
dye removal of 98.41%.
1 INTRODUCTION
Dyestuff waste generally has non-biodegradable
properties because it contains complex aromatic
compounds that are difficult to decompose by
microbes. The dye waste is harmful to human health
and biota that live around polluted water bodies.
Generally, these organic compounds are also
teratogenic (causing defects in the fetus during
pregnancy), carcinogenic and mutagenic, so that they
can pose a serious threat to human health (Irawati et
al., 2018). One of the dyes that are very dangerous for
human health is methylene blue. Methylene blue can
cause skin irritation, irritation to the digestive tract. If
inhaled, it can cause cyanosis (Fayazi et al., 2016).
To reduce environmental pollution caused by the
use of methylene blue, it is important to treat the dye
wastewater before being discharged into the
environment. One of the methods used to reduce
methylene blue dye wastewater that is easy and
economical is the adsorption process. Adsorption is an
efficient technique to remove odors and reduce the
concentration of dyes from solutions perfectly without
converting them into more dangerous compounds.
Adsorption based on carbon materials is
considered the best available and low-cost technology
for the removal of several organic and nonorganic
toxic contaminants from aqueous solutions
(Yanagisawa et al., 2010). Coal is one of the widely-
known carbonaceous raw materials that could be act
as a potentially low-cost adsorbent material for toxic
water contaminants (A. Gu¨ rses et al, 2014).
The adsorption of dyes using several ranks of coal
as adsorbent was studied by several authors. Most
research so far has focused on medium and high rank
coals, thus paid less attention to low rank coals. In this
research the dye removal of methylene blue use low-
rank coal of East Kalimantan as adsorbent.
A good adsorption performance for methylene
blue also achieved by Yu et al. (2020) in their
research “Adsorptive removal of cationic methylene
blue and anionic Congo red dyes using wet-torrefied
microalgal biochar : Equilibrium, kinetic and
mechanism modeling”. The maximum adsorption
capacities and percent removal for methylene blue
were 113.00 mg/g and 26.23% respectively.
268
Patmawati, Y. and Rahayu, I.
Synthetic Dye Removal of Methylene Blue on Adsorption Process using Low-rank Coal of East Kalimantan.
DOI: 10.5220/0010943800003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 268-271
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
Research was conducted by Myneni et al. (2019)
has studied “Modelling and Optimization of
Methylene Blue (MB) Adsorption onto Magnesium
Oxide Nanoparticles loaded onto Activated Carbon
(MgONP-AC): Response Surface Methodology and
Artificial Neural Networks” obtained MB removal of
94.34% at pH 5.91, MgONP-AC dosage 0.47 g/L,
initial concentration of MB in solution 15mg/L and
temperature 313 K and 207 mg/g for MgONP-AC
adsorption capacity.
The other research has done by Shokry and
Elkady (2019) used Maghara coal which impregnates
to nano-activated carbon was inspected for the
removal of methylene blue (MB) dye from aqueous
solutions. This study obtained the adsorption
equilibrium of MB onto prepared nano-activated
carbon (NAC) was fitted well with the Langmuir
isotherm indicating monolayer coverage of dye
molecules on NAC with a maximum mono-layer
adsorption capacity of 28.09 mg/g. NAC produced
from Maghara coal is an effective adsorbent and low
cost material for removal of organic pollutants from
wastewater.
Another study related to the absorption of
methylene blue dye was carried out by Rizki et al.
(2019). By using tamarind seeds as adsorbents with
variations in contact time of 30, 60, 120, and 180 min,
the adsorbent dosage was 0.3, 0.4, 0.6, and 0.9 g. The
best sorption removal of methylene blue was
98.827% with a contact time of 120 min and pH 6 at
0.9 g biosorbent.
This article discusses the dye removal of
methylene blue on adsorption process using low rank
coal of East Kalimantan as an adsorbent.
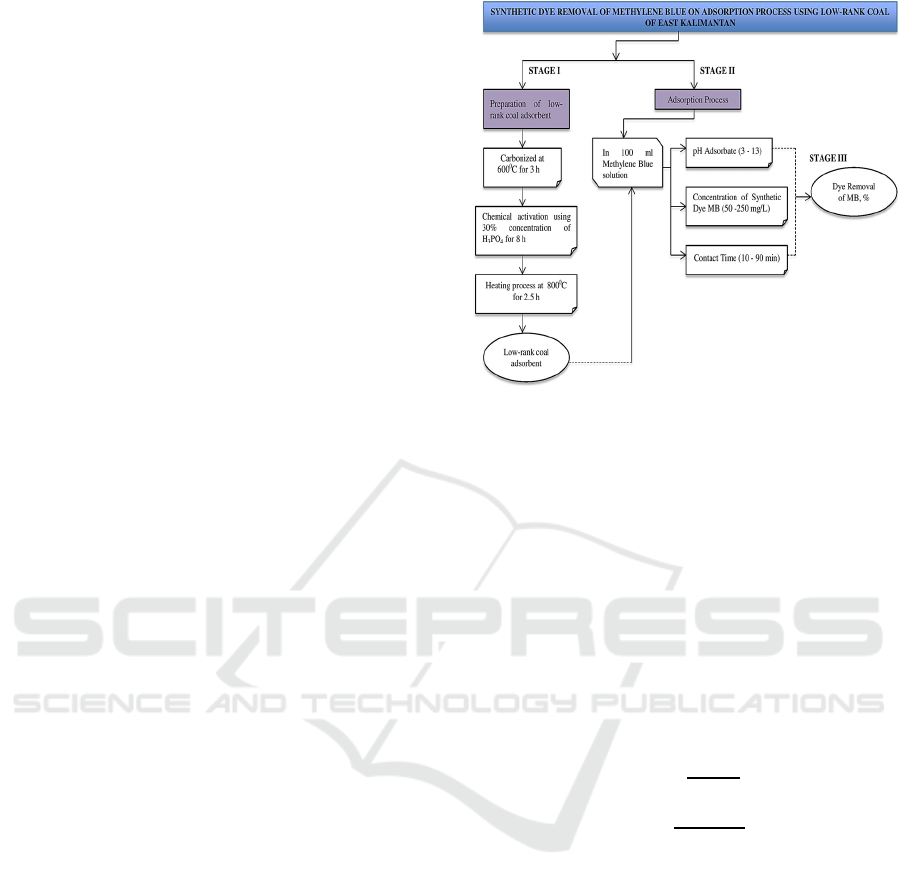
2 METHODOLOGY
Firstly, Low rank coal is carbonized at 600
0
C for 3 h,
then after cold it was activated using 30%
concentration of H
3
PO
4
for 8 h. The immersion
results were then washed to neutral pH and continued
with the heating process at 800
0
C for 2.5 h. Second,
the investigation of the dye removal of methylene
blue using the low rank coal as adsorbent runs in a
batch adsorption process at room temperature with
the adsorbent dosages 100 mg, variations in pH of
methylene blue solution (3 to 13), the initial
concentration of (50 to 250 mg/L) and the contact
time (10 to 90 min). 100 mg of low rank coal
adsorbent was added to 100 mL of methylene blue
solution at 30 °C were shaker at 150 rpm based on
different contact times and initial concentration of
methylene blue. Initial pH effect adjusted with 0.10
Figure 1: Research framework and design.
mol/L of HCl or NaOH. At the end of the period, the
shaker was stopped, and the supernatants were
filtered through a Whatman 40 filter paper to separate
the liquid from the solid phase. The remaining
synthetic dye concentrations of methylene blue were
determined using UV-Vis Spectrophotometer at 664
nm maximum wavelength (λ
max
) of adsorption.
Third, determine of dye adsorption performance
are expressed by methylene blue dye removal based
on the amount of methylene blue adsorbed per gram
of the low rank coal adsorbent at equilibrium, q
e
(mg/g), were obtained using Equation (1) and
Equation (2).
𝑅𝑒𝑚𝑜𝑣𝑎𝑙, %
𝑥 100% (1)
𝑞
(2)
Where C
0
and
C
e
(mg/L)
refer to methylene blue
concentration at initial and equilibrium,
respectively. Meanwhile,
V symbol refers to the
volume of methylene blue solution (Liter) and W
symbol refers to the adsorbent dosage used (mg).
3 RESULT AND DISCUSSION
The low-rank coal of East Kalimantan is used as an
adsorbent in the adsorption process of synthetic dye
methylene blue. The characteristics of low rank coal
to be processed into adsorbent are investigated by
proximate analysis to determine the parameters of
moisture content, ash content, volatile matter, fixed
carbon, and calorific value respectively as follows
33.66%, 3.72%, 32.53%, 33.09%, and 4208 cal/g.
Synthetic Dye Removal of Methylene Blue on Adsorption Process using Low-rank Coal of East Kalimantan
269
The characteristics of adsorbents were obtained after
the activation process consists of moisture content,
ash content, volatile matter, fixed carbon and iodine
adsorption number respectively as follows 0.45%,
1.12%, 4.2%, 84.23%, and 761 mg/g.
The methylene blue adsorption process results at
100 mg/L concentration of methylene blue dye
solution, pH 12, adsorbent dosage 100 mg, and
different contact times can be seen in Table 1 and
Figure 2.
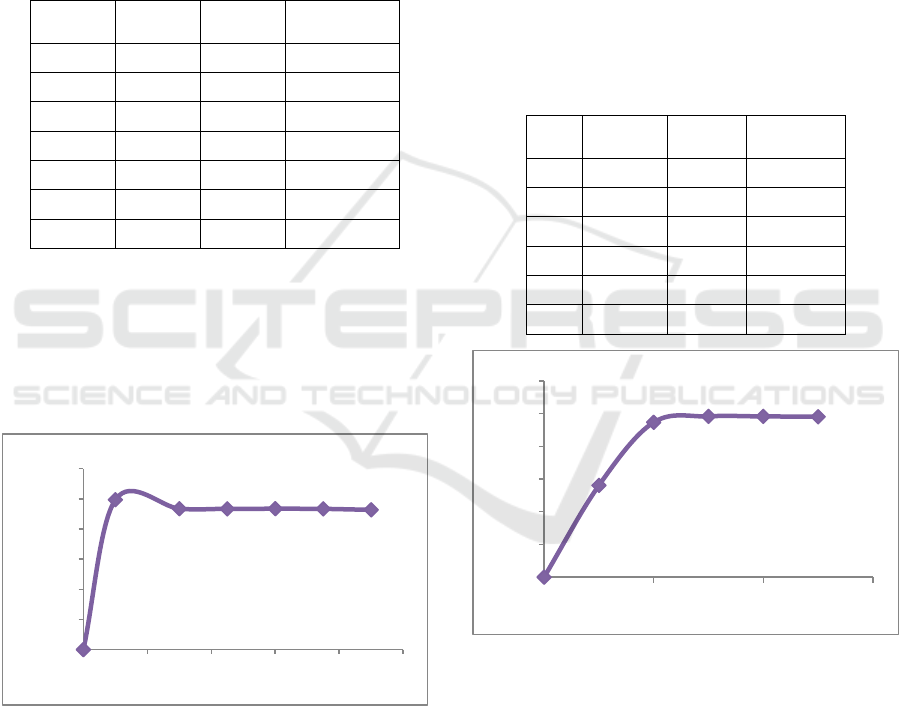
Table 1: The effect of contact time on methylene blue dye
removal.
Time
(minute)
C
0
(mg/L)
C
e
(mg/L)
Removal
(%)
0 100 0 0
10 100 0.60 99.40
30 100 6.57 93.43
45 100 6.64 93.36
60 100 6.61 93.39
75 100 6.70 93.30
90 100 7.34 92.66
Contact time is one of the factors that affect the
adsorption process of methylene blue. The reaction
rate depends on the number of collisions per unit
time. The more collisions that occur, the faster the
reaction takes place until equilibrium conditions
occur. The equilibrium occurs when the rate of
adsorption is equal to the desorption rate.
Figure 2: The effect of contact time on methylene blue dye
removal.
Figure 2 shows that the methylene blue dye
removal increased with the increase of contact time
until a certain time and then tends to be constant. In
Figure 2, methylene blue dye solution adsorption
using low-rank coal as adsorbent occurs very quickly
in the first 10 minutes. After that, the methylene blue
dye removal decreased slowly and could be assumed
to be constant at 30 minutes of the adsorption process.
After the adsorption reached equilibrium at the
optimum contact time, the addition of the contact time
between the adsorbent and the adsorbate further did
not significantly affect the absorption of the dye.
Hastuti et al. (2012) said that too long physical
contact between the dye and the adsorbent causes the
dye to be released from the solution over time
(desorption). The optimum contact time was obtained
at 10 minutes of the adsorption process. Furthermore,
the adsorption process for the synthetic dye
methylene blue was carried out with variations in the
initial concentration of methylene blue at 10 minutes
of contact time. The pH of the dye solution was 12
and 100 mg adsorbent dosage.
Table 2: The effect of initial concentration on methylene
blue dye removal.
No
C
0
(
m
g
/L
)
C
e
(
m/L
)
Removal
(
%
)
1 0 0 0
2 50 21.90 56.20
3 100 5.26 94.74
4 150 2.38 98.41
5 200 3.28 98.36
6 250 4.62 98.15
Figure 3: The effect of initial concentration on methylene
blue dye removal.
Based on Figure 3, the dye removal increases as
the initial concentration of the methylene blue dye
solution increases. At 50 mg/L concentration, the
methylene blue dye removal was 56.20 %. It is due to
the large number of empty spaces on adsorbent
surfaces or pores that dyes have not occupied. At 100
mg/L concentration, the percent removal has
increased to 94.74%. It indicates that there are still
empty spaces on the surface of the adsorbent, namely
the availability of active groups from the adsorbent to
0
20
40
60
80
100
120
0 20406080100
Removal of MB, %
Contact Time, min
0
20
40
60
80
100
120
0 100 200 300
Removal of MB, %
Initial Concentration, mg/L
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
270

bind the methylene blue dye. At 150 mg/L
concentration, the increase of methylene blue dye
removal was not significant because almost all the
pores of the low-rank coal adsorbent had been filled
with methylene blue (almost saturated). Meanwhile,
at a concentration of more than 150 mg/L, the
adsorbent is already saturated, so adding the dye
concentration will decrease the adsorption ability.
The concentration of the dye is related to the active
sites on the surface of the adsorbent. If the number of
active sites is large enough compared to the amount
or concentration of dye, the dye removal will be high
until the number of active sites is the same as the dye
concentration. Therefore, when the optimum
concentration has been reached, increasing the
concentration can reduce the adsorption ability. From
this research, the optimum methylene blue dye
removal of 98,41% was obtained at 150 mg/L
concentration, 12 pH of dye solution, 100 mg
adsorbent dosage, and 10 minutes contact time of
adsorption process.
4 CONCLUSIONS
1. Characteristics of low-rank coal adsorbents used
in the methylene blue adsorption process have
moisture content, ash content, volatile matter,
fixed carbon, and iodine adsorption number
respectively as follows 0.45%, 1.12%, 4.2%,
84.23%, and 761 mg/g.
2. The optimum synthetic dye removal of methylene
blue of 98,41% was obtained at 150 mg/L
concentration, 12 pH of dye solution, 100 mg
adsorbent dosage, and 10 minutes contact time of
adsorption process.
ACKNOWLEDGEMENTS
The author would like to acknowledge the Center for
Research and Community Service at Polytechnic
State of Samarinda which has provided funding for
this research as well as to the Chemical Engineering
Laboratory of Polytechnic State of Samarinda as a
place for the research to be carried out.
REFERENCES
A. Gürses, A. Hassani, M. K. O. A. S. K. (2014). Removal
of Methylene Blue from Aqueous Solution Using by
Untreated Lignite as Potential Low-Cost Adsorbent:
Kinetic, Thermodynamic and Equilibrium Approach.
Journal of Water Process Engineering, 2, 10–21.
https://doi.org/10.1016/j.jwpe.2014.03.002.
Fayazi, M., Taher, M. A., Afzali, D., & Mostafavi, A.
(2016). Enhanced Fenton-like Degradation of
Methylene Blue by Magnetically Activated
Carbon/Hydrogen Peroxide with Hydroxylamine as
Fenton Enhancer. Journal of Molecular Liquids, 216,
781–787. https://doi.org/10.1016/j.molliq.2016.01.093
Hassan Shokry, Marwa Elkady, H. H. (2019). Nano
Activated Carbon from Industrial Mine Coal as
Adsorbents for Removal of Dye from Simulated Textile
Wastewater: Operational Parameters and Mechanism
Study. Journal of Materials Research and Technology,
8(5), 4477–4488. https://doi.org/10.1016/j.jmrt.2019.
07.061.
Heni Irawati, Nurul Hidayat Aprilita, dan E. S. (2018).
Adsorpsi Zat Warna Kristal Violet Menggunakan
Limbah Kulit Singkong (Manihot esculenta). Bimipa,
25(1), 17–31.
Hiroki Yanagisawa, Yuki Matsumoto, M. M. (2010).
Adsorption of Zn (II) and Cd (II) Ions Onto Magnesium
and Activated Carbon Composite in Aqueous Solution.
Applied Surface Science, 256(6), 1619–1623.
Kai Ling Yu, Xin Jiat Lee, Hwai Chyuan Ong, W.-H. C., &
Jo-Shu Chang, Chih-Sheng Lin, Pau Loke Show, T. C.
L. (2020). Adsorptive Removal of Cationic Methylene
Blue and Anionic Congo Red Dyes Using Wet-
Torrefied Microalgal Biochar: Equilibrium, Kinetic and
Mechanism Modeling. Environmental Pollution, xxxx,
115986. https://doi.org/10.1016/j.envpol.2020.115986.
Rizki, A., Syahputra, E., & Pandia, S. (2019). “Pengaruh
Wajtu Kontak dan Massa Adsorben Biji Asam Jawa
(Tamarindus indica) dengan Aktivator H3PO4 terhadap
Kapasitas Adsorpsi Zat Warna Methylene Blue,.” J.
Tek. Kim. USU, 8(2), 54–60.
V. R. Myneni, Thanusha Punugoti, N. Sasi Kala, N. R.
Kanidarapu, M. V. (2019). Modelling and Optimization
of Methylene Blue Adsorption onto Magnesium Oxide
Nanoparticles Loaded onto Activated Carbon
(MgONP-AC): Response Surface methodology and
artificial neural networks. Materials Today:
Proceedings, 18, 4932–4941. https://doi.org/10.1016/
j.matpr.2019.07.485.
Synthetic Dye Removal of Methylene Blue on Adsorption Process using Low-rank Coal of East Kalimantan
271
