
Effects of Herbal Ingredients (Allium sativum, Punica granatum,
Curcuma longa, Curcuma xanthorrhiza) on FATP3 Gene Expression
in Aorta of High Fat Diet-fed Rats: A Preliminary Study
Diana Krisanti Jasaputra
1,2 a
, Julia Windi Gunadi
2,3 b
, Penny Setyawati Martioso
4
, Larissa
4
,
Yenny Noor
5
, Irna Permanasari Gani
6
, Erik Dwikurnia Saiman
7
, Desman Situmorang
8
and Andi Haryanto
9
1
Department of Pharmacology, Faculty of Medicine, Maranatha Christian University, Jl. Suria Sumantri, Bandung,
Indonesia
2
Maranatha Biomedical Research Laboratory, Maranatha Christian University, Jl. Suria Sumantri, Bandung, Indonesia
3
Department of Physiology, Faculty of Medicine, Maranatha Christian University, Jl. Suria Sumantri, Bandung, Indonesia
4
Department of Clinical Pathology, Faculty of Medicine, Maranatha Christian University, Jl. Suria Sumantri, Bandung,
Indonesia
5
Department of Ophthalmology, Faculty of Medicine, Maranatha Christian University, Jl. Suria Sumantri, Bandung,
Indonesia
6
Department of Psychiatry, Faculty of Medicine, Maranatha Christian University, Jl. Suria Sumantri, Bandung, Indonesia
7
Department of Obstetric Gynecology, Faculty of Medicine, Maranatha Christian University, Jl. Suria Sumantri, Bandung,
Indonesia
8
Department of Obstetric Gynecology, Faculty of Medicine, Maranatha Christian University, Jl. Suria Sumantri, Bandung,
Indonesia
9
Department of Internal Medicine, Faculty of Medicine, Maranatha Christian University, Jl. Suria Sumantri, Bandung,
Indonesia
yennynoor@yahoo.com, Irna_genie@yahoo.com, eriksaiman72@gmail.com, dman2912@gmail.com,
andi_agape@yahoo.com
Keywords: Curcuma, Allium Sativum, Punica Granatum, FATP3.
Abstract : FATP (Fatty Acid Transport Protein) is a protein that facilitates uptake of LCFA (Long Chain Fatty Acid) by
activating it into CoA-thioester and trapping them in the cell. FATP3 is critical for LCFA uptake in endothelial
cells. Herbal ingredients are well known as anti-hyperlipidemic and anti-atherosclerotic agents, but the
molecular mechanism for these effects are still unclear. Twenty-eight male Wistar rats used in this study were
divided into negative control, positive control (HFD), and treatments (175 mg/kg BW Allium sativum, Punica
granatum, Curcuma longa, Curcuma xanthorrhiza, and 1.8 mg/kg BW Rosuvastatin), each group consisted
of 4 rats. The rats were given vitamin D 700.000 mg/kg BW single dose to all groups except for negative
control, continued with HFD combined with herbal ingredients for twelve weeks. After treatments, the rats
were sacrificed, RNA was extracted from the aorta to perform semi-quantitative PCR (FATP3 and GAPDH).
We found no significant differences in FATP3 gene expression between all groups. In summary, herbal
ingredients (Allium sativum, Punica granatum, Curcuma longa, Curcuma xanthorrhiza) do not influence
FATP3 gene expression in the aorta of high fat diet-fed rats.
1 INTRODUCTION
Population around the world has been through a
modern transition, where the trends of a sedentary
a
https://orcid.org/0000-0001-5608-6112
b
https://orcid.org/0000-0003-3645-7486
lifestyle and over calories become more prominent
than under-nutrition (Shao et al., 2017). This
transition might lead to obesity that served as a risk
factor for developing metabolic syndrome, thus
328
Jasaputra, D., Gunadi, J., Mar tioso, P., Larissa, ., Noor, Y., Gani, I., Saiman, E., Situmorang, D. and Haryanto, A.
Effects of Herbal Ingredients (Allium sativum, Punica granatum, Curcuma longa, Curcuma xanthorrhiza) on FATP3 Gene Expression in Aorta of High Fat Diet-fed Rats: A Preliminary Study.
DOI: 10.5220/0010753800003113
In Proceedings of the 1st International Conference on Emerging Issues in Technology, Engineering and Science (ICE-TES 2021), pages 328-332
ISBN: 978-989-758-601-9
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

increasing the risk for atherosclerosis (Aboonabi,
Meyer, & Singh, 2019). Recent research has shown
that metabolic syndrome promotes atherosclerotic
lesions, and endothelium might mediate some of the
effects (Aboonabi et al., 2019; Goldberg & Bornfeldt,
2013). The main risk factor for developing obesity
that might contribute to the incidence of
atherosclerosis is dietary fat intake (Csige et al.,
2018). The previous study has shown that dietary
intake of Long Chain Fatty Acid (FATP) in large
amounts might induce lesions of atherosclerosis
(Blair, Sepulveda, & Papachristou, 2016).
LCFA, mostly found in our dietary lipid intake,
require active transport into the blood flow (Dallinga-
Thie et al., 2010). FATP (Fatty Acid Transport
Protein), a family of transmembrane proteins, has
been proven to improve LCFA cellular uptake (C.
Hagberg, Mehlem, Falkevall, Muhl, & Eriksson,
2013; Stahl, Gimeno, Tartaglia, & Lodish, 2001).
FATP has 6 members, ranging from FATP1 until
FATP6, that could be found in many organs utilizing
fatty acid (Stahl et al., 2001). FATP3 is expressed in
endothelial cells including aorta and works
synergically with FATP4 to induce LCFA uptake (C.
E. Hagberg et al., 2010). Abnormal LCFA influx into
skeletal muscle, heart, the liver might lead to insulin
resistance, oxidative stress, and eventually apoptosis,
therefore it is important to study the mechanism of
LCFA uptake by FATP to identify potential therapy
for a metabolic disease that might lead to
atherosclerosis (Aboonabi et al., 2019; Anderson &
Stahl, 2013; Kazantzis & Stahl, 2012).
Herbal ingredients, such as Allium sativum,
Punica granatum, and Curcumin has been known for
having the effect of anti-atherosclerosis (Koscielny et
al., 1999; Majeed, Ghafil, Fatima, Hadi, & Mahdi,
2021; Supekar & Kale, 2015). Low-dose curcumin
reduce atherogenesis in a mouse model of human
atherosclerosis through the suppression of CD36 (a
FATP) in macrophages (Hasan et al., 2014). Allium
sativum inhibited the thickening of neointimal in
rabbits given high cholesterol diet, and in cell culture
treated with atherosclerosis patient’s serum, reduced
atherogenic potential was shown (Sobenin et al.,
2016; Sobenin, Myasoedova, Iltchuk, Zhang, &
Orekhov, 2019). Punica granatum reduced the
progression of atherosclerosis in
hypercholesterolemic mice (de Nigris et al., 2007). A
clinical trial of Curcuma longa in patients at risk of
CVD showed evidence of Curcuma xanthorrhiza
beneficial effects on serum TG and LDL-C levels
(Qin et al., 2017). The study of Curcuma
xanthorrhiza has proven that C. xanthorrhiza
decreased LDL-Cholesterol level and Total-
Cholesterol level, and increased HDL-Cholesterol
level in dyslipidemic Sprague Dawley rats (Budiarto
et al., 2017). Although many kinds of research have
proven the anti-hyperlipidemic and anti-
atherosclerotic effect of these herbal ingredients, little
is known about the detailed molecular mechanism
involving FATP3 in the aorta.
In the present study, we want to elaborate on the
effect of herbal ingredients in the aorta of high-fat
diet-fed rats. According to previous study,
atherosclerosis could be induced by vitamin D3 single
dose and three months of high lipid diets in rats (Pang
et al., 2010). Therefore, in this study, we aim to know
whether a high-fat diet would influence FATP3 in the
aorta of Wistar rats after supplementation of herbal
ingredients (Allium sativum, Punica granatum,
Curcuma longa, and Curcuma xanthorrhiza).
2 METHODS (AND MATERIALS)
2.1 Animals
Twenty-eight male Wistar rats, aged 8 weeks, weight
200-220 grams, were divided into seven groups
(negative control, positive control, A. sativum, P.
granatum, C. longa, C.xanthorrhiza, and
Rosuvastatin). The rats were put in a cage per group
and given a high-fat diet. The temperature was
maintained between 22-24°C each day and light-dark
cycle every 12 hours. The rats were environmentally
habituated for 1 week, continued with vitamin D3
700.000 mg/kg BW orally single dose, then high-fat
diet for 12 consecutive weeks, except the negative
control group that was given standard chow diet
(Pang et al., 2010). The treatment was given for 12
weeks and there was 175 mg/kg BW of A. sativum, P.
granatum, C. longa, C. xanthorrhiza ethanol extract,
and 1.8 mg/kgB Rosuvastatin. On the final day of
treatments, rats were terminated, and aorta was taken,
then stored in a -80°C refrigerator until further use for
RNA extraction and PCR.
All procedures were conducted according to the
use and care of laboratory animal guidelines
(Committee for the Update of the Guide for the Care
and Use of Laboratory Animals, Institute for
Laboratory Animal Research, Division on Earth and
Life Studies, 2011). Ethical approval was obtained
from the Faculty of Medicine’s Research Ethics
Committee in Universitas Kristen Maranatha-Rumah
Sakit Immanuel Bandung with the number
160/KEP/XI/2020.
Effects of Herbal Ingredients (Allium sativum, Punica granatum, Curcuma longa, Curcuma xanthorrhiza) on FATP3 Gene Expression in
Aorta of High Fat Diet-fed Rats: A Preliminary Study
329
2.2 RNA Extraction and
Semi-quantitative PCR
We conducted extraction of RNA from stored aorta
using Trisure reagent, with the proportion of 200 ul
Trisure per 10-20 mg sample (BIO-38033, Bioline,
London). After measuring the purity and
concentration of the extracted RNA using 260/280 nm
absorbance spectrophotometry (51119300, Multiskan
Go Microplate Spectrophotometer, Thermo,
Netherland), we conducted semi-quantitative PCR
using One-Step RT PCR Kit (BIO-65409, Bioline,
London). We used GAPDH as the housekeeping gene.
After PCR, we continued with electrophoresis, then
visualization of the gels using Bluepad, and image
quantification using Image J. Primer sequences used
in this study were as follows: for FATP3:
Fwd 5’- CTGGGACGAGCTAGAGGAAG -3’,
Rev 5’- GCTGAGGCCAGAGGTCTAAC -3’
(Lee et al., 2017)
GAPDH:
Fwd 5’- GTTACCAGGGCTGCCTTCTC-3’,
Rev 5’- GATGGTGATGGGTTTCCCGT-3’
(Wang et al., 2017).
2.3 Statistical Analysis
The result of the study (relative ratio of gene
expression) is presented as mean ± SEM. Statistical
analysis was done using SPSS 26.0 statistical
software (IBM, United States). ANOVA continued
with LSD post hoc analysis was used for testing the
difference between the groups, and p<0.05 is
considered as significant.
3 RESULTS AND DISCUSSION
We presented the result One Way Anova analysis for
the mean relative ratio of FATP3 gene expression
normalized by GAPDH in table 1 below.
Table 1: One Way Anova of FATP3 Gene Expression in
Aorta of Wistar Rats.
Groups
FATP3
Relative
Ratio ± SEM
N F p
Negative control 0.88 ± 0.06 4
1.524 0,219
Positive control
0.74 ± 0.07 4
A
llium sativum
0.97 ± 0.06 4
Punica granatum
0.89 ± 0.04 4
Curcuma longa
1.01 ± 0.07 4
Rosuvastatin
1.02 ± 0.09 4
Curcuma xanthorrhiza
0.97 ± 0.07 4
The result of
semi-quantitative PCR and the graphical
result of the study is presented in figure 1 below
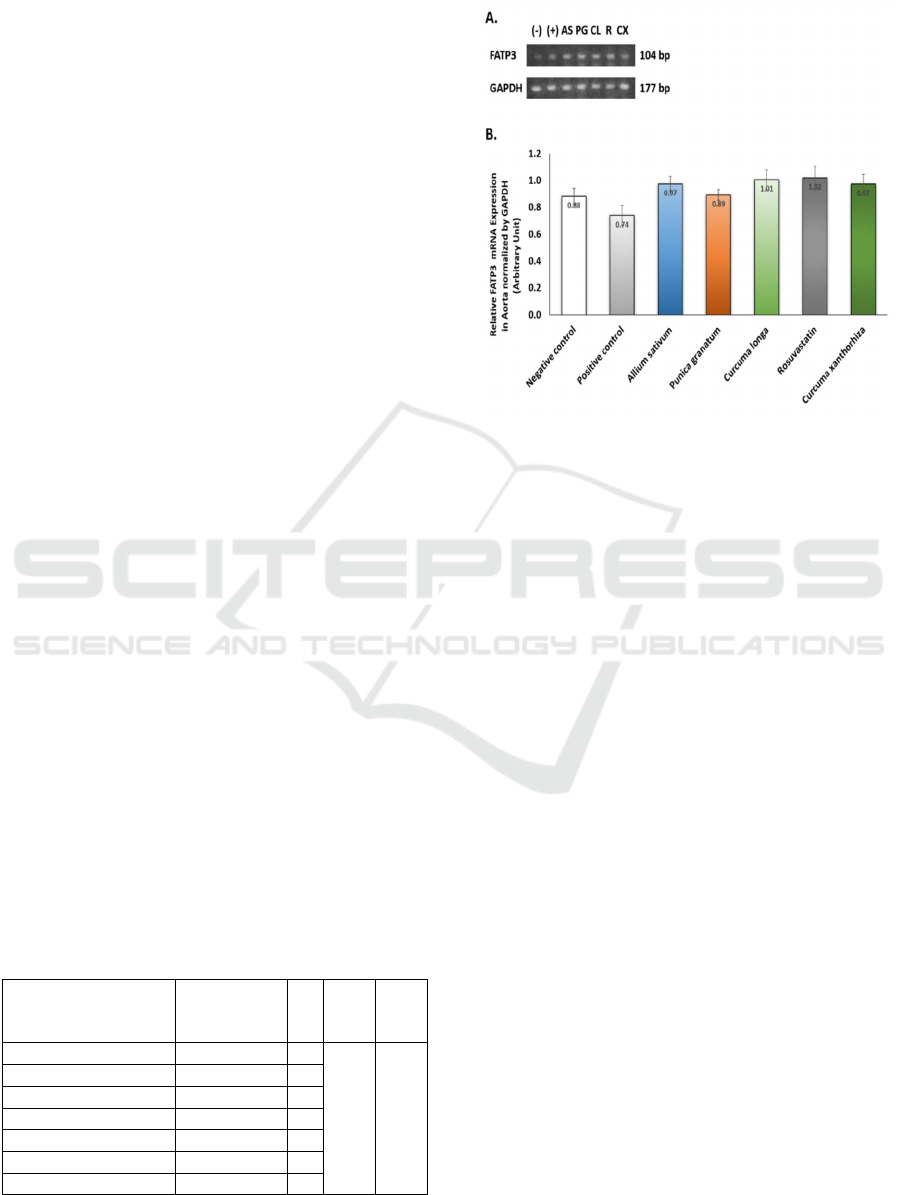
Figure 1: A. FATP3 and GAPDH Gene Expression from
Aorta of High Fat Diet-fed Rats; B. Relative Ratio of
FATP3 Gene Expression from Aorta of High Fat Diet-fed
Rats. (-) = Negative control, (+) = Positive control, AS =
Allium sativum, PG = Punica granatum, CL = Curcuma
longa, R = Rosuvastatin, CS = Curcuma xanthorrhiza
Protein-mediated transport active has been proven
to be the major route for the entering of LCFA into
the cells. Therefore, a comprehensive understanding
of FATPs would guide the possibility of making them
a promising target therapy for treating metabolic
diseases (Glatz, Luiken, & Bonen, 2010). FATP3 and
FATP4 are endothelial fatty acid transport, both
required for effective LCFA transport through the
barrier of the vascular endothelium (C. Hagberg et al.,
2013). Research showed that a high-fat diet induces
an increase of FATP1 in soleus muscle, but a decrease
in gastrocnemius muscles and this contrary effect
might be caused by different fiber types correlated
with distinct PPAR gamma sensitivity (Marotta et al.,
2004). In the intestine of humans, FATP4 is
upregulated after 3 days of a high-fat diet (Tremblay
et al., 2013). But research about the effect of a high-
fat diet on FATP3 gene expression is still limited.
In this study, we found no significant difference
in FATP3 gene expression between groups (figure 1).
This is a preliminary study that aims to investigate
whether FATP3 in the aorta might change after given
a high-fat diet and vitamin D3 that might induce
atherosclerosis and after supplementation of herbal
ingredients. This result might show that a high-fat diet
and herbal ingredients might not influence FATP3
gene expression in the aorta, but there is a tendency
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
330

of increase of FATP3 gene expression in treatment
groups compared to control. This tendency to
increase might show increased activity of FATP3 on
LCFA uptake as compensation to prevent lipid
deposition in non-adipose tissue. While in the
positive control, high fat diet and vitamin D3 that
potentially induce atherosclerosis decrease FATP3
gene expression. We hypothesize this effect (table 1)
may occur because of endothelial dysfunction that
might potentially found in early atherosclerosis
(Gimbrone Jr & García-Cardeña, 2016), but further
investigation needs to be conducted to support this
hypothesis.
The limitation of this study is: (1) time duration of
HFD
and vitamin D3 induce atherosclerosis might be
too short, therefore we suggest a longer time duration
to achieve a better perspective of the molecular
mechanism behind fatty acid transport protein
alteration in aorta after supplementation of herbal
ingredients; (2) herbal ingredients formulation might
not be appropriate and need to be adjusted for better
result of the experiments; (3) microscopic evaluation
to confirm atherosclerosis, such as Weigert and van
Kossa staining (Pang et al., 2010) is not provided in
this study.
4 CONCLUSIONS
In summary, herbal ingredients (Allium sativum,
Punica granatum, Curcuma longa, and Curcuma
xanthorrhiza) do not influence FATP3 gene
expression in the aorta of high-fat diet-fed Wistar rats.
Further study needs to be conducted to investigate the
detailed mechanism of LCFA transport change in
hyperlipidemia states and the role of herbal
ingredients as anti-atherosclerotic agents.
ACKNOWLEDGEMENTS
We would like to thank Nenden, dr Ardo, Demes for
the technical assistance for molecular procedures.
And we also would like to thank dr. Ronny, dr.
Hanna, Azis, Pak Nana, Pak Kris for their assistance
in studying the Wistar rats.
REFERENCES
Aboonabi, A., Meyer, R. R., & Singh, I. (2019). The
association between metabolic syndrome components
and the development of atherosclerosis. Journal of
Human Hypertension, 33(12), 844–855.
https://doi.org/10.1038/s41371-019-0273-0
Anderson, C. M., & Stahl, A. (2013). SLC27 fatty acid
transport proteins. Molecular Aspects of Medicine,
34(2–3), 516–528.
https://doi.org/10.1016/j.mam.2012.07.010
Blair, H. C., Sepulveda, J., & Papachristou, D. J. (2016).
Nature and nurture in atherosclerosis: The roles of
acylcarnitine and cell membrane-fatty acid
intermediates. Vascular Pharmacology, 78, 17–23.
https://doi.org/10.1016/j.vph.2015.06.012
Budiarto, A., Wibowo, A., Putri, S., Shabrina, N.,
Ngestiningsih, D., & Tjahjono, K. (2017). Pengaruh
Pemberian Ekstrak Rimpang Temulawak (Curcuma
Xanthorrhiza Roxb.) dan Jintan Hitam (Nigella Sativa)
terhadap Profil Lipid Tikus Sprague Dawley
Dislipidemia. Majalah Kedokteran Bandung, 47, 8–14.
https://doi.org/10.15395/mkb.v47n1.982
Committee for the Update of the Guide for the Care and Use
of Laboratory Animals, Institute for Laboratory Animal
Research, Division on Earth and Life Studies, & N. R.
C. (2011). Guide for the care and use of laboratory
animals (8th ed.). Washington (DC).
https://doi.org/10.17226/12910
Csige, I., Ujvárosy, D., Szabó, Z., Lőrincz, I., Paragh, G.,
Harangi, M., & Somodi, S. (2018). The Impact of
Obesity on the Cardiovascular System. Journal of
Diabetes Research, 2018, 3407306.
https://doi.org/10.1155/2018/3407306
Dallinga-Thie, G. M., Franssen, R., Mooij, H. L., Visser,
M. E., Hassing, H. C., Peelman, F., … Nieuwdorp, M.
(2010). The metabolism of triglyceride-rich
lipoproteins revisited: new players, new insight.
Atherosclerosis, 211(1), 1–8.
https://doi.org/10.1016/j.atherosclerosis.2009.12.027
de Nigris, F., Williams-Ignarro, S., Sica, V., Lerman, L. O.,
D’Armiento, F. P., Byrns, R. E., … Napoli, C. (2007).
Effects of a pomegranate fruit extract rich in
punicalagin on oxidation-sensitive genes and eNOS
activity at sites of perturbed shear stress and
atherogenesis. Cardiovascular Research, 73(2), 414–
423. https://doi.org/10.1016/j.cardiores.2006.08.021
Gimbrone Jr, M. A., & García-Cardeña, G. (2016).
Endothelial Cell Dysfunction and the Pathobiology of
Atherosclerosis. Circulation Research, 118(4), 620–
636.
https://doi.org/10.1161/CIRCRESAHA.115.306301
Glatz, J. F. C., Luiken, J. J. F. P., & Bonen, A. (2010).
Membrane fatty acid transporters as regulators of lipid
metabolism: implications for metabolic disease.
Physiological Reviews, 90(1), 367–417.
https://doi.org/10.1152/physrev.00003.2009
Goldberg, I. J., & Bornfeldt, K. E. (2013). Lipids and the
endothelium: bidirectional interactions. Current
Atherosclerosis Reports, 15
(11), 365.
https://doi.org/10.1007/s11883-013-0365-1
Hagberg, C. E., Falkevall, A., Wang, X., Larsson, E.,
Huusko, J., Nilsson, I., … Eriksson, U. (2010).
Vascular endothelial growth factor B controls
Effects of Herbal Ingredients (Allium sativum, Punica granatum, Curcuma longa, Curcuma xanthorrhiza) on FATP3 Gene Expression in
Aorta of High Fat Diet-fed Rats: A Preliminary Study
331

endothelial fatty acid uptake. Nature, 464(7290), 917–
921. https://doi.org/10.1038/nature08945
Hagberg, C., Mehlem, A., Falkevall, A., Muhl, L., &
Eriksson, U. (2013). Endothelial fatty acid transport:
role of vascular endothelial growth factor B. Physiology
(Bethesda, Md.), 28(2), 125–134.
https://doi.org/10.1152/physiol.00042.2012
Hasan, S. T., Zingg, J.-M., Kwan, P., Noble, T., Smith, D.,
& Meydani, M. (2014). Curcumin modulation of high
fat diet-induced atherosclerosis and steatohepatosis in
LDL receptor deficient mice. Atherosclerosis, 232(1),
40–51.
https://doi.org/10.1016/j.atherosclerosis.2013.10.016
Kazantzis, M., & Stahl, A. (2012). Fatty acid transport
proteins, implications in physiology and disease.
Biochimica et Biophysica Acta, 1821(5), 852–857.
https://doi.org/10.1016/j.bbalip.2011.09.010
Koscielny, J., Klüssendorf, D., Latza, R., Schmitt, R.,
Radtke, H., Siegel, G., & Kiesewetter, H. (1999). The
antiatherosclerotic effect of Allium sativum.
Atherosclerosis, 144(1), 237–249.
https://doi.org/10.1016/s0021-9150(99)00060-x
Lee, Y. Bin, Choi, J. H., Kim, E. N., Seok, J., Lee, H.-J.,
Yoon, J.-H., & Kim, G. J. (2017). Human Chorionic
Plate-Derived Mesenchymal Stem Cells Restore
Hepatic Lipid Metabolism in a Rat Model of Bile Duct
Ligation. Stem Cells International, 2017, 5180579.
https://doi.org/10.1155/2017/5180579
Majeed, M. L., Ghafil, F. A., Fatima, G., Hadi, N. R., &
Mahdi, H. F. (2021). Anti-Atherosclerotic and Anti-
Inflammatory Effects of Curcumin on
Hypercholesterolemic Male Rabbits. Indian Journal of
Clinical Biochemistry, 36(1), 74–80.
https://doi.org/10.1007/s12291-019-00858-5
Marotta, M., Ferrer-Martnez, A., Parnau, J., Turini, M.,
Macé, K., & Gómez Foix, A. M. (2004). Fiber type- and
fatty acid composition-dependent effects of high-fat
diets on rat muscle triacylglyceride and fatty acid
transporter protein-1 content. Metabolism: Clinical and
Experimental, 53(8), 1032–1036.
https://doi.org/10.1016/j.metabol.2004.03.011
Pang, J., Xu, Q., Xu, X., Yin, H., Xu, R., Guo, S., … Cao,
J.-M. (2010). Hexarelin suppresses high lipid diet and
vitamin D3-induced atherosclerosis in the rat. Peptides,
31(4), 630–638.
https://doi.org/10.1016/j.peptides.2009.11.007
Qin, S., Huang, L., Gong, J., Shen, S., Huang, J., Ren, H.,
& Hu, H. (2017). Efficacy and safety of turmeric and
curcumin in lowering blood lipid levels in patients with
cardiovascular risk factors: a meta-analysis of
randomized controlled trials. Nutrition Journal, 16(1),
68. https://doi.org/10.1186/s12937-017-0293-y
Shao, A., Drewnowski, A., Willcox, D. C., Krämer, L.,
Lausted, C., Eggersdorfer, M., … Griffiths, J. C.
(2017). Optimal nutrition and the ever-changing dietary
landscape: a conference report. European Journal of
Nutrition, 56
(1), 1–21. https://doi.org/10.1007/s00394-
017-1460-9
Sobenin, I. A., Andrianova, I. V, Lakunin, K. Y.,
Karagodin, V. P., Bobryshev, Y. V, & Orekhov, A. N.
(2016). Anti-atherosclerotic effects of garlic
preparation in freeze injury model of atherosclerosis in
cholesterol-fed rabbits. Phytomedicine : International
Journal of Phytotherapy and Phytopharmacology,
23(11), 1235–1239.
https://doi.org/10.1016/j.phymed.2015.10.014
Sobenin, I. A., Myasoedova, V. A., Iltchuk, M. I., Zhang,
D.-W., & Orekhov, A. N. (2019). Therapeutic effects of
garlic in cardiovascular atherosclerotic disease.
Chinese Journal of Natural Medicines, 17(10), 721–
728. https://doi.org/10.1016/S1875-5364(19)30088-3
Stahl, A., Gimeno, R. E., Tartaglia, L. A., & Lodish, H. F.
(2001). Fatty acid transport proteins: a current view of
a growing family. Trends in Endocrinology and
Metabolism: TEM, 12(6), 266–273.
https://doi.org/10.1016/s1043-2760(01)00427-1
Supekar, A. R., & Kale, A. J. (2015). Anti-Atherosclerosis
Activity of Seed oil of Punica Granatum Linn in Triton
X-100 induced hyperlipidemic rats. International
Journal of Advanced Research, 3, 1276–1280.
Retrieved from https://www.journalijar.com/article/
6105/anti-atherosclerosis-activity-of-seed-oil-of-
punica-granatum-linn-in-triton-x-100-induced-
hyperlipidemic-rats/
Tremblay, A. J., Lamarche, B., Guay, V., Charest, A.,
Lemelin, V., & Couture, P. (2013). Short-term, high-fat
diet increases the expression of key intestinal genes
involved in lipoprotein metabolism in healthy men. The
American Journal of Clinical Nutrition, 98(1), 32–41.
https://doi.org/10.3945/ajcn.113.060251
Wang, K., Wang, F., Bao, J.-P., Xie, Z.-Y., Chen, L., Zhou,
B.-Y., … Wu, X.-T. (2017). Tumor necrosis factor α
modulates sodium-activated potassium channel SLICK
in rat dorsal horn neurons via p38 MAPK activation
pathway. Journal of Pain Research, 10, 1265–1271.
https://doi.org/10.2147/JPR.S132185
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
332
