
Factorial Identification on Surface Hardness of Cut Low Carbonsteel
with Hardening Process using Manganese Minerals Powder
Oktovianus Dharma Rerung, Fransiskus Sapar and Roymons Jimmy Dimu
Dept. of Mechanical Engineering, Kupang State polytechnic, Kupang, Indonesia
Keywords: Factorial, Hardening, Steel Cleavage, Hardness.
Abstract: This study wanted to ensure the diffusion of manganese on the surface of the carbon steel cleavage into the
steel core using high heat with a hardening process and a pack carburizing approach. The purpose of the pack
carburizing process approach is the hardening of the steel surface at high temperatures using manganese rock
powder mineral as a substitute for carbon such as solid charcoal. Previous research showed that the chemical
composition test formed elemental Manganese (Mn) and several other elements on the steel surface from the
pyrometallurgical oxidation and reduction process of manganese rock powder at a temperature of 1000 °C.
The purpose of this test is to determine the factorial effect of steel cleavage surface hardness with factors,
temperature, coal composition, and holding time. Control of chemical composition by using Energy
Dispersive X-ray (EDX). The ANOVA test results show that the percentage of coal has a significant effect
on increasing the hardness of the test sample. Control of chemical composition showed that there was an
atomic diffusion process with increasing levels of carbon (C), Manganese (Mn) and other elements, Silicon
(Si), Aluminum (Al). EDX data information explains that to form a hard steel surface not only by using carbon
but also with manganese rock powder minerals.
1 INTRODUCTION
Developers and seekers of mineral alloy steel will be
interested in discussing the manganese mineral found
on the island of Timor. Not because of the abundant
minerals but also the high content of manganese
(Mn). It is known as one of the best manganese rock
minerals with an Mn content of around 20% -5%
(Panjaitan, 2011). One of these mining locations is the
Koa area and its surroundings in the Mollo Barat
District, Timor Tengah Selatan Regency (TTS) with
a total deposit of 454,123,065.8 m³ (Harjanto, 2011).
There did two types of manganese ores found in the
area, namely manganese layers and manganese
nodules (Idrus et al., 2013). Manganese minerals did
widely distributed in various forms such as oxides,
silicates, carbonates of the most common compounds.
Both types of manganese minerals can be able formed
into powder for this research. In addition to other
technical functions, the function of manganese in
steel is as a special alloy that causes steel to be
friction-resistant, impact-resistant, and has high
hardness. That is why manganese is so important in
the metal or machinery industry. Accordingly, the
manufacturing industry is very interested in
developing new types of technological equipment
that allow the application of methods to modify the
surface of parts by processing them with lower
resources (Skeeba, Ivancivsky and Martyushev,
2021). Previous studies by researchers tried to link
manganese rock powder and high heat. This research
is to ensure the presence of metal between iron and
manganese atoms on the surface of carbon steel with
the help of high-temperature heat with pack
carburizing and pyrometallurgical approaches. Pack
Carburizing is a form of hardening of steel using a
medium containing carbon at a temperature of 800 -
950 °C then quenching quickly. For example,
ordinary gears use AlNas embedding particles, whose
carburizing temperature is in the range of 930–980
°C(Liu et al., 2021). At that temperature in an
environment containing activated carbon atoms,
activated carbon atoms will diffuse to the surface of
the steel and reach a certain depth (Mirantie
Dwiharsanti, 2016). Other tests concluded that the
optimumaustenitizing temperature is about 840◦C for
the test steel(Chen et al., 2021). Pyrometallurgy is a
metal extraction process with heat energy at a general
temperature used in the range of 500 °C-1600 °C
(Wibawa, 2018).
Rerung, O., Sapar, F. and Dimu, R.
Factorial Identification on Surface Hardness of Cut Low Carbonsteel with Hardening Process using Manganese Minerals Powder.
DOI: 10.5220/0010941700003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 167-173
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
167
Previous research with Energy Dispersive X-ray
(EDX) showed the formation of Manganese (Mn) and
several other elements on the surface of steel samples
in the oxidation-reduction process at a temperature of
1000 °C (Rerung, Sapar and Dimu, 2019). Next,
identify the factors that affect the hardnessof the steel
cleavage after receiving the previous hardening
treatment. The purpose of testing steel pieces is to
analyze effect of hardness from the steel surface
towards the steel core. The control variable remains
using the chemical composition with EDX. The
results test will be important to answer industry needs
regarding surface hardening and steel resistance to
friction. The addition of Manganese (Mn) will make
the steel test sample hard, friction-resistant and high-
strength. (Bleck and Haase, 2019). The usefulness of
the processed steel is to produce steel that can work
on various special equipment such as attack-resistant
excavator bucket teeth (Winarto et al., 2019).
The development of heavy-duty manufacturing
technology such as automotive and heavy equipment
has driven the engineering of Advanced High
Strength Steels (AHSS), namely high-strength
manganese alloy steels (SimoneKaar 1,*,
DanielKrizan 2, ReinholdSchneider 1, 2019). The
description of the AHSS data starts from traditional
high-strength steels which include manganese carbon
steel (C-Mn), hardened steel, high-strength free
intergroup steel (HSS-IF), and high-strength low-
alloy steel (HSLA). . The concept of Advanced High
Strength Steel (AHSS) had divided into high strength
steel into conventional HSS and advanced high
strength steel. The strength of AHSS ranges from 500
Mpa to 1500 Mpa as and one of the techniques used
to achieve AHSS is to increase the hardness or tensile
stress using hardening in the form of heat treatment
(Herwandi, 2005).
The next research step is to make a factorial
design. The design of factorial experiments is a
procedure for placing treatments into experimental
units with the main objective of obtaining data that
meet scientific requirements. Thus research can be
measured and strict control inprocesses and methods.
2 METHODOLOGY
This research is a continuation of previous research
that focuses on the interaction of manganese and iron
on the surface of carbon steel with a hardening
approach with pack carburizing and pyrometallurgy.
Furthermore, this study will analyze the diffusion of
manganese from the steel surface into the steel core
by measuring the hardness and analyzing the
chemical
composition of each element. Measuring the hardness
of the cut part of the test sample by selecting the
position of the outermost part of the surface of the
steel shell. The type of penetration that has been used
is Rockwell B with a spherical penetration measuring
1/16". Furthermore, testing the chemical composition
at these points. This is to ensure the influence of
manganese in manganese powder on the hardness of
carbon steel.
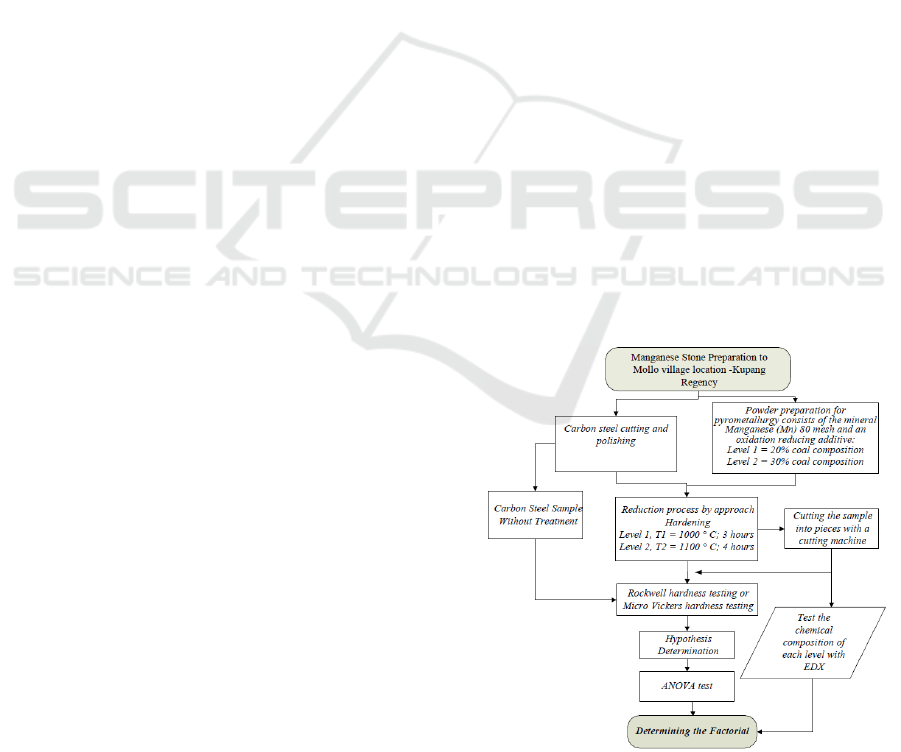
2.1 Flow Chart Diagram
Experimental research tries to explain the benefits of
manganese on the island of Timor (East Nusa
Tenggara) in this study. Another important thing is
the workflow chart from sampling, the methods, to
experimental processes. The description can be
illustrating in a research flow chart. Furthermore,
developing the research with critical thinking with
statistical rules to help find the factors that affect the
hardness of the test sample. Many experiments
involve two or more the factors. This concept creates
a factorial design in which every possible level of a
combination of all the factors will be available.
(Salomon et al., 2017). The design process will place
independent variables with two different levels
namely temperature, holding time, and percentage of
coal catalyst. The dependent variable design is the
hardness value. Factorial designs design concurrent
trials of two or more single trials with two factors
often found in multilocation trials (Lawson, 2016). To
further elaborate the research steps as shown in the
flow chart in Figure 1 below.
Figure 1: Research flow chart.
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
168
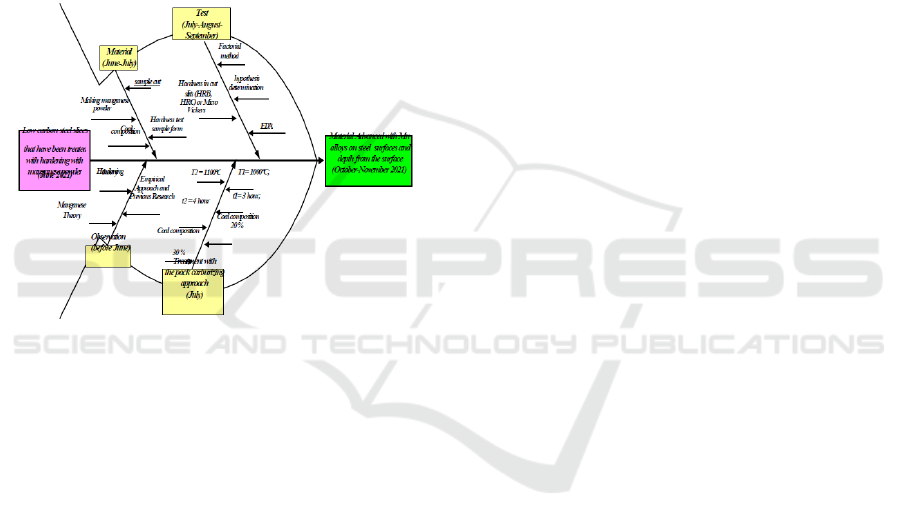
2.2 Research Roadmap
Further explanation of the research explains the
research journey from input to output with a cause-
and-effect diagram. Cause-and-Effect Diagram is a
research Road Map tool that helps identify, sort, and
display the possible causes of a certain quality or
characteristic. (Deshpande, 2008). The diagram
shows the whole concept of thinking by connecting
theory with several methods. These methods are
experimental and statistical with tentative
assumptions. The diagram picture will describe the
research journey from the beginning to the end of the
study. The information displayed is time, process,
cause and effect relationship, and achievement.
Figure 2: Road Map with Cause Effect Diagram.
2.3 Research Design
2.3.1 Research Site
The location was carried out at the Kupang State
Polytechnic Materials Testing Laboratory and testing
of chemical composition and Energy Dispersive X-
ray (EDX) was carried out at the Materials Testing
Laboratory of the Tenth Institute of Technology (ITS)
November Surabaya.
2.3.2 Research Methods
Experimental research may be of three types; Pre
experiment, Quasi experiment and True experiment.
All these types have contrastive characteristics
(Qasim, Imtiaz and Alvi, 2014). Study, experimental,
or research design is the backbone of good research.
It directs the experiment by orchestrating data
collection, defines the statistical analysis of the
resultant data, and guides the interpretation of the
results (Knight, 2010). The experimental design of
the action starts from the preparation of materials,
namely the manufacture of manganese powder, the
manufacture of coal powder, the manufacture of heat
treatment boxes, and the cutting of test samples. All
processes take place with the team by working
directly in the Kupang State Polytechnic Material
Testing Laboratory. The next step is to conduct an
experiment using the true experiment method,
namely a study of the possibility of causality between
the treated group and the untreated control group and
compare the two. The experiment in question is
hardening with pack carburizing and
pyrometallurgical processes. The last step is to carry
out hardness testing and chemical composition testing
with EDX. To explain the factors that affect the
hardness of materials and their interactions using the
factorial experimental method. Factorial
Experimental Method is an experiment that combines
or crosses all certain the factors with each other at the
factor level in the experiments. Factorial
identification will look at the influence of individual
factor and the interaction of the factors on the
measurement of the target object in the study.
Determination of significance with going test the
significant level of the influence of the factors and
their interactions using the ANOVA method. The
ANOVA test used one-way ANOVA with a
significance level (α) = 5%. If F-count < F-table, then
the null hypothesis is accepted and, the first
hypothesis was rejected and, if F-count > F-table,
then the null hypothesis is rejected and, the first
hypothesis is accepted.
2.3.3 Research Variable
Factorial design is probably the most powerful
statistical technique for research into any
manufacturing process for the purpose of quality
improvement (Teow, 2005). This article discusses the
practical aspects of using a full factorial design
optimization of heat treatment variables to increase
the cleavage hardness of carbon steel. Variables The
research variables are arranged as follows:
Independent Variables:
Level 1: Hardening temperature T1 = 1000ºC with
20% coal and holding time 3 hours Level 2:
Hardening temperature T2 = 1100ºC with 30% coal
and holding time 4 hours –
Dependent variable:
Hardness value with Rockwell Hardness on the HRC
scale.
Control variable
Chemical composition test results and Scanning
Electron Microscopy (SEM)
Factorial Identification on Surface Hardness of Cut Low Carbonsteel with Hardening Process using Manganese Minerals Powder
169

3 DISCUSSION
Researchers arrange relationships between variables
according to the concept of causal relationships in the
previous section. If one independent variable affects
the dependent variable, it is call a one-factor
experiment because there is only one modifier. This
kind of relationship is a simple relationship between
variables. The causal relationship will be more
complicated if it is a combination of several factors.
We can say that factorial is an experiment consisting
of two or more independent variables. Two
independent variables that influence is called a two-
factor factorial, and if three independent variables
affect it is called a three-factor factorial and so on. In
this study, three independent factors are considered to
have an effect on the hardness of carbon steel after
hardening treatment, namely temperature (T), holding
time (t), and coal percentage C (%). have been carried
out are as follows:
3.1 Experiment Planning
The response variable is the hardness of the low
carbon steel piece hardened by the pack carburizing
approach. The Factors using three independent
variables, namely temperature holding time and coal
composition. A process is also a form of
pyrometallurgical approach process. Each factor has
two levels with austenitizing temperatures consist of
1000 °C and 1100 °C and holding time factors of 3
hours and 4 hours, respectively. The carburizing
medium uses manganese powder with a coal
percentage of 20% and 30%.
The experimental design table format and the
table of hardness test results on treated carbon steel in
the form of a matrix are as follows:
Table 1: Experimental Design.
Independent Variables: Level
Hardening temperature (T) 1000°C 1100°C
Holding Time ( t ) 3 hour 4 hour
Coal Percentage ( C ) 20 % 30%
One of the reasons for using the table format is
because in carrying out heat treatment tests where the
temperature requires variations in holding time.
Although the format of the relationship between these
variables is quite complex and may not be common,
this study tries to answer the influence of these factors
on the sample test.
3.2 Experiment Execution
In general, pyrometallurgical and hydrometallurgical
processes are two technologies for extracting and
separating metals from minerals to produce refined
metals. Pyrometallurgy is a process that utilizes high
temperatures to chemically change minerals,
separating the desired metal from other materials and
ultimately reducing metal oxides to free metals (Sara
Yasipourtehrani, Vladimir Strezov*, Tim Evans,
2020). The experimental implementation of
hardening and pyrometallurgical processes with a
pack carburizing approach on low carbon steel is
follow the experimental design. The entire test takes
place under strict measurement controls to obtain
accurate data. If you pay attention to the table of
hardness results, it can be see that there is a
distribution of data between the hardness of 95.44
HRB - 106.12 HRB. The data shows there is an
increase in hardness from the original sample, namely
low carbon steel with a hardness ranging from 180
HB ~ 89 HRB. Support theory is the Fe - C phase
diagram at austenite temperatures above 800 C which
shows a diffusion process in carbon steel. Two
elements of the diffusion process into hope are
elements of Carbon (C) and elements of Manganese
(Mn). This provisional assumption will be
meaningful in engineering the properties of steel to
become friction-resistant steel. Friction-resistant steel
is a special steel that is rich in the element
Manganese. The hardness results from the pack
carburizing process can be seen in table 2.
Table 2: Hardness Test Results table.
Treatment
Hardness ( HRB )
Temperature ( T )
1000 °C ( T1 ) 1100 °C ( T2 )
Holding Time ( t )
3 hours (t1) 4 hours (H2) 3 hours (t1) 4 hours (t2)
Treatment Interaction
T1t1 T1t2 T2t1 T2t2
20% (C1 )
1 102,77 96,66 97,69 103,30
2 97,30 102,55 102,10 99,36
3 95,44 102,66 96,46 101,79
coal
4 100,05 99,67 100,12 99,65
composition to
5 99,80 98,93 99,80 102,89
manganese
30% ( C2 )
1 103,90 105,55 104,88 106,12
p
owde
r
2 98,77 99,01 103,44 104,90
3 105,02 103,55 105,77 98,64
4 103,11 105,77 98,99 104,25
5 102,00 100,82 104,50 105,22
3.3 Determination of Hypothesis
H01: There is no significant difference between the
temperature factors in influencing the hardness of
carbon steel
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
170
H11: There is a significant difference between the
temperature factors in influencing the hardness of
carbon steel.
H02: There is no significant difference between
holding time factors in influencing the hardness of
low carbon steel.
H12: There is a significant difference between the
holding time factors in influencing the hardness of
carbon steel.
H03: There is no significant difference between the
percentage of coal factors in influencing the hardness
of carbon steel.
H13: There is a significant difference between the
percentage of coal factors in influencing the hardness
of carbon steel.
H04: There is no significant difference between the
interaction of temperature and holding time factors in
influencing the hardness of carbon steel.
H14: There is a significant difference between the
interaction of temperature and holding time factors in
influencing the hardness of carbon steel.
H05: There is no significant difference between the
interaction of temperature factor and coal percentage
factor in influencing the hardness of carbon steel.
H15: There is a significant difference between the
interaction of temperature factor and coal percentage
factor in influencing the hardness of carbon steel.
H06: There is no significant difference between the
interaction of holding time factor and coal percentage
factor in influencing the hardness of carbon steel.
H16: There is a significant difference between the
interaction of holding time and percentage of coal in
influencing the hardness of carbon steel.
H07: There is no significant difference between the
interaction of temperature factor, holding time and
coal percentage factor in influencing the hardness of
carbon steel.
H17: There is a significant difference between the
interaction of temperature factors, holding time and
coal percentage factors in influencing the hardness of
carbon steel.
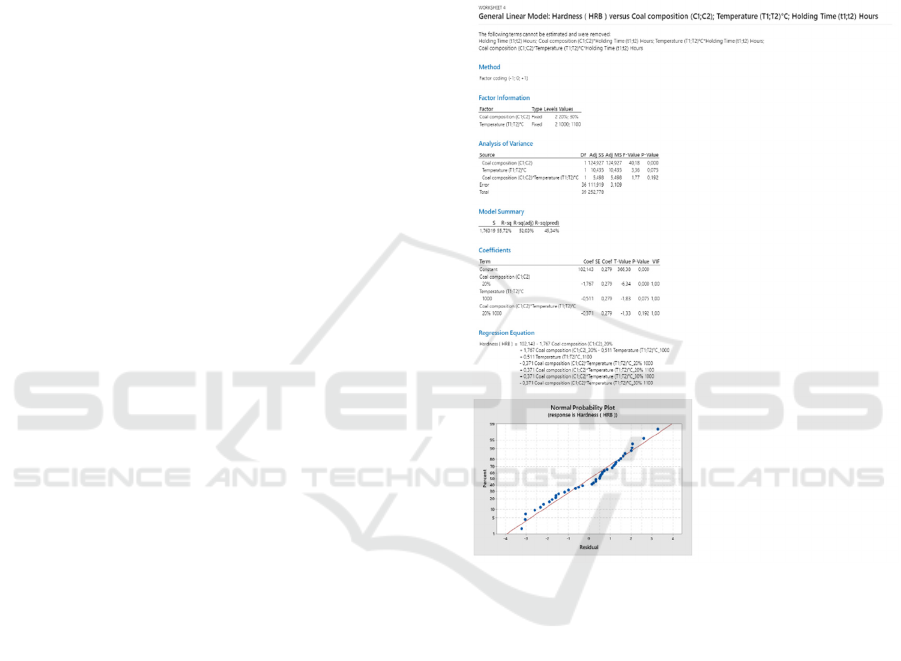
3.4 ANOVA Test
The basis of the experimental trial is a design matrix
with a factorial design. Development of a significant
regression model of process parameters to predict
quality characteristics by Design Of Experiment
(DOE) (Chauhan et al., 2017). The first step is to do
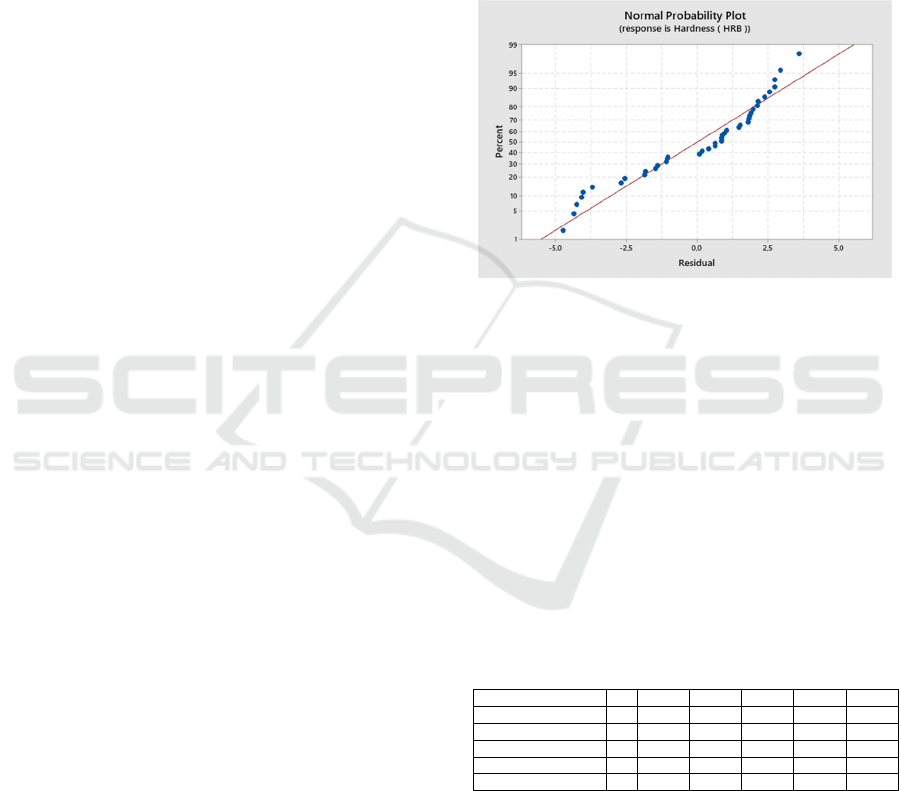
the Normality test before doing the ANOVA test. The
normality test aims to determine: "Is the data
presented is normally distributed or not?". The test
results show a p-value > 0.1 or greater than the 0.05
significance level, then the data can be the key to the
normal distribution as shown in Figure 3. Next,
perform an ANOVA test on the hardness value of the
test sample steel.
The ANOVA test results in the ANOVA table
provide information about three variables as sources
of variation and their interactions that affect steel
hardness. A more in-depth explanation with ANOVA
numbers are degrees of freedom (df), the sum of
squares (SS), mean square (MS), F-count, and F-
table.
Figure 3: Normal Probability Plot.
This test will be more interesting by integrating
experimental design and factorial testing that can
scientifically produce mathematical equations. Then
control it by displaying the results of the chemical
composition test on the hardening steel section as a
test sample.
Furthermore, it will be equipped with more
detailed and complete data and discussions.
The results of the OF Variance Analysis
(ANOVA) test show that coal has a very dominant
effect on the hardness of steel that has been treated
compared to temperature and holding time or its
interactions.
Table 3: ANOVA test.
Source DF Adj SS Adj MS F-Value F-Table P-Value
C (C1;C2) 1 106,341 106,341 17,37 4,1 0
T (T1;T2)°C 1 9,39 9,39 1,53 4,2 0,224
C (C1;C2)*T(T1;T2)°C 1 3,894 3,894 0,64 4,2 0,43
Error 36 220,398 6,122
Total 39 340,023
The effect of coal causes an increase in hardness in
the approach of the pack carburizing process because
coal contains a lot of carbon. Carbon is one of the
elements that increase the hardness of steel. The
treatment temperature above the austenite
temperature in the iron-carbon phase diagram
generally has no significant effect on the test model.
An interesting thing for the study is: "What is the role
of manganese (Mn) or manganese rock powder in the
Factorial Identification on Surface Hardness of Cut Low Carbonsteel with Hardening Process using Manganese Minerals Powder
171

process of increasing the hardness of steel?". To
explain it, let's look at the results of the following
chemical composition test: formed not only with the
elements but also with manganese rock powder in
hardening and pyrometallurgical processes.
3.5 Chemical Composition Test
Chemical Test is useful to support hardness test
results. The chemical composition will provide
information about: "How Manganese can diffuse into
the steel cleavage. It this in line with the expectations
of the research design. Besides Manganese, another b
important element is Carbon (C) for steel hardening.
Table 5 shows the hardness of the Mn diffusion
process. which occurs on a small scale of about 1%
around the point of intersection of carbon steel. In
addition to the elements Mn, there are also elements
of Carbon (C), Silicon (Si), and Aluminum (Al).
Table 4 is the chemical composition of the original
sample of carbon steel shows that no there is an
element of Mn and other additives.
Table 4: Low Carbon Steel Chemical Composition.
El
AN
Series
unn.C norm. C CAtom CError
(wt.%) (wt.%) (at.%) (%)
Fe 26 K-Series 87,36 98,47 93,27 4,1
C 6 K-Series 1,36 1,53 6,73 0,1
Total: 88,72 100,00 100,00
Table 5: Chemical composition of treated carbon steel cut
cleavage.
El
AN
Series
unn.C norm. C CAtom CError
(wt.%) (wt.%) (at.%) (%)
Mn 25 K-Series 24,19 25,30 14,25 1,04
Fe 26 K-Series 55,50 60,79 44,14 2,13
O 8 K-Series 8,30 8,55 28,80 2,88
C 6 K-Series 4,06 4,19 12,60 0,54
Si 14 K-Series 0,15 1,16 0,20 0,00
Al 13 K-Series 0,01 0,01 0,01 0,00
Total: 92,21 100,00 100,00
Observing the treatment gives a strong impression
that the hardening process is going well. The
diffusion process into the steel cleavage is not only
the C element but the Mn element. The Mn element
was importanted in the engineering of specialed steel
materials with the ability to withstand friction and
withstand impact.
Supporting data from the carbon composition test
results found that pursuing steel surfaces can be
formed not only with the elements but also with
manganese rock powder in hardening and
pyrometallurgical processes.
4 CONCLUSIONS
After conducting research and discussion resulted in
several important things as conclusions, namely:
- Hardening factorial design in hardening and
pyrometallurgical processes shows that coal
composition, temperature, and holding time
factors increase the hardness of the carbon steel
cleavage sheath.
- The most dominant factor in increasing the
hardness of steel is the percentage of coal
composition. Coal is a solid mineral that is rich in
carbon.
- Chemical composition test results show the
formation of Manganese (Mn), Carbon (C), in
small amounts of silicon (Si), and Aluminum (Al)
in the steel cleavage sheath.
- The general explanation of this research is that the
steel hardening process by pyrometallurgy shows
an increase in the hardness of carbon steel not
only because of the content ofCarbon (C) but also
the element Manganese (C) as one of the
constituents of alloy steel strength. Manganese
alloy steel is useful for spare parts for heavy work
industrial equipment such as crushers, excavator
buckets, wheel loaders, marine ship plates,
sprockets and so on.
ACKNOWLEDGEMENTS
I would like to thank my fellow research members,
including Mr. Fransiskus Sapar, Mr. Roymonds DJ
Dimu, and two students who have faithfully assisted
in the research phase. We would also like to thank the
head of the materials testing laboratory - Kupang
State Polytechnic (PNK) who helped with several
laboratory tests. Finally, thanks to ICAST and P3M
PNK who helped in the publication.
REFERENCES
Bleck, W., & Haase, C. (2019). Physical metallurgy of high
manganese steels. Metals, 9(10), 1053.
Chen, W. et al. (2021) ‘Microstructure, hardness, and
tensile properties of vacuum carburizing gear steel’,
Metals, 11(2), pp. 1–15. doi: 10.3390/met11020300.
Harjanto, A. (2011) ‘PERHITUNGAN CADANGAN
TEREKA MANGAN (Mn) DI DAERAH KOA DAN
SEKITARNYA, KECAMATAN MOLLO BARAT,
KABUPATEN SOE, NTT’, in PROCEEDING SEMINAR
NASIONAL KEBUMIAN 2011. Available at: http://
psdg.bgl.esdm.go.id/index.php?view=article&cat
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
172
id=52%3Acontent-menu-
utama&id=470%3Apenyelidikan-endapan-mangan-di-
pulau-doi-kecamatan-loloda-
kepulauan&tmpl=component&print=1&page=&option
=com_content&Itemid=475.
Herwandi (2005) ‘Analisa Perubahan Struktur Akibat Heat
Treatment’, Jurnal Teknik Mesin, 7, pp. 57–62.
Idrus, A. et al. (2013) ‘Characteristics and Origin of
Sedimentary-Related Manganese Layers in Timor
Island , Indonesia Sedimen di Pulau Timor , Indonesia’,
8(4), pp. 191–203.
Knight, K. L. (2010) ‘Study/Experimental/Research
Design: MuchMoreThanStatistics’, 45(1),pp.98–100.
Liu, H. et al. (2021) ‘Precipitation criterion for
inhibiting austenite grain coarsening during
carburization of al‐ containing 20CR gear steels’,
Metals, 11(3), pp. 1–19.doi: 10.3390/met11030504.
Lawson, J., & Erjavec, J. (2016). Basic experimental
strategies and data analysis for science and engineering.
Chapman and Hall/CRC.
Mirantie Dwiharsanti, W. S. J. dan S. V. (2016)
‘PERANCANGAN EKSPERIMEN BAJA KARBON
RENDAH HASIL PROSES PACK CARBURIZING
DENGAN METODE EKSPERIMEN DESIGN OF
EXPERIMENT OF LOW CARBON STEEL
RESULTED FROM PACK’, Jurnal Riset Industri Vol.
(2016) 10(2) 92-97, 10, pp. 92–97.
Panjaitan, R. R. (2011) ‘Kajian Pemanfaatan Batu Mangan
/ Senyawa Mangan Dalam Industri’, Berita Litbang
Industri, 48(2), pp. 45–53.
Qasim, S., Imtiaz, Z. and Alvi, U. (2014) ‘Review of True
Experimental Research Studies in Applied Linguistics’,
Research on Humanities and Social Sciences, 4(22), pp.
146–150. Available at: www.iiste.org.
Rerung, O., Sapar, F. and Dimu, R. (2019) ‘Experimental
Design Of Manganese Stone Minerals On Carbon Steel
With Pack Carburizing Approach Using Factorial
Methods’, ICESC 2019, proceeding. doi:
10.4108/eai.18-10-2019.2289977.
Salomon, L. L., Kosasih, W., & Jap, L. (2017). Peningkatan
Kualitas Benang Dty Single 150d/48f Pada Mesin Cone
Wender Menggunakan Metode Six Sigma Dan
Factorial Design Di Pt. Gemilang Texindotama. Jurnal
Ilmiah Teknik Industri, 2(2).
Sara Yasipourtehrani, Vladimir Strezov*, Tim Evans, and
H. M.A. (2020) ‘Macquarie UniversityPURE Research
Management System’, 41, pp. 933–961.
SimoneKaar 1,*, DanielKrizan 2, ReinholdSchneider 1, C.
3 andChristofSommitsch 3 (2019) ‘Effet of Manganese
on the Cavitation.Pdf’. Metals 2019, 9, 1122; doi:
10.3390/met9101122.
Skeeba, V. Y., Ivancivsky, V. V and Martyushev, N. V
(2021) ‘Peculiarities of High-Energy Induction Heating
during Surface Hardening in Hybrid Processing
Conditions’.
Teow, E. L. (2005) ‘Quality Improvement Using Factorial
Design’, Pakistan’s 9th International Convention on
Quality Improvement, pp. 1–7.
Wibawa, A. (2018) ‘TRANSFORMASI MINERAL
PIROLUSIT PADA TEMPERATUR TINGGI
Transformation of Pirolusite Mineral at High
Temperature’, 14(September), pp. 179–186. doi:
10.30556/jtmb.Vol14.No3.2018.682.
Winarto, E. W. et al. (2019) ‘The Improvement of Impact
on Manganese Steel for Bucket Tooth Product’, 2019,
pp. 78–83. doi: 10.18502/kss.v3i23.5138.
APPENDIX
Factorial Identification on Surface Hardness of Cut Low Carbonsteel with Hardening Process using Manganese Minerals Powder
173
