
Substantially Improved Antioxidant Activity of Modified Polymeric
Nanostructure Entrapping Curcumin
Deni Rahmat
1
, Wahyu Widowati
2,*
, Etik Mardliyati
3
, Eny Kusrini
4
, Abdi Wira Septama
5
,
Yati Sumiyati
1
, Mita Restinia
1
, Sjaikhurrizal El Muttaqien
3
, Cintani Dewi Wahyuni
6
,
Hanna Sari Widya Kusuma
6
, Muhammad Aldi
6
, Tri Handayani
6
and Rizal Rizal
6,7
1
Faculty of Pharmacy, Pancasila University, Jl. Srengseng Sawah, Jagakarsa, Jakarta Selatan, 12640, West Java,
Indonesia
2
Faculty of Medicine, Maranatha Christian University, Jl. Surya Sumantri no 65, Bandung 40164, West Java, Indonesia
3
Pharmacy Technology and Medic Center of BPPT, Kawasan Puspiptek Serpong Tangerang Selatan, Banten, Indonesia
4
Faculty Engineering, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Depok 16424,
West Java, Indonesia
5
Chemistry Research Center, LIPI, Kawasan PUSPITEK, Serpong, Tangerang Selatan, 15314, Banten, Indonesia
6
Biomolecular and Biomedical Research Center, Aretha Medika Utama, Jl. Babakan Jeruk 2 no 9, Bandung 40163,
West Java, Indonesia
7
Biomedical Engineering, Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia,
Depok 16426, West Java, Indonesia
abdiwiraseptama@gmail.com, yati.sumiyati@yahoo.com, mita_restinia09@ymail.com, sjaikhurrizal.el@bppt.go.id,
cintanidewi@amubbrc.co.id, hannasariw@amubbrc.co.id, aldimaulana.srl@gmail.com, mbaktrihandayani@gmail.com,
rizal_biotek@yahoo.com
Keywords: Curcumin, Nanoparticle, Antioxidant, Free Radicals.
Abstract: BACKGROUND: Chronic and degenerative diseases due to free radicals cause oxidative stress in the body.
The body requires natural antioxidants to cope with the negative effects of free radicals. Curcumin is a
compound that has been shown to have pharmacological potential, such as antioxidant, anti-inflammatory,
and anti-tumor properties. In recent years, the nanoparticle system for drug administration has become one of
the most frequently studied methods of treating the disease. OBJECTIVE: This study aimed to formulate
nanocurcumin (NC) to enhance its antioxidant activity. METHODS: The antioxidant activity of Curcumin
and NC was evaluated using 2,2-diphenyl-1-pycrylhydrazyl (DPPH), 2,2’-azinobis-3-ethylbenzo-thiazoline-
6-sulfonic acid (ABTS), H2O2, NO scavenging activities and ferric reducing antioxidant power (FRAP)
assay. RESULTS: The results showed that the median Inhibitory Concentration (IC50) for DPPH, ABTS,
H2O2, NO scavenging activities of NC was 0.68; 15.59; 24.98; 19.61 µg/mL, respectively. While the IC50
value for curcumin was 3.20; 18.54; 38.40; 24.94 µg/mL, respectively. The FRAP activity of NC and
curcumin was 502.92 and 256.50 μM Fe(II)/μg, respectively, at the highest concentration of 50 µg/mL.
CONCLUSION: The antioxidant activity of the NC was higher than that of curcumin alone. Thus, the
nanoparticle system may enhance the antioxidant activity of curcumin.
1 INTRODUCTION
Free radicals are unstable and highly reactive
molecules due to the lack of electron pairs in the
atomic orbits (Yildiz, 2020). To become stable, free
radicals can either accept electrons or give electrons
to other molecules. This results in the target molecule
*
Corresponding author
losing electrons and becoming free radicals, which
triggers a chain reaction and ultimately harms living
cells (Phaniendra, et al., 2015). Free radicals can be
produced in the body through metabolic processes or
occur due to environmental factors such as X-ray
exposure, smoking, air pollution, and industrial
chemical (Zubieta-Calleja & Zubieta-DeUrioste,
2017).
344
Rahmat, D., Widowati, W., Mardliyati, E., Kusrini, E., Septama, A., Sumiyati, Y., Restinia, M., El Muttaqien, S., Wahyuni, C., Kusuma, H., Aldi, M., Handayani, T. and Rizal, R.
Substantially Improved Antioxidant Activity of Modified Polymeric Nanostructure Entrapping Curcumin.
DOI: 10.5220/0010754000003113
In Proceedings of the 1st International Conference on Emerging Issues in Technology, Engineering and Science (ICE-TES 2021), pages 344-350
ISBN: 978-989-758-601-9
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

An imbalance between antioxidants and free
radicals in the body can cause oxidative stress, which
will lead to various diseases such as heart disease,
stroke, gastric ulcers, hypertension, preeclampsia,
and neurological disorders (Alzheimer's disease and
Parkinson's disease) (Zubieta-Calleja & Zubieta-
DeUrioste, 2017).
Curcumin is one of the compounds contained in
the rhizomes of Curcuma longa. Curcumin is an
extremely potent antioxidant that has been studied by
many scientists over the world. Antioxidant activity
was related to the phenolic groups that are presence
in curcumin (Sokmen & Khan, 2016). The U.S. Food
and Drug Administration (FDA) has stated that
curcumin is a safe compound, with a daily intake of
curcumin at a dose of 0.10003 mg/kg BW. However,
the use of curcumin is limited because curcumin has
poor absorption, a short half-life, and rapid
metabolism in the digestive system (Ghosh et al.,
2015).
Nanocurcumin, chitosan-coated curcumin,
liposome-encapsulated curcumin, cyclodextrin
encapsulated curcumin and polylactic-coglycolic acid
encapsulated curcumin are structural modifications of
curcumin that have been tried to increase the
bioavailability of curcumin (Ghosh et al., 2015). In a
study conducted by Aditya et al. (2015), curcumin in
the form of nanosuspension could increase the
bioavailability of curcumin. Nanoparticles containing
curcumin compounds can also be a promising
strategy for delivering drugs to target organs and
increasing antidiabetic activity. This has been proven
in research by Rahmat et al. (2020), which stated that
nanoparticles containing curcumin could reduce
blood sugar levels in Alloxan-induced diabetic rats.
The objective of this study was to determine the
antioxidant activity of nanoparticle curcumin (NC) by
comparing it to curcumin alone. This research was a
preliminary study, the continued research evaluated
Curcumin and NC as anti-inflammatory and
antioxidant potential on acute lung injury rat model.
2 MATERIALS AND METHODS
2.1 Preparation of Nanoparticles
The preparation of nanoparticles was carried out as
described by Rahmat et al. (2020) with modifications.
A 1 g of chitosan was dissolved in 100 mL of glacial
acetic acid 1% (v/v) to obtain 1% chitosan. A 1 g of
ethylcellulose was dissolved in 100 mL of 96%
ethanol to obtain 1% ethylcellulose. Curcumin was
dissolved in the final concentration of 60 mg/mL in a
mixed solvent containing 20 mL of 10% dimethyl
sulfoxide (DMSO), 20 mL of 70% ethanol, and 20
mL of polypropylene glycol (PPG). Subsequently, the
curcumin solution was added by 40 mL of chitosan
solution. The mixture was then stirred for ten mins. A
40 mL of ethylcellulose was added to the preparation.
Sodium tripolyphosphate 0.2% was added per drop to
the final mixture up to 3 mL while being stirred.
2.2 DPPH Scavenging Assay
Briefly, 50 μL of samples, 200 μL DPPH solution
(Sigma Aldrich, D9132) were added to 96-well.
microplate. The plate then incubated for 30 mins in
dark condition at room temperature. A microplate
reader was used for measuring the absorbance at 517
nm (Multiskan GO Microplate Spectrophotometer,
Thermo Scientific) (Widowati et al., 2017; Prahastuti
et al., 2019; Prahastuti et al., 2020). The DPPH
scavenging activity was calculated using the equation
below:
%𝑆𝑐𝑎𝑣𝑒𝑛𝑔𝑖𝑛𝑔 𝑎𝑐𝑡𝑖𝑣𝑖𝑡𝑦 =
𝑐𝑜𝑛𝑡𝑟𝑜𝑙 𝑎𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒 − 𝑠𝑎𝑚𝑝𝑙𝑒 𝑎𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
𝑐𝑜𝑛𝑡𝑟𝑜𝑙 𝑎𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
𝑥 100
(1)
2.3 ABTS Reducing Activity Assay
To obtain ABTS•+ solution, 14 mM ABTS (Sigma
Aldrich, A1888) was mixed with 4.9 mM potassium
persulfate (Merck, EM105091) with a volume ratio of
1:1. The solution was incubated in dark condition at
room temperature for 16 h. The solution was added
by 5.5 mM Phosphate Buffered Saline (PBS) at pH
7.4 until the solution’s absorbance was 0.70±0.02 at
745 nm. A 198 µL of ABTS•+ solution and 2 µL of
samples was added into several well in 96-well
microplate. The plate then incubated at 30°C for 6
mins (Widowati et al., 2017; Prahastuti et al., 2019;
Prahastuti et al., 2020). ABTS reducing activity was
calculated using the equation below:
%𝑆𝑐𝑎𝑣𝑒𝑛𝑔𝑖𝑛𝑔 𝑎𝑐𝑡𝑖𝑣𝑖𝑡𝑦 =
𝑐𝑜𝑛𝑡𝑟𝑜𝑙 𝑎𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒 − 𝑠𝑎𝑚𝑝𝑙𝑒 𝑎𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
𝑐𝑜𝑛𝑡𝑟𝑜𝑙 𝑎𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
𝑥 100
(2)
2.4 H2O2 Scavenging Activity
Briefly, 3 μL of 5 mM H2O2 (Merck 1,08597), 12 μL
of 1 mM ferrous ammonium sulphate (Sigma Aldrich,
215406) were added to 96-well microplate.
Subsequently, the mixture then added by 60 µL of
samples, 63 μL of Dimethyl sulfoxide (DMSO)
(Supelco, 1.02952.1000) was added in the control
well and 90 μL in the blank well. The plate was put in
Substantially Improved Antioxidant Activity of Modified Polymeric Nanostructure Entrapping Curcumin
345
dark condition, room temperature for 5 mins. Amount
75 μL of 10-phenanthroline (Sigma Aldrich, 131377)
was added to the mixture, and the plate was re-
incubated for 10 mins. The sample absorbance was
measured at 510 nm using a microplate reader (Utami
et al., 2017; Prahastuti et al., 2020). The H2O2
scavenging activity was calculated according to the
following equation:
%𝑆𝑐𝑎𝑣𝑒𝑛𝑔𝑖𝑛𝑔 𝑎𝑐𝑡𝑖𝑣𝑖𝑡𝑦 =
𝑐𝑜𝑛𝑡𝑟𝑜𝑙 𝑎𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒 − 𝑠𝑎𝑚𝑝𝑙𝑒 𝑎𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
𝑐𝑜𝑛𝑡𝑟𝑜𝑙 𝑎𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
𝑥 100
(3)
2.5 NO Scavenging Activity Assay
The samples were mixed with 40 µL of 10 mM
sodium nitroprusside (Merck, 106541) in PBS.
(Gibco, 1740576). The mixture was then incubated
for 2 h at room temperature. Into the mixture, add 100
µL Griess reagent containing 1% sulfanilamide
[Merck 111799, Germany], 2% H3PO4 (Merck,
100573), and 0.1% N-(1-naphthyl) ethylenediamine
dihydrochloride (Sigma Aldrich, 222488) was added.
The absorbance was measured by using a microplate
reader at a wavelength of 546 nm (Utami et al., 2018
Prahastuti et al., 2019; Prahastuti et al., 2020). NO
scavenging activity was calculated using the
following equation:
%𝑆𝑐𝑎𝑣𝑒𝑛𝑔𝑖𝑛𝑔 𝑎𝑐𝑡𝑖𝑣𝑖𝑡𝑦 =
𝑐𝑜𝑛𝑡𝑟𝑜𝑙 𝑎𝑏𝑠 − 𝑠𝑎𝑚𝑝𝑙𝑒 𝑎𝑏𝑠
𝑐𝑜𝑛𝑡𝑟𝑜𝑙 𝑎𝑏𝑠
𝑥100
(4)
2.6 FRAP Assay
Briefly, 10 mL of 300 mM acetate buffer was mixed
with 1 mL of 10 mM 2,4,6-Tris (2-pyridyl)-s- triazine
(TPTZ) (Sigma Aldrich, T1253), and 1 mL of 20 mM
ferric chloride hexahydrate (Merck 1.03943.0250) at
pH 3.6 to obtain FRAP reagent. Amount 142.5 μL of
FRAP reagent and 7.5 μL of samples were then added
into several well in a 96-well microplate. The plate
was then incubated for 6 mins at 37°C and the
absorbance was measured at 593 nm (Prahastuti et al.,
2019; Prahastuti et al., 2020).
2.7 Statistical Analysis
The data were statistically analyzed using Oneway
ANOVA and Tukey's HSD Post-hoc test (IBM SPSS
Statistics for Windows, version 20.0, Armonk, NY)
at a significance level of P < 0.05. The results were
expressed as mean ± standard deviation.
3 RESULT AND DISCUSSION
3.1 Preparation of Nanoparticles
The nanoparticles were generated by the ionic
gelation method. The positively charged substructure
of chitosan interacted with the negative charge of TPP
ions. The resulting nanoparticles showed a diameter
of 330 nm.
3.2 DPPH Scavenging Assay
The radical DPPH is typically used as a substrate for
the observation the antioxidant activity. The DPPH is
typically used to attribute the radical scavenging
activity of plant extracts or organic compounds
(Widowati et al., 2016; Widowati et al., 2018). When
a hydrogen donor is present, it becomes paired and
absorption at 517 nm will be reduced (Widowati et
al., 2018). Stable DPPH radicals will be reduced to
diphenyl picrylhydrazine (DPPH-H) during the
DPPH assay. The concentration that allowed an
antioxidant to scavenge 50% of the free radical of
DPPH corresponds to the median inhibitory
concentrations (IC50) value. The lower the IC50
value, indicating higher the antioxidant activity.
Table 1: The IC50 value of Antioxidant Activities of
Curcumin and Nanocurcumin.
Sample
IC50 of
DPPH
(μg/mL)
IC50 of
ABTS
(μg/mL)
IC50 of
H
2O2
(μg/mL)
IC
50 of
NO
(μg/mL)
Curcumin 3.20 18.54 38.40 24.94
Nano
curcumin
0.68 15.59 24.98 19.61
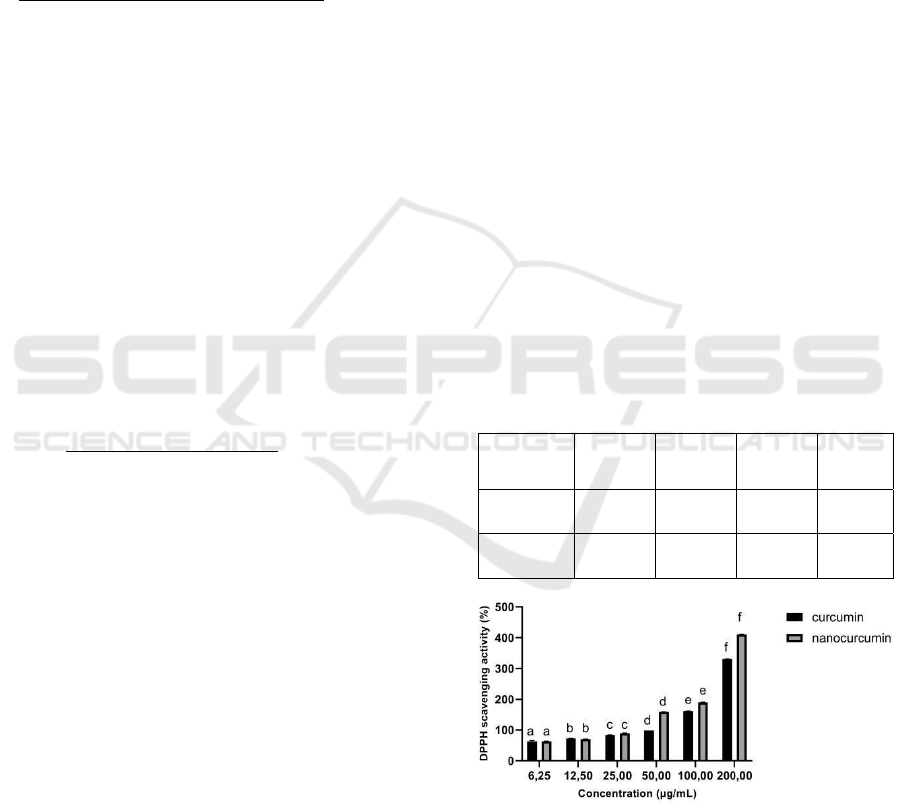
Figure 1: DPPH Scavenging Activity of Curcumin and
Nanocurcumin. The data was presented as mean ± SD.
Different letters (a,b,c,d,e,f) indicate a significant
difference among concentration both curcumin and
nanocurcumin based on Tukey’s post hoc test (p < 0.05).
Based on the results, both curcumin and
nanocurcumin were successful to show antioxidant
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
346
activity by DPPH scavenging assay. The results of
this study were in line with some previous studies.
Curcumin’s antioxidant activity has been revealed in
biological models by many researchers. There is
several scientific evidence that proven curcumin's
free radicals trapping capability on living cells
(Rafiee et al., 2019). The IC50 DPPH scavenging
activity of curcumin was 3.2 μg/mL, whereas the
IC50 for nanocurcumin was 0.68 μg/mL. According
to Widowati et al. (2017), the smaller the IC50 of a
sample, the better the sample’s ability to trap free
radicals. The DPPH scavenging activity of curcumin
and nanocurcumin were categorized very active when
the IC50 value < 50 μg/mL (Marjoni and Zulfisa,
2017).
It was discovered that nanocurcumin was more
active in the DPPH scavenging activity than curcumin
because it provided the lower IC50 value (Table 1).
Nanocurcumin has a better reduction activity than
curcumin (Figure 1). This finding was was in line
with the previous study, which concluded that the
antioxidant activity of nanocurcumin was improved
over that of curcumin. Moghaddasi et al. (2018)
tested the synthesis of the nanocurcumin system
(Nano- CUR) using the O/W nanoemulsion method.
The antioxidant activities of Nano-CUR have more
potential than its native curcumin and it has a potent
candidate for treating chronic diseases (Moghaddasi
et al., 2018). In another study, Hosseini et al. (2019)
studied the effect of curcumin and nanocurcumin on
the oxidant and antioxidant system of liver
mitochondria using an aluminum phosphide (AIP)-
induced toxicity model in rats. It was found that
nanocurcumin enhanced the oxidative stress factors
and protected the liver against the adverse effects of
AlP by scavenging the free radicals and stabilizing
the oxidative status (Hosseini et al., 2019).
Several physico-chemical properties considered
to make nanocurcumin more effective than native
curcumins are surface area, particle size,
hydrophobicity, and surface charge. Previous studies
have demonstrated that these properties can enhance
solubility, bioavailability, and active targeting
(Biswas et al., 2014). The reduction in particle size
greatly increases the efficiency of nanocurcumin and
makes it superior to curcumin. Nanocurcumin is an
ideal drug compared to normal curcumin because of
its larger surface area (Flora et al., 2013; Rahmat et
al., 2020).
3.3 ABTS Reducing Activity Assay
ABTS is generated by the reaction between ABTS
salt and a strong oxidizing agent (potassium
permanganate/potassium persulphate). An ABTS
reduction activity test was performed to measure the
relative capacity of antioxidants to trap the ABTS
produced. The reduction of the ABTS solution by
antioxidants is measured by the spectrum of long-
wave absorption. The effectiveness of the 50%
trapping activity (IC50) is the concentration required
for the sample to trap 50% of the ABTS radical. The
lower the IC50 value, the higher the antioxidant
activity. An ABTS reduction activity test in this study
use the sample with the final concentration of 50
μg/mL; 25 μg/mL; 12.5 μg/mL; 6.25 μg/mL; 3.13
μg/mL; and 1.56 μg/mL. The ABTS IC50 reduction
values are given in Table 1. The results of curcumin
and nanocurcumin ABTS reduction activities are
presented in Figure 2.
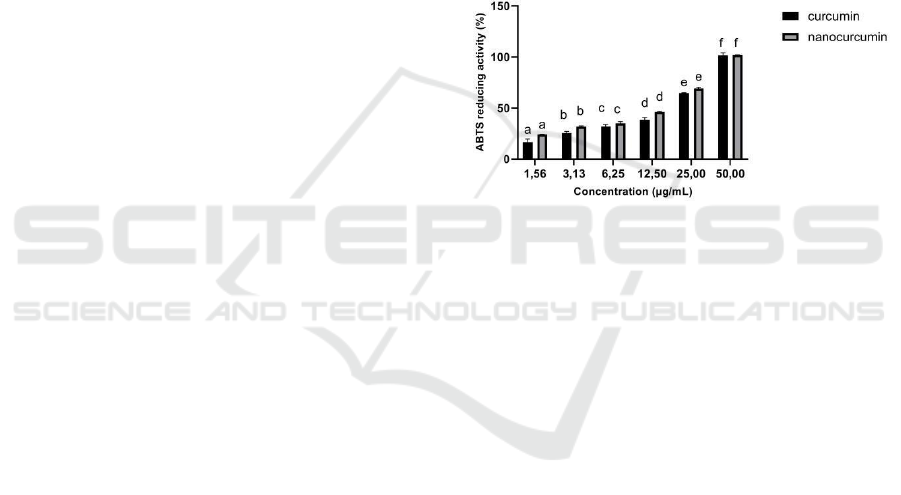
Figure 2: ABTS Reducing Activity of Curcumin and
Nanocurcumin. The data was presented as mean ± SD.
Different letters (a,b,c,d,e,f) indicate a significant
difference among concentration both curcumin and
nanocurcumin based on Tukey’s post hoc test (p < 0.05).
The results indicated that the IC50 for the ABTS
reducing activity assay was 18.54 μg/mL, while the
IC50 for nanocurcumin was 15.59 μg/mL. Both
curcumin and nanocurcumin were categorized very
active toward ABTS reducing activity (Marjoni and
Zulfisa, 2017). Nanocurcumin has higher ABTS
reducing activity compared to curcumin since it has a
lower IC50 (Table 1). This finding is also consistent
with other previous study that concluded that the
antioxidant activity of nanocurcumin was higher than
curcumin (Hosseini et al., 2019).
3.4 H2O2 Scavenging Activity
Hydrogen peroxide (H2O2) plays an important role in
the production of energy such as phagocytosis, in vivo
systems, cell growth control, intercellular signal
transfer, and the synthesis of essential biological
compounds. H2O2 is a byproduct of normal aerobic
metabolism that has generated and increased during
training, infections, and stressful conditions
(Mukhopadhyay et al., 2016). The concentration
Substantially Improved Antioxidant Activity of Modified Polymeric Nanostructure Entrapping Curcumin
347
allowed by an antioxidant to scavenge 50% of the
H2O2 free radical is the IC50 value. The smaller the
IC50 value indicating higher the antioxidant activity.
The IC50 value of the H2O2 radical scavenging
activity of curcumin and nanocurcumin were
presented in Table 1. The results of H2O2 reduction
activities of curcumin and nanocurcumin are shown in
Figure 3.
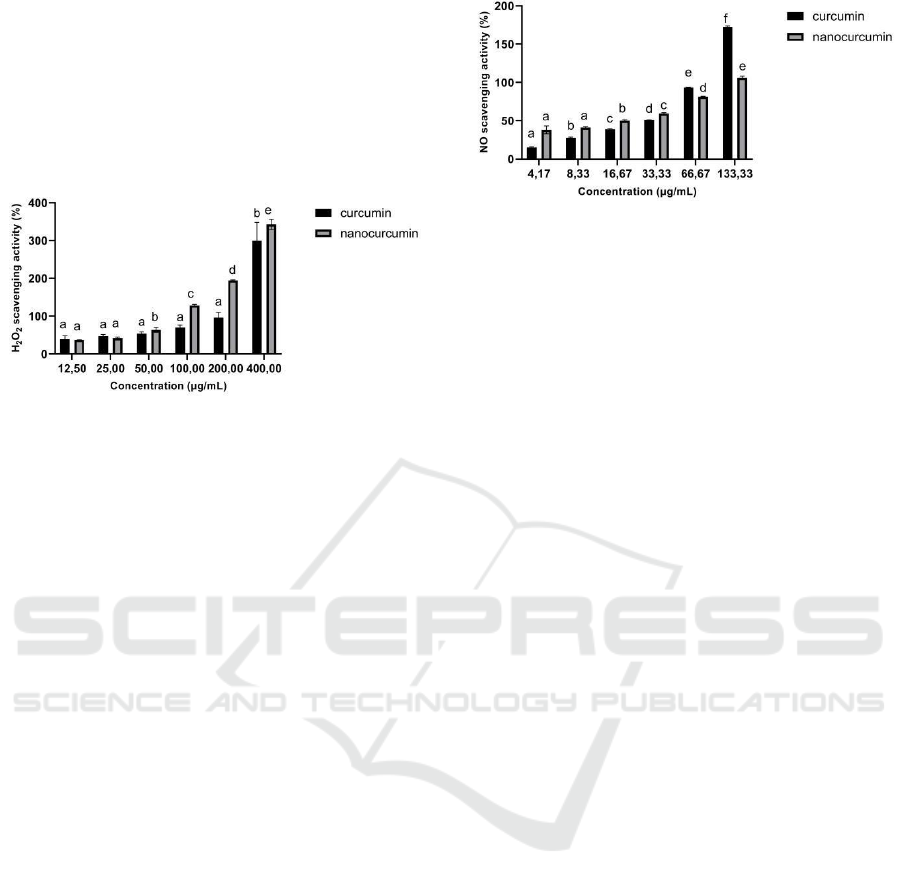
Figure 3: H2O2 Scavenging Activity of Curcumin and
Nanocurcumin. The data was presented as mean ± SD.
Different letters (a,b) for curcumin and different letters
(a,b,c,d,e) indicate a significant difference among
concentration based on Tukey’s post hoc test (p < 0.05).
Based on the results, the IC50 of H2O2 scavenging
activity for curcumin was 38.40 μg/mL, whereas the
IC50 for nanocurcumin was 24.98 μg/mL. According
to Widowati et al. (2017), the lower the IC50 for a
sample, the higher the sample’s ability to trap free
radicals. Both curcumin and nanocurcumin were
categorized as very active toward H2O2 scavenging
activity (Marjoni and Zulfisa, 2017). Nanocurcumin
was found to be more active in H2O2 scavenging
activity than curcumin because it had the lower IC50
(Table 1). Nanocurcumin has a better reduction
activity in comparison to curcumin (Figure 3). This
finding was appropriate for an earlier study that
concluded that the antioxidant activity of
nanocurcumin was improved over curcumin (Hosseini
et al., 2019).
3.5 NO Scavenging Activity
Nitric oxide (NO) is a potent signaling mediator in
several cellular processes. This molecule acts as a
mediator in the regulation of inflammation,
neurotransmission, host defense mechanisms, and
vascular tonus (Utami et al., 2018). In this study, NO
scavenging activity was tested by using the sample
with final concentration of 133.33 μg/mL; 66.67
μg/mL; 33.33 μg/mL; 16.67 μg/mL; 8.33 μg/mL; and
4.17 μg/mL. The IC
50 value of NO radical scavenging
activity of curcumin and nanocurcumin are presented
in Table 1. The results of NO reduction activities from
curcumin and nanocurcumin are shown in Figure 4.
Figure 4: NO Scavenging Activity of Curcumin and
Nanocurcumin. The data was presented as mean ± SD.
Different letters (a,b,c,d,e,f) for curcumin and different
letters (a,b,c,d,e) indicate a significant difference among
concentration based on Tukey’s post hoc test (p < 0.05).
The result indicated that the IC50 value of the NO
reducing activity was 24.94 μg/mL, whereas the IC50
of nanocurcumin was 19.61 μg/mL. Both curcumin
and nanocurcumin were categorized as very active
toward NO scavenging activity (Marjoni and Zulfisa,
2017).
Nanocurcumin showed a higher NO scavenging
activity compared to curcumin because it has a lower
IC50 (Table 1). The reduction activity of this assay
differed from that of the other assay. Curcumin had a
trapping activity of 172.72±1.22%, higher than
nanocurcumin trapping activity of 106.44±2.22%,
especially at the highest concentration (133 μg/mL)
(Figure 4). This finding is also in line with another
previous study that concluded that nanocurcumin had
higher antioxidant activity than curcumin (Hosseini et
al., 2019).
3.6 FRAP Activity
FRAP assays are commonly used for antioxidant
properties based on the electron transfer potential
of the existing antioxidants. The FRAP method
was based on the reduction of analog ferroin in an
acid medium, the TPTZ3
+
in the colored Fe
2+
complex of Fe(TPTZ)
2+
(greatly blue) by
antioxidant (Widowati et al., 2018). A reduction in
the tripyridyltriazine Fe(III) complex at 593 nm
results from the absorbance of the Fe(II) complex.
The results of FRAP reduction activities from
curcumin and nanocurcumin are shown in Figure
5.
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
348
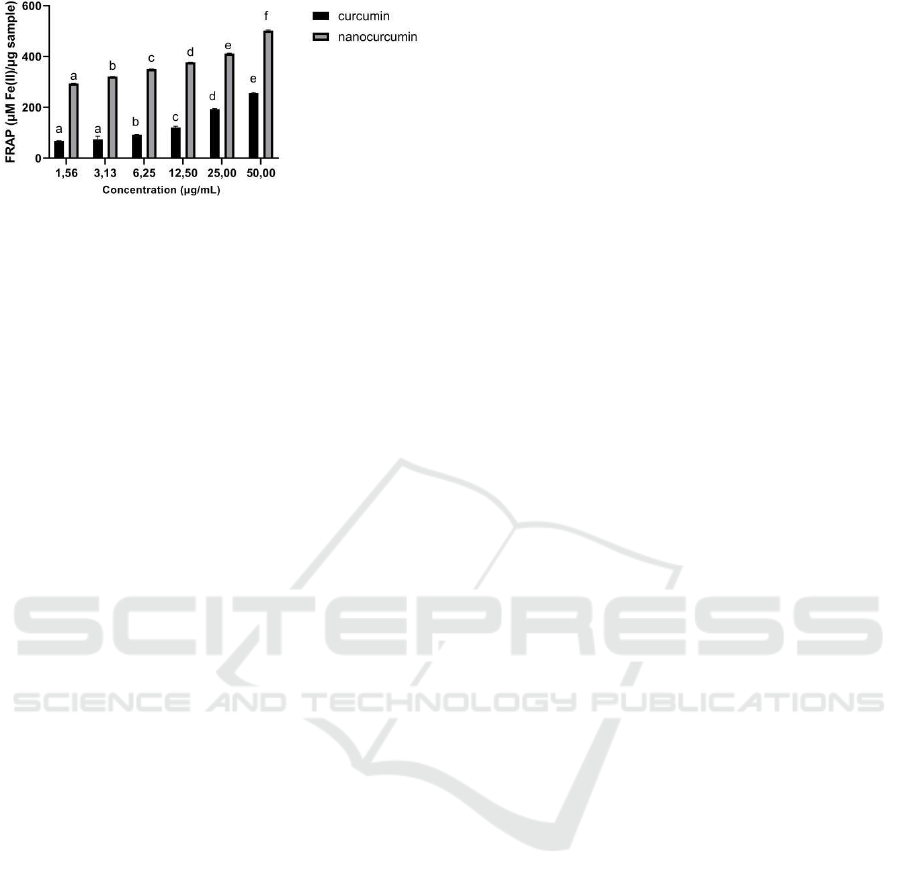
Figure 5: FRAP Scavenging Activity of Curcumin and
Nanocurcumin. The data was presented as mean ± SD.
Different letters (a,b,c,d,e) for curcumin and different
letters (a,b,c,d,e,f) indicate a significant difference among
concentration based on Tukey’s post hoc test (p < 0.05).
Based on the results, nanocurcumin is more active
in FRAP scavenging activity than curcumin because
it provides a lower IC50 value (Table 1).
Nanocurcumin has a better reduction activity than
curcumin (Figure 5). At the highest concentration (50
μg/mL), nanocurcumin had a trapping activity of
502.92±2.55%, higher than the curcumin trapping
activity of 256.50±3.68%.
This finding was appropriate for a previous study
that concluded that the antioxidant activity of
nanucurcuma was improved over curcumin (Hosseini
et al., 2019). Our current findings could demonstrate
the antioxidant activity of curcumin and
nanocurcumin. Nevertheless, this study has not been
able to describe the effect of curcumin and
nanocurcumin treatment on cells. Future research is
expected to be able to test curcumin and
nanocurcumin’s effect on cells or in vivo.
4 CONCLUSIONS
Nanocurcumin was found to be more effective than
native curcumins in the free radical scavenging
activities of DPPH, ABTS, H2O2, NO, and FRAP.
Nanocurcumin showed higher antioxidants reduction
activity in DPPH, ABTS, H O, and FRAP compared
to curcumin. Both curcumin and nanocurcumin have
very active antioxidants.
ACKNOWLEDGEMENTS
This study was funded by the LPDP Covid-19
Consortium of Minister of Finance of Republic
Indonesia and supported by Aretha Medika Utama,
Biomolecular and Biomedical Research Center,
Bandung, Indonesia. We also acknowledge the
technical support of Ervi Afifah, Cahyaning Riski
Wijayanti of Aretha Medika Utama-Biomolecular
and Biomedical Research Center, Bandung,
Indonesia.
REFERENCES
Aditya, N. P., Aditya, S., Yang, H. J., Kim, H. W., Park, S.
O., Lee, J., Ko, S. (2015). ‘Curcumin and catechin co-
loaded water-in-oil-in-water emulsion and its beverage
application’. J. Funct. Foods, 15, 35-43.
Biswas, A. K., Islam, M. R., Choudhury, Z. S., Mostafa, A.,
Kadir, M. F. (2014). ‘Nanotechnology based
approaches in cancer therapeutics’. Adv. Nat. Sci:
Nanosci. Nanotechnol., 5, 43001.
Flora, G., Gupta, D., Tiwari, A. (2013). ‘Nanocurcumin: a
promising therapeutic advancement over native
curcumin’. Crit Rev Therap Drug Carrier Syst, 30.
Ghosh, S., Banerjee, S., Sil, P. C. (2015). ‘The beneficial
role of curcumin on inflammation, diabetes and
neurodegenerative disease: A recent update’. Food
Chem. Toxic., 83, 111-124.
Hosseini, A., Rasaie, D., Soleymani, S., Nili, A., Ranjbar,
A. (2019). ‘Evaluation of the protective effects of
curcumin and nanocurcumin against lung injury
induced by sub-acute exposure to paraquat in
rats’.Toxin. Rev., 1-9.
Kharia, A. A., Singhai, A. K., Verma, R. (2012).
‘Formulation and evaluation of polymeric nanoparticles
of an antiviral drug for gastroretention’. Int.J. Pharm.
Sci.Nanotechnol., 4(4), 1557-1562.
Marjoni, M.R. Zulfisa, A. (2017). ‘Antioxidant activity of
methanol extract/fractions of Senggani leaves
(Melastoma candidum D. Don)’. Pharmaceuti.
Analytic. Acta. 8(8):1-6.
Moghaddasi, F., Housaindokht, M. R., Darroudi, M.,
Bozorgmehr, M. R., Sadeghi, A. (2018). ‘Synthesis of
nano curcumin using black pepper oil by O/W
nanoemulsion technique and investigation of their
biological activities’. LWT. 92, 92-100.
Mukhopadhyay, D., Dasgupta, P., Roy, D. S.,
Palchoudhuri, S., Chatterjee, I., Ali, S., Dastidar, S. G.
(2016). ‘A sensitive in vitro spectrophotometric
hydrogen peroxide scavenging assay using 1, 10-
phenanthroline’. Free Rad. Antiox., 6(1), 124-132.
Munawar, H., Smolinska-Kempisty, K., Cruz, A. G.,
Canfarotta, F., Piletska, E., Karim, K., Piletsky, S. A.
(2018). ‘Molecularly imprinted polymer nanoparticle
based assay (MINA): application for fumonisin B1
determination’. Analyst., 143 (14), 3481-3488.
Phaniendra, A., Jestadi, D. B., Periyasamy, L. (2015). ‘Free
radicals: properties, sources, targets, and their
implication in various diseases’. ‘Indian J. Clin.
Biochem, 30(1), 11-26.
Prahastuti, S., Hidayat, M., Hasianna, S. T., Widowati, W.,
Amalia, A., Yusepany, D. T., Kusuma, H. S. W. (2019).
‘Antioxidant potential ethanolic extract of Glycine max
Substantially Improved Antioxidant Activity of Modified Polymeric Nanostructure Entrapping Curcumin
349

(l.) Merr. Var. Detam and daidzein’. Int. J. Phys: Conf.
Ser., 1374(1), 012020.
Prahastuti, S., Hidayat, M., Hasianna, S. T., Widowati, W.,
Handayani, A. S., Rizal, R., & Kusuma, H. S. W.
(2020). ‘The ethanol extract of the bastard cedar
(Guazuma ulnifolia L.) as antioxidants’. Pharmaciana,
10 (1), 77-78.
Rafiee, Z., Nejatian, M., Daeihamed, M., Jafari, S. M.
(2019). ‘Application of curcumin-loaded nanocarriers
for food, drug and cosmetic purposes. Trends Food Sci.
Tech., 88, 445-458.
Rahmat, D., Farida, Y., Brylianto, A. T., Sumarny, R.,
Kumala, S. (2020). ‘Antidiabetic activity of
nanoparticles containing javanese turmeric rhizome
extract: the strategy to change particle size. Int. J. App.l
Pharm., 90-93.
Sasikumar, V. Kalaisezhiyen, P. (2014). ‘Evaluation of free
radical scavenging activity of various leaf extracts from
Kedrostis foetidissima (Jacq.) Cogn. Biochem. Analytic
Biochem., 3(2), 150-157.
Sokmen, M., Khan, M. A. (2016). ‘The antioxidant activity
of some curcuminoids and chalcones’.
Inflammopharmacol., 24(2), 81-86.
Utami, S., Sachrowardi, Q. R., Damayanti, N. A.,
Wardhana, A., Syarif, I., Nafik, S., Widowati, W.
(2018). ‘Antioxidants, anticollagenase and antielastase
potentials of ethanolic extract of ripe sesoot (Garcinia
picrorrhiza Miq.) fruit as antiaging’. J.Herbmed
Pharmacol., 7(2), 88-93.
Utami, S., Adityaningsari, P., Sosiawan, I., Endrini, S.,
Sachrowardi, Q. R., Laksono, S. P., Nafik, S.,
Arrahmani, B. C., Afifah, E., Widowati, W. (2017).
‘Antioxidants and anticholinesterase activities of the
characterized ethanolic of ripe sesoot (Garcinia
picrorrhiza Miq.) fruit extract (GpKar) and xanthone’.
MOT, 22(3), 160-165
Widowati, W., Fauziah, N., Herdiman, H., Afni, M., Afifah,
E., Kusuma, H. S. W., Nufus, H., Arumwardana, S,.
Rihibiha, D.D. (2016). Antioxidant and anti-aging
assays of Oryza sativa extratcs, vanilin and coumaric
acid. J. Nat. Remed. 16(3), 88-99
Widowati, W., Rani, A.P., Hamzah R. A., Arumwardana,
S., Afifah, E., Kusuma, H.S.W., & Amalia, A. (2017).
Antioxidant and antiaging assays of Hibiscus sabdariffa
extract and its compounds. Nat. Prod. Sci. 23(3), 192-
200.
Widowati, W., Janeva, B. W., Nadya, S., Amalia, A.,
Arumwardana, S., Kusuma, H. S. W., and Arinta, Y.
(2018). Antioxidant and antiaging activities of
Jasminum sambac extract, and its compounds. J. Rep.
Pharm. Sci. 7(3), 270.
Yildiz, S. C. (2020). Innovative theories in science and
environment. Iksad Publishing.
Zubieta-Calleja, G. R. and Zubieta-DeUrioste, N. A.
(2017). Extended longevity at high altitude: benefits of
exposure to chronic hypoxia. BLDE Univ. J. Health
Sci
., 2(2), 80.
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
350
