
The Effect of Agarwood Leaves Ethanol Extract on Porphyromonas
gingivalis Growth Inhibition and in Vitro Cytotoxicity Assay on
Fibroblast
Vinna Kurniawati Sugiaman
1a
, Henry Yonatan Mandalas
2b
, Ethan Yeshael Tanamal
3c
,
Nathalia Cahya Calista
3d
and Natallia Pranata
1e
1
Department of Oral Biology, Faculty of Dentistry, Maranatha Christian University, Jalan Prof. Suria Sumantri, Bandung,
Indonesia
2
Department of Periodontology, Maranatha Christian University, Bandung, Indonesia
3
Faculty of Dentistry, Maranatha Christian University, Jalan Prof. Suria Sumantri, Bandung, Indonesia
Keywords: Aquilaria Malaccensis Lamk., Poprhyromonas Gingivalis, Inhibition Assay, Fibroblast, Cytotoxicity.
Abstract: Agarwood leaves (Aquilaria malaccensis Lamk.) have an antibacterial activity that could be used as wound
healing agent. Porphyromonas gingivalis were the main pathogens in periodontitis. This was a laboratory
experimental study post-test only control group design. Agarwood leaves were obtained from Ibun Garden,
Majalaya District, West Java. Sample identified and determined by Biology Research Center, LIPI Indonesia.
Extraction and phytochemical test were conducted at Aretha Medika Utama BBRC Bandung and BPTRO
Bogor. Fibroblast ATCC 3T3 Balb/C obtained and cultured at Aretha Medika Utama BBRC. Cytotoxicity
test was carried out by using MTS Assay method, and the results are adjusted to ISO 10993-5. IC
50
was
obtained using PROBIT analysis. Inhibition assay was carried out by well-diffusion method and the results
are adjusted to Davis and Stout criteria. Research and P. gingivalis (ATCC 33277) was carried out at
Microbiology Laboratory Faculty of Dentistry, Universitas Padjadjaran. Results were analysed with ANOVA.
The results indicate agarwood leaves had weak inhibitory ability at under concentration of 50% and moderate
inhibition at a concentration of 100%. The cytotoxicity results showed no toxic effect at under concentrations
of 62.5 µg/mL. The IC
50
at a concentration of 215.54 µg/mL.
1 INTRODUCTION
Periodontal disease is defined as various types of
conditions that affect the supporting structures of the
teeth, including gingiva, alveolar bone, and
periodontal ligament (Kinane et al., 2017).
This
disease is the 11th most common disease in the world
and can be classified into gingivitis and periodontitis
(Nazir, 2017). Periodontitis is an inflammatory
disease in dental support tissue caused by specific
microorganisms resulting in progressive destruction
of the periodontal ligament and alveolar bone by
pocket formation and recession (Newman et al.,
2012).
a
https://orcid.org/0000-0002-3688-6718
b
https://orcid.org/0000-0002-9799-3824
c
https://orcid.org/0000-0002-5634-1942
d
https://orcid.org/0000-0002-4548-2395
e
https://orcid.org/0000-0001-7970-1915
Periodontitis has a prevalence of 10.8% or is
experienced by approximately 743 million
individuals in the world and is the sixth highest
prevalence disease according to Global Burden
Disease (Wijaksana, 2019; Séverin, 2018). The
pathophysiology of periodontitis is an imbalance of
microorganisms in the oral cavity that causes chronic
exposure to several pathogenic bacteria periodontitis,
including Porphyromonas gingivalis, Actinobacillus
actinomycetemcomitans, Tannerella forsythia, and
Treponema denticola (How et al., 2016).
P. gingivalis is one of the main etiologic agents in
the development of periodontitis with a prevalence of
92% (How et al., 2016; Liu et al., 2013). P. gingivalis
112
Sugiaman, V., Mandalas, H., Tanamal, E., Calista, N. and Pranata, N.
The Effect of Agarwood Leaves Ethanol Extract on Porphyromonas gingivalis Growth Inhibition and in Vitro Cytotoxicity Assay on Fibroblast.
DOI: 10.5220/0010745500003113
In Proceedings of the 1st International Conference on Emerging Issues in Technology, Engineering and Science (ICE-TES 2021), pages 112-121
ISBN: 978-989-758-601-9
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

is a gram-negative, anaerobic, saccharolytic, and non-
motile bacteria. These bacteria are shaped like cocci
or rod and belong to the group of black pigmented
bacteria (How et al., 2016). The main habitat of P.
gingivalis is plaque in the subgingival pocket of the
oral cavity. The proportion of P. gingivalis was found
to be higher in the deep periodontal pocket compared
to the shallower periodontal pocket. This is due to the
availability of amino acid fermentation requirements
to produce bacterial energy in the periodontal pocket,
such as low sugar levels, low oxygen levels, rich in
blood and serum protein, and has a stable pH that is
slightly alkaline (How et al., 2016)
P. gingivalis bacteria have several factors that
play a role in the virulence process of human cells,
namely fimbriae, lipopolysaccharide (LPS), capsular
polysaccharide (CPS), hemagglutinin, and gingipain.
In addition, P. gingivalis interacts with body tissues
by adhesion and coaggregation so that these bacteria
can invade the body's epithelial cells. However, the
low biological activity of P. gingivalis, especially its
endotoxicity, causes these bacteria to colonize and
grow in sterile tissue without being detected by the
body (Klein et al., 2012).
The treatment that has been carried out against P.
gingivalis infection still has many drawbacks. The
use of medications such as the antiseptic
Chlorhexidine can cause staining of the teeth and
some other disadvantages. In addition, P. gingivalis
is also known to be resistant to antibiotics, including
amoxicillin, clindamycin, and metronidazole (Gerits
et al., 2017). This has led to research on new and
natural substances in the treatment of periodontitis
due to P. gingivalis infection.
In addition to medicament, periodontal disease
treatment also varies depending on extent of the
affected periodontal tissue and can be performed with
and/or without surgery. Some of the most common
non-surgical procedures are scaling and root planing
and medication either locally or systemically with the
aim of infection control, inhibition of microbial
growth, and restoring the healthy state of periodontal
tissue. The most common surgical procedure is the
periodontal flap, aimed to restore the clinical
attachment of the periodontal ligament. This surgery
involves incisions and requires wound healing as well
as postoperative tissue regeneration (Newman et al.,
2012; Williams et al., 2016; Hudwekar et al., 2019).
Fundamentally wound healing is a complex
cellular process, focuses on restoring the structure
and function of damaged tissues through 3 (three)
phases, namely the inflammatory phase, proliferation
phase, and remodelling phase. Fibroblasts are
important cells in the wound healing process, derived
from undifferentiated mesenchymal cells. Fibroblasts
produce mucopolysaccharides, amino-glycine, and
proline acids which are the basic ingredients for
linking the edges of the wound. Inflammatory signals
activate the proliferation and maturation of the
fibroblasts, which are responded by collagen
synthesis and cross-bond initiation to form an
extracellular matrix as well as differentiate into
myofibroblast phenotype to facilitate wound closure
(Sugiaman, 2011; Gonzales et al., 2016).
Agarwood (Aquilaria malaccensis Lamk.) is a
plant that grows in the forests of Indonesia. The final
product of agarwood known as gubal contain resin as
a result from mushroom infection in induction
process (Janshen et al., 2017).
Induction process is an
outcome from a long term and complex
microorganism interaction, makes gubal extremely
rare and high in value. Agarwood resin often used as
ingredient in perfume and cosmetic industry (Musir
et al., 2016; Nugraha and Ginting, 2013).
The use of
resin that is too dominant, make the other part of
agarwood often become overlooked and resulted as
waste, especially the leaf (Wangiyana, 2020).
In traditional medicine, agarwood leaves tend to
be used empirically by Indonesians as a treatment for
malaria, diabetes, asthma, abdominal pain
(constipation), and skin care. These potencies
achieved by drinking the leaf’s brew or inhaling the
scent of burned leaves and stems (Wangiyana, 2020;
Syamsul et al., 2020).
Agarwood leaf still can’t reach
its maximum use yet in Indonesia due to the lack of
information about the goods contained (Janshen et al.,
2017).
Agarwood leaf extract as an antibacterial
facilitated by the presence of flavonoids as main
compound that intervene in the destruction of
bacterial cell membranes (Nomer et al., 2019;
Warganegara and Restina, 2016). Along with
alkaloids, flavonoids shall change the protein
structure found on the outer surface of bacteria
namely fimbriae, resulting in the decrease of its
hydrophobic property and inhibits bacterial adhesion
with host cell (Pratiwi et al., 2015).
Furthermore,
there are saponins with active substances to lower the
surface tension of bacterial cell walls. The substance
will bind to the cytoplasmic membrane to destabilize
the bacterial cell membrane, causing cytoplasm
leakage, resulted in bacterial lysis Dennis et al.,
2017).
Phytochemical screening of agarwood leaf
extract shows presence of tannins and
steroids/triterpenoids that invade the bacterial cell
membrane, causing the membrane becomes brittle
and easily destroyed (Sari et al., 2017).
The Effect of Agarwood Leaves Ethanol Extract on Porphyromonas gingivalis Growth Inhibition and in Vitro Cytotoxicity Assay on
Fibroblast
113

Wound healing-wise, agarwood leaf extract
proven to accelerate the inflammatory process, as
well as re-epithelization in mice with DM. Main
compounds that act as mentioned in agarwood are
flavonoids, saponins, and tannins (Suhardiman and
Juanda, 2019; Wahid and Safwan, 2019; Fauzi et al.,
2017).
Flavonoids act in the activation and
proliferation of fibroblasts and induce the production
of collagen fiber in order to accelerate wound healing
process. Along with flavonoids, saponins stimulates
blood vessels proliferation, while tannins act as
homeostat by inhibits the production of prostaglandin
and stimulating vasoconstriction (Suhardiman and
Juanda, 2019; Fauzi et al., 2017; Rahmadhani et al.,
2020).
Research on the comparison of total phenol levels
in agarwood leaf steeping and agarwood leaf ethanol
extract showed that the ethanol extract has higher
flavonoids level, namely 62.9 mg GAE/gram and
28.5 GAE/gram in agarwood leaf steeping. Other
studies showed that there are 8342.82 mg/100 gram
flavonoids contained with antioxidant activity
ranging from 28.50 – 40.30 ppm, classified as very
strong (Nurmiati and Wijayanti, 2018; Komang et al.,
2018; Harahao et al., 2015).
Therefore, agarwood leaf has the potential sources
of antibacterial, antioxidant and anti-inflammatory
that in addition to inhibiting the growth of P.
gingivalis, shall be effective in wound healing
process.
2 METHODS AND MATERIALS
2.1 Agarwood Leaf Extraction
Agarwood leaves was obtained from Ibun Garden,
Majalaya District, West Java and had been identified
by Biology Research Center, Lembaga Ilmu
Pengetahuan Indonesia, Bogor. Extraction was
carried out by maceration method and ethanol as
solvent. For cytotoxicity assay, 5 mg total of extract
was dissolved in 1 mL DMSO 10% and become seven
different extract concentration (500 µg/mL, 250
µg/mL, 125 µg/mL, 62.5 µg/mL, 31.25 µg/mL, 15.63
µg/mL, and 7.81 µg/mL). As for inhibition assay,
serial dilution method used to make working
concentration. 100% extract was dissolved with 10
mL aquadest to 50%, 25%, 12.5%, 6.25%, 3.13%,
1.56% agarwood leaf extract. Both final
concentrations were filtered using 0.22 µm tissue
culture pore syringe resulted in sterile sample.
2.1.1 Phytochemical Test
The qualitative phytochemical assay was carried out
by Farnsworth method. Results showed Agarwood
leaf ethanol extract indeed contain flavonoid,
saponin, tannin, alkaloid, triterpenoid, steroid, and
phenol.
2.2 Cytotoxicity Assay (Viability Test
using MTS Assay)
Fibroblast cell (3T3 Balb/C) ATCC CCL-163 was
obtained from Aretha Medika Utama BBRC Bandung
as collection. Thawing and subculture process was
conducted, and cells were cultured in a complete
medium, contained of 10% FBS (Biowest, S81B-
500), 1% ABAM (Biowest, L0010-100), 1%
Amphotericine B (Biowest, L0009-050), 1% MEM
Vitamins (Biowest, X0556-100), 1% L-Glutamine
(Biowest, X0551-100), 0.2% Nanomycopulitine
(Biowest, LX16-100), 0.1% Gentamicin (Gibco,
15750060) and basal medium DMEM High Glucose
(Biowest, L0103-500).
Cells were harvested and calculated using
hemacytometer after it reached the confluency of
70%, then implanted with 5 x 10
3
density in a 96 well-
plate. After incubated for 24 h, the old medium was
replaced with 200 µL new medium and 20 µL
agarwood leaf ethanol extract, then proceeded to be
incubated under 37˚C with 5% CO
2
. Furthermore, 20
µL of MTS reagent was added on each well and was
incubated for 3 h. Absorbance was measured using
spectrophotometer with 490 nm. Cells death was
calculated based on the absorbance and integrated to
standard curve of 3T3 Balb/C.
2.2.1 Statistical Analysis
Results that obtained as data processed with IBM
SPSS 21.0 ver. Normality test was carried out then
proceeded to One-way ANOVA and Tuckey HSD
(Post-Hoc). IC
50
value was obtained using PROBIT
analysis.
2.3 Inhibitory Assay
P. gingivalis which had been made into a suspension
were taken with a cotton swab and spread into the
blood agar medium. Then a hole is made using a
perforator in the inoculated agar, forming a well.
Furthermore, the wells will be filled with each
treatment, specifically positive control with antiseptic
chlorhexidine solution, negative control with
aquadest, and ethanol extract of agarwood leaves with
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
114

various concentrations. The filled media were then
incubated for 24 hours at 37
0
C.
The same procedure will be repeated three times.
The inhibition zone that formed around the well was
measured using a calliper with units of mm as
research data. Results were further categorized
according to inhibition zone category according to
Davis and Stout.
2.3.1 Statistical Analysis
Results that obtained as data processed with IBM
SPSS 21.0 ver. Normality test was carried out using
Shapiro-Wilk then proceeded to One-way ANOVA
and Dunnett T3 (Post-Hoc).
3 RESULTS AND DISCUSSION
3.1 Results
3.1.1 Cytotoxicity Assay
Phytochemical analysis of agarwood leaf ethanol
extract showed positive result from the presence of
flavonoid, saponin, tannin, alkaloid, triterpenoid, and
phenol as shown in Table 1.
Table 1: Phytochemical Analysis Results.
Compound Results
Flavonoids (+)
Saponins (+)
Tannins (+)
Terpenoids (+)
Triterpenoids/Steroids (+) Triterpenoid
Phenols (+)
Alkaloids (+)
The outcomes of cytotoxicity assay included
mean of absorbance, corrective absorbance, number
of viable cells, percentage of viability, and percentage
of inhibition obtained through calculations and
spectrophotometer measurements.
Parameters used in this study were the percentage
of viability cell and IC
50
. Cell viability was obtained
and calculated from the number of cells that are still
alive (viable) after treated, while IC
50
value was
obtained through PROBIT analysis with the aim of
knowing the concentration of agarwood leaf extract
which can inhibit fibroblasts growth by as much as
half the population. Results of cytotoxicity assay
shown in Appendix.
In vitro cytotoxicity assay according to ISO
10993-5: Biological Evaluation of Medical Devices
states that, if the relative cell viability for the extract
concentration of a sample is more than equal to 70%,
then the material must consider as non-toxic
(International Standard Organization, 2009;
International Standard Organization, 2012). Based
on these standards in this study, the concentration of
agarwood leaf extract that did not toxic on 3T3
Balb/C fibroblasts were concentrations of 62.5
µg/mL, 31.25 µg/mL, 15.63 µg/mL and 7.81 µg/mL.
Meanwhile, concentrations of 500 µg/mL, 250
µg/mL, and 125 µg/mL were toxic.
Standard curve of 3T3 Balb/C cells was created
(Figure 1) as a reference for calculating the number
of cells based on the absorbance obtained. The curve
made by regressing the absorbance value and the
number of cells into the standard curve line equation
y = ax + b. The y-axis is the absorbance value, while
the x-axis is the number of cells.
Figure 1: 3T3 Balb/C Standard Curve.
The regression curve shows linear relationship
between the number of cells and the absorbance
value. This could also be seen in observations on well
plates. The darker the color produced on the well
plate, the higher the absorbance value and the number
of viable cells. This curve is then used as a reference
for calculating the number of viable cells in each
treatment.
Furthermore, statistical analysis conducted
towards these data resulting in normality distributed
and homogenous data. Analysis proceeded to
ANOVA and Post-Hoc Tuckey HSD. Results showed
that each treatment significantly affecting the
difference in cell viability by significance (p)<0.5.
The IC50 value obtained was at a concentration of
215.54 µg/mL using PROBIT analysis.
y = 7E-05x + 0,0329
R² = 0,9811
0,0000
0,1000
0,2000
0,3000
0,4000
0,5000
0,6000
0 2000 4000 6000 8000 10000
Absorbance Value
Number of cells
3T3-Balb/C Standard Curve
The Effect of Agarwood Leaves Ethanol Extract on Porphyromonas gingivalis Growth Inhibition and in Vitro Cytotoxicity Assay on
Fibroblast
115
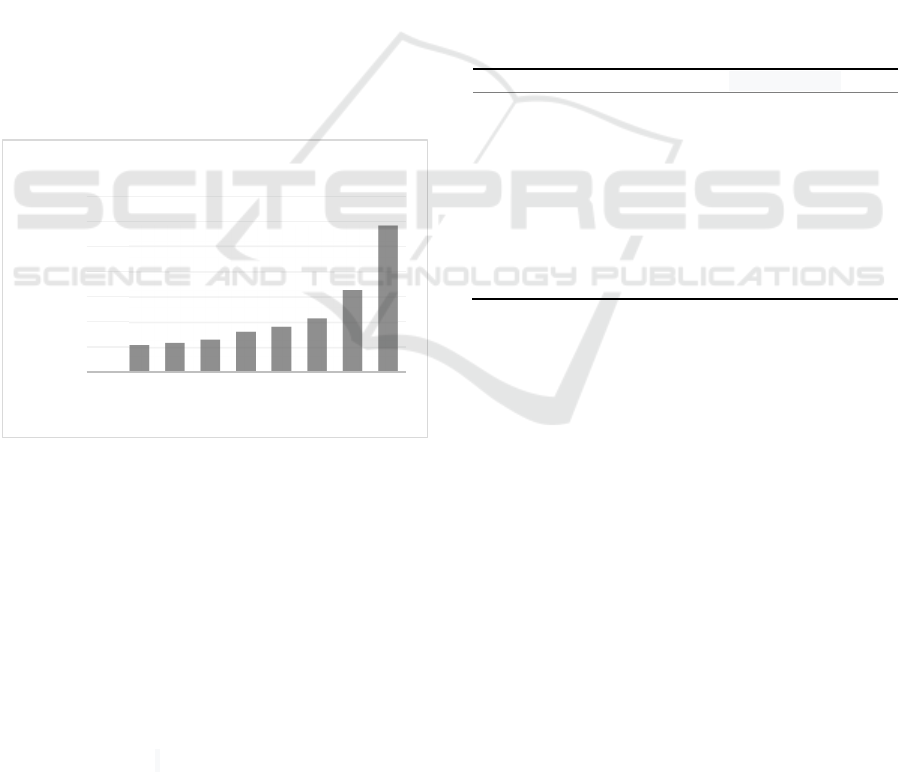
3.1.2 Inhibitory Assay
In this study, 9 treatments were repeated three times
on P. gingivalis. There were 7 concentrations of
agarwood leaf ethanol extract, namely 100%, 50%,
25%, 12.5%, 6.25%, 3.13%, and 1.56%. Other
treatments given were positive control with antiseptic
chlorhexidine solution and negative control with
aquadest.
Parameter used in this study was the diameter of
the inhibition zone produced in each treatment as the
form of a clear zone on agar media. The results of this
inhibition zone measurement will then be interpreted
into the inhibition category according to Davis and
Stout.
As shown in appendix and Figure 2,
measurements of inhibition zone diameter showed
that the largest diameter of the inhibition zone was in
group 9, namely chlorhexidine as positive control.
Then followed by a group of 8, namely the ethanol
extract of gaharu leaves with a concentration of
100%. Meanwhile, the smallest diameter of the
inhibition zone was in group 1, namely the negative
control of aquadest.
Figure 2: Mean of inhibition zone diameter.
Note:
1 treatment group for (-) control (aquadest)
2 treatment group for agarwood leaf ethanol extract 1.56%
3 treatment group for agarwood leaf ethanol extract 3.13%
4 treatment group for agarwood leaf ethanol extract 6.25%
5 treatment group for agarwood leaf ethanol extract 12.5%
6 treatment group for agarwood leaf ethanol extract 25%
7 treatment group for agarwood leaf ethanol extract 50%
8 treatment group for agarwood leaf ethanol extract 100%
9 treatment group for (+) control (chlorhexidine)
According to the Davis and Stout inhibition
category, the result will be categorized as weak if the
inhibition zone formed is 5 mm or less. Followed by
moderate inhibitory ability if 5-10 mm inhibition
zone formed. While strong inhibitory ability when
10-20 mm zone is formed. Lastly, inhibitory ability
will be categorized as very strong if more than 20 mm
zone formed (Rastina et al., 2015). Based on these
categories, ethanol extract of agarwood leaf has weak
inhibitory ability at concentrations of 50%, 25%,
12.5%, 6.25%, 3.13%, and 1.56%. While the
concentration of 100% has moderate inhibitory
ability against the growth of P. gingivalis.
Normality test was performed resulting in
normally distributed data by significance of p<0.05.
Data analysis was proceeded to One-way ANOVA
and it was found to support H
0
, states that agarwood
leaf extract has significance effect on P. gingivalis
growth. Further analysis was carried out by using
Post-Hoc Dunnett T3 method. Results showed that
each concentration of agarwood leaf extract has
different significance effect toward P. gingivalis
growth.
Table 2: Post-Hoc Dunnett T3 Analysis.
Treatment Inhibition Zone
(-) Control 0 ± 0.00
a
1,56% 2.18 ± 0.08
b
3,13% 2.35 ± 0.15
bc
6,25% 2.63 ± 0.08
bdc
12,50% 3.23 ± 0.34
cde
25% 3.62 ± 0.10
def
50% 4.30 ± 0.39
ef
100% 6.55 ± 0.23
g
(+) Control 11.62 ± 0.60
h
3.2 Discussion
3.2.1 Cytotoxicity Assay
This study results showed that the highest percentage
of cell viability was in the group with concentration
of 7.81 µg/mL, and the lowest was in the group with
concentration of 500 µg/mL. These data indicate that
there is decrease in cell viability which is inversely
proportional to the increase in concentration. The
differences in cell viability at each concentration
could indicate that the cell response towards each
concentration was also different, which is thought to
be due to the variance in the amount of active
compound content at each concentration.
Qualitative phytochemical testing carried out in
this study did not prove the exact amount and
proportion of active compounds contained in
agarwood leaf extract. In addition, the dilution of
agarwood leaf extract is also thought to result in a
decrease in the number of active compounds along
with the smaller the concentration, since the amount
0,00
2,00
4,00
6,00
8,00
10,00
12,00
14,00
123456789
Diameter (mm)
Treatment Group
Mean of Inhibition Zone Diameter
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
116

of extract keep reduced during the dilution process.
Referring to several previous studies, the active
compounds that play a role in this research are
saponins, flavonoids, and tannins.
Saponins are derivatives of glycosides that can act
as antioxidant agents capable of neutralizing free
radicals by binding to active oxygen. The lyobipolar
properties possessed by saponins allows their
interaction with cell membranes by reducing the
surface tension of cells (Yildirim and Kutlu, 2015).
Saponins also could increase the expression and
activation of TGF-β, FGF, and VEGF via monocyte
proliferation. Activation of FGF will increase
fibroblast proliferation and stimulate fibronectin
synthesis. Fibronectin synthesis will induce fibroblast
migration. The fibroblasts will then be responsible for
the synthesis of collagen in the extracellular matrix
(Suharto and Etika, 2019; Ardiana et al., 2015).
Signaling performed by FGF plays an important
role in the regulation of cell pluripotency through
interaction with FGFRs. FGF/FGFRs will then be
responsible for cellular processes such as
proliferation, migration, embryonic development,
tissue regeneration, and cell metabolism. This entire
process is mediated by activation of RAS - mytogen-
activates protein kinase (MAPK), Phospholipase C
Gamma, and signal transducers and activators of
transcription (STAT). These various signaling
pathways will also work together with signaling from
other growth factors, such as TGF-β (Mohammadi et
al., 2020).
In molecular level, flavonoids could induce
extracellular signal-regulated kinase (ERK). ERK can
specifically recognize various growth factor receptors
on the cell surface such as FGFRs, KGFR, and EGFR
also significantly activate these rexeptors (Etika et al.,
2017). Arginine, which is a derivative of flavonoids,
has been shown to affect the proliferation of human
gingival fibroblasts and fibroblasts through activation
of amino acid receptors and cyclic-AMP response
element binding (CREB). This activation will then
stimulate the secretion of various growth factors and
also the extracellular matrix (Kurahashi and Fujii,
2015).
Flavonoids contributed in macrophage activation
which will stimulate the synthesis of several growth
factors such as PDGF, FGF, EGF, TGF-β, and TGF-
α. Macrophages together with neutrophils can
synthesize Reactive Oxygen Species (ROS) which is
a chemically reactive molecule, formed due to the
acceptance of electrons by the O
2
molecule. ROS
produced in the wound healing process is superoxide
radical anion which will then be broken down into
H
2
O
2
(hydrogen peroxide) and oxygen molecules
through the superoxide dismutase mechanism. The
disintegration of superoxide radical anion aims to
prevent the formation of destructive ROS with high
concentrations such as peroxynirite (ONOO--) or
hydroxyl radical (-OH) Jiang et al., 2018; Ningrum
and Kurniawaty, 2019).
Tannins contributed to the differentiation of
fibroblasts into myofibroblasts along with the
production of an extracellular matrix which important
in wound contraction. But along with this process,
tannins can also control the differentiation and
proliferation of fibroblasts through the expression of
genes involved in extracellular matrix production.
This is performed by induction of Smad2 and Erk
proteins on the TGF-β1 signaling pathways. This
regulation of fibroblast differentiation and
proliferation aims to prevent fibrosis forming in the
wound. The molecular mechanism of the anti-
proliferative effect of fibroblasts by tannins is thought
to be due to the regulation of cyclin gene expression
All these mechanisms suggest that both
differentiation and inhibition of fibroblast
proliferation depend on inhibition of Smad2 and Erk
activation on the TGF-β1 signaling pathways
(Pattarayan et al., 2018).
Toxicity to fibroblasts in this study not only could
be caused by the level of the active compound at each
concentration, mechanism of action, and structure of
the active compound in the agarwood leaf extract. In
vitro, the mechanism of cytotoxicity can be in the
form of cell membrane destruction, prevention of
protein synthesis, binding with irreversible receptors,
prolonged inhibition of polydeoxynucleotide and
enzyme reactions (Aslanturk, 2018). It has also been
shown that toxic agents can induce excessive nitric
oxide production, ROS followed by oxidative stress,
and mitochondrial dysfunction as a result of oxidative
stress. Toxic agents can also potentially release
components that can directly result in DNA damage,
followed by apoptosis (Stammenković-Radak and
Andjelković, 2016; Zhang, 2018; Spindola et al.,
2018).
The determination of IC
50
value is important in
determining and understanding the pharmacological
and biological characteristics of a chemotherapeutic
agent. The IC
50
value is a measurement of drug
efficacy indicating the amount of concentration of a
chemotherapeutic agent required to inhibit half the
biological processes. This suggests a description of
the antagonistic potential of chemotherapeutic agents
in a study Aykul and Martinez-Hackert, 2016; He et
al., 2016).
In this study, the IC
50
value obtained was at a
concentration of 215.54 µg / mL, which indicates that
The Effect of Agarwood Leaves Ethanol Extract on Porphyromonas gingivalis Growth Inhibition and in Vitro Cytotoxicity Assay on
Fibroblast
117

this concentration can inhibit fibroblast proliferation
by up to 50% of the population. These results can be
used as a reference for further research regarding the
potential of agarwood leaf extract as a wound healing
agent by using the IC
50
value as the minimum
concentration of agarwood leaf extract.
3.2.2 Inhibitory Assay
Based on the mean of inhibition zone measured, it can
be concluded that the diameter of the inhibition zone
is directly proportional towards the concentration of
agarwood leaf extract.
Phytochemical test results showed that agarwood
leaf extract contain several active compounds that can
inhibit the growth of P. gingivalis. The main
compounds contained in the ethanol extract of gaharu
leaves are flavonoids. These compounds contributed
in the destruction of bacterial cells due to damage to
the permeability of their cell membranes (Nomer et
al., 2019; Warganegara and Restina, 2016). The action
mechanism of flavonoids as antimicrobial is divided
into 3, that is nucleic acid synthesis inhibition,
inhibiting the function of bacterial cell membranes,
and inhibiting energy metabolism from amino acids
(Nomer et al., 2019).
Flavonoids can inhibit nucleic acid synthesis in
bacterial cells because of their A and B rings. These
rings contributed in the intercalation process or the
process of hydrogen bonding by accumulating nucleic
acid bases so that the formation process of DNA and
RNA is inhibited (Nomer et al., 2019). These
flavonoid compounds can also cause damage to the
permeability of bacterial cell walls so that bacterial-
toxic substances can enter these bacterial cells. In
addition, flavonoids also form complex compounds
with extracellular proteins that can damage cell
membranes owned by bacteria resulting in leakage of
intracellular compounds. Flavonoids are also able to
inhibit the energy metabolism process of bacteria by
interfering with the macromolecular biosynthesis
process of these bacteria. Due to inhibited metabolic
processes, these molecules cannot develop properly to
meet the needs of these bacteria (Nomer et al., 2019;
Sapara and Waworuntu, 2016).
There are various types of flavonoids, such as
genkwanin, apigenin, and luteolin. Some of these
compounds are a class of flavonoids which are found
in agarwood leaves (Wangiyana, 2020). Genkwanin,
apigenin, and luteolin are known to have antibacterial
activity. Apigenin can affect the cytoplasmic
membrane of bacteria, this compound will interfere
with the metabolic process of bacteria and ultimately
inhibit energy production in bacteria (Xie et al., 2014).
Other active compounds found in agarwood leaf
extract are alkaloids, tannins, saponins, and
triterpenoids/steroids. These compounds are involved
in the destruction of bacterial cell membranes (Sari et
al., 2017). Alkaloids penetrate the bacterial
lipopolysaccharide membrane, causing depolarization
of the cytoplasmic membrane. This compound will
then affect the production of enzymes in bacteria and
cause leakage in the cytoplasm of the bacteria
(Cushnie et al., 2014). Meanwhile, tannins invade the
polypeptides present in the cell walls, these
compounds can denature proteins and eventually lead
to bacterial lysis (Zeniusa et al., 2019).
Saponins would bind water molecules and dissolve
fat so that it could disrupt the surface tension of
bacterial cells and eventually cause cell destruction. In
addition, there are triterpenoids/steroids that can bind
to lipid molecules on the bacterial cell membrane.
These compounds will disrupt the integrity of the
bacterial cell membrane and can change the
morphology of the cell membrane. In the end,
bacterial cells will be fragile and will undergo lysis
(Sari et al., 2017).
Previous research states that agarwood leaf extract
produced moderate to strong inhibition of
Staphylococcus aureus bacteria, a gram-positive
bacterium (Liana, 2014). While research conducted
upon gram-negative bacteria showed that the
inhibition power of agarwood leaf extract was weaker
when compared to gram-positive bacteria. This is
caused by 3 layers of the wall owned by gram-negative
bacteria. Those are the outer lipoprotein layer, the
middle lipopolysaccharide layer, and the outer
peptidoglycan layer (Septiani et al., 2017).
In this study, the inhibitory ability produced
towards P. gingivalis was weak to moderate. This
bacterium is an encapsulated gram-negative which
makes it more resistant to antibacterial activity
compared to other bacteria. This is supported by other
research which states that agarwood leaf extract has
weak to moderate inhibitory power against several
other encapsulated gram-negative bacteria such as
Escherichia coli, Klebsiella pneumoniae and Vibrio
mimicus (Hendra et al., 2016; Begum, 2016; Jihadi et
al., 2020).
In this study, the diffusion method used was agar
well diffusion method which is widely used to
evaluate the antimicrobial activity of extracts derived
from plants. In this method, a hole with a diameter of
8 mm is made which will then be filled with agarwood
leaf extract. The agar well diffusion method is used
with the aim that the results obtained directly
reflecting the agarwood leaf extract inhibitory ability.
This is ought due to the diffusion process is better and
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
118

the volume of material placed is more than the agar
disk diffusion method Balouiri et al., 2016).
There are several factors that can affect the
diameter of the bacterial growth inhibition zone. The
first factor is the temperature used at the time of
incubation. If during the incubation process the agar
media are stacked, there is a possibility that there is a
temperature difference between the plates. In addition,
the thickness of the agar also affects the diameter of
the inhibition zone. The best agar thickness is 4 mm.
More than that, the diffusion process of the extract will
be slower (Zeinusa et al., 2019). The dilution of the
extract could also affect the diffusion ability. Higher
concentration means decreasing solubility or thicker
extract. Therefore, the extract diffusion process with
higher concentrations will be slower.
Another factor that can affect the diameter of
inhibition zone is the turbidity of the bacterial
suspension. In this study, the measurement of the
turbidity level was only visually performed by
comparison with the 0.5 Mc Farland solution. If the
bacterial suspension is too turbid, the resulting
inhibition zone diameter will be smaller and vice
versa.
The phytochemical test carried out in this study
was a qualitative test. Hence, it is difficult to
determine with certainty the number of compounds
contained in agarwood leaf extract which can inhibit
P. gingivalis growth. In addition, the solvent used in
diluting the ethanol extract of gaharu leaves was
distilled water (aquadest) which was also used as a
negative control in this study. It was found that this
treatment did not produce a clear zone on the agar
medium, meaning that distilled water confirmed had
no inhibitory ability and did not affect P. gingivalis
growth.
In this study, the concentration of agarwood leaf
extract was made in percent units makes the range of
concentrations that could be made as a treatment was
less than optimal. Making the concentration in units of
PPM (parts per million) might be better because this
method refers more to the unit of concentration. This
is a way to measure the concentration of a substance
that is both very low and high, which 1 ppm is
equivalent to 1 milligram per liter, or the concentration
in percent is 0.0001%.
4 CONCLUSIONS
This study showed that there was a cytotoxicity effect
of the agarwood leaf extract towards fibroblasts in
vitro at concentrations of 500 µg/mL, 250 µg/mL, and
125 µg/mL, and there was no cytotoxicity effect of
agarwood leaf extract towards fibroblasts in vitro at
concentrations of 62.50 µg/mL, 31.25 µg/mL, 15.63
µg/mL, and 7.81 µg/mL. Agarwood leaf extract also
had effect towards P. gingivalis growth with the
maximum inhibition at a concentration of 100%
which is classified into the moderate inhibition
category.
ACKNOWLEDGEMENTS
We thank Faculty of Dentistry of Maranatha Christian
University for the technical support during the
research. This research received grant from
Maranatha Christian University.
REFERENCES
Kinane DF, Stathopoulou PG, Papapanou PN. (2017).
Periodontal diseases. Nat Rev Dis Prim. 3(1), 1–14.
Nazir MA. (2017). Prevalence of periodontal disease, its
association with systemic diseases and prevention. Int J
Health Sci (Qassim). 11(2), 72–80.
Newman MG, Takei HH, Klokkevold PR, Carranza FA.
(2012). Carranza’s clinical periodontology, Saunders
Elsevier. St. Louis, 12
th
ed.
Wijaksana Evan IK. (2019). Periodontal chart dan
periodontal risk assessment sebagai bahan evaluasi dan
edukasi pasien dengan penyakit periodontal. J Kesehat.
Gigi. 6(3), 19–25.
Séverin T. (2018). Periodontal health and disease: A
practical guide to reduce the global burden of
periodontal disease, FDI World Dental Federation.
Switzerland.
How KY, Song KP, Chan KG. (2016). Porphyromonas
gingivalis: An overview of periodontopathic pathogen
below the gum line. Front. Microbiol. 7(1), 1–14.
Liu Y, Zhang Y, Wang L, Guo Y, Xiao S. (2013).
Prevalence of porphyromonas gingivalis four rag locus
genotypes in patients of orthodontic gingivitis and
periodontitis. PLoS One. 8(4), 1–6.
Klein BA, Tenorio EL, Lazinski DW, et al. (2012).
Identification of essential genes of the periodontal
pathogen porphyromonas gingivalis. BMC Genomics.
13(8), 1–17.
Gerits E, Verstraeten N, Michiels J. (2017). New
approaches to combat porphyromonas gingivalis
biofilms. J. Oral Microbiol. 9(1), 1–11.
Williams AJ, Wang Z, Taylor SF. (2016). Prevention and
treatment of periodontal diseases in primary care-dental
clinical guidance. Scott. Med. J. 22(5), 1–57.
Hudwekar AD, Beldar A, Murkute S, Lendhey SS, Thamke
M. (2019). Aloe vera on wound healing after
periodontal flap surgery in chronic periodontitis
patient: A randomized control trial. J. Oral Res. Rev.
11(7), 72-76.
The Effect of Agarwood Leaves Ethanol Extract on Porphyromonas gingivalis Growth Inhibition and in Vitro Cytotoxicity Assay on
Fibroblast
119

Sugiaman VK. (2011). Peningkatan penyembuhan luka di
mukosa oral melalui pemberian aloe vera (Linn.) secara
topikal. Maranatha J Med Heal. 11(1), 70–9.
Janshen YR, Sidharta BR, Swasti R. (2017). Aktivitas
antibakteri ekstrak daun gaharu (Aquilaria malaccensis
Lamk.) terhadap pseudomonas aeruginosa dan
staphylococcus aureus. J. Biotechnol. Biosci. 53(9), 1–
16.
Musir A, Winarti W, Siregar SHP. (2016). Phytochemical
screening and toxicity test BSLT of 70% ethanol extract
of gaharu leaves (Aquilaria beccariana Tiegh.).
Pharmacol. Sci. Res. 13, 34–9.
Nugraha R, Ginting H. (2013). Uji aktivitas antioksidan
ekstrak etanol daun gaharu (Aquilaria malaccensis
Lamk) berdasarkan umur pohon. Peronema. For. Sci. J.
6(3), 1–9.
Wangiyana IGAS. (2020). Medicinal effect review of
agarwood leaves from aquilaria and gyrinops genera. J.
Silva. Samalas. 3(1), 36–9.
Syamsul ES, Amanda NA, Lestari D. (2020). Perbandingan
ekstrak lamur aquilaria malaccensis dengan metode
maserasi dan refluks. J. Ris. Kefarmasian. Indones.
2(2), 97–104.
Nomer NMGR, Duniaji AS, Nocianitri KA. (2019).
Kandungan senyawa flavanoid dan antosianin ekstrak
kayu secang (Caesalpinia sappan L.) serta aktivitas
antibakteri terhadap vibrio cholerae. J. Ilmu dan
Teknol. Pangan. 8(2), 216-225.
Warganegara E, Restina D. (2016) Getah jarak (Jatropha
curcas L.) sebagai penghambat pertumbuhan bakteri
streptococcus mutans pada karies gigi. Med. J.
Lampung Univ. 5(3), 64–5.
Pratiwi EW, Praharani D, Mahdiyah Y. (2015) Daya
hambat ekstrak daun pepaya (Carica papaya L.)
terhadap adhesi bakteri porphyromonas gingivalis pada
neutrofil. 3(2), 193–8.
Dennis, Nurliza C, Savitri W. (2017). Antibacterial effect
of ethanol extract of the avocado seed (Persea
Americana Mill.) as an alternative root canal irrigants
against porphyromonas gingivalis (in vitro). Int. J.
Appl. Dent. Sci. 3(1), 90-92.
Sari R, Muhani M, Fajriaty I. (2017). Uji aktivitas
antibakteri ekstrak etanol daun gaharu (Aquilaria
microcarpa Baill.) terhadap bakteri staphylococcus
aureus dan proteus mirabilis. Pharmacol. Sci. Res.
4(3), 143–54.
Suhardiman A, Juanda D. (2019). Pengembangan obat
herbal fraksi daun gaharu (aquilaria malaccensis Lam)
dalam bentuk gel untuk penyembuhan luka bakar. J.
Sains dan Teknol. Farm. Indones. 3(1), 16–24.
Wahid AR, Safwan S. (2019). Efek antioksidan ekstrak
etanol daun gaharu (Aquilaria malaccensis L.) pada
tikus jantan galur sprague dawley yang diinduksi
paracetamol (kajian aktivitas enzim katalase, SGOT
dan SGPT). Pharmauho. J. Farm. Sains, dan Kesehat.
4(2), 22-4.
Fauzi R, Cahaya N, Hidayaturrahmah. (2017). Pengaruh
pemberian ekstrak etanol daun gaharu terhadap waktu
perdarahan pada tikus putih jantan galur wistar. J. Ilm.
Ibnu Sina. 2(2), 169–73.
Rahmadhani N, Yudaniayanti IS, Saputro AL, Triakoso N,
Wibawati PA, Yudhana A. (2020) Efektivitas krim
ekstrak buah naga merah (Hylocereus polyrhizus)
dalam meningkatkan jumlah sel fibroblas luka bakar
derajat II pada tikus putih (Rattus norvegicus). J. Med.
Vet. 3(1), 65–72.
Nurmiati N, Wijayanti E. (2018). Perbandingan kadar
fenolik total antara seduhan daun gaharu dan kombucha
daun gaharu. JC-T. 2(1), 6–10.
Komang N, Septiani A, Oka IM, Parwata A, Bawa A.
(2018). Penentuan kadar total fenol, kadar total
flavonoid dan skrining fitokimia ekstrak etanol daun
gaharu. J. Mat. Sains, dan Pembelajarannya. 12(1), 78–
87.
10993-5 I. (2009). Biological evaluation of medical devices
- Part 5: Test for in vitro cytotoxicity. Int. Stand. Organ.
3(5), 17–8.
10993-12 I. (2012). Biological evaluation of medical
devices - Part 12: Sample preparation and reference
materials. Int. Stand. Organ. 4(12), 7–8.
Rastina, Sudarwanto M, Wientarsih I. (2015). Aktivitas
antibakteri ekstrak etanol daun kari terhadap
staphylococcus aureus, escherichia coli, dan
pseudomonas sp. J. Kedokt. Hewan. 9(2), 185–188.
Yildirim I, Kutlu T. (2015). Anticancer agents: saponin and
tannin. Int. J. Biol. Chem. 9(6), 333–5.
Suharto I, Etika AN. (2019). Pengaruh ekstrak jahe
(Zingiber officinale roscoe) terhadap kepadatan serabut
kolagen luka insisi. J. Ilmu Kesehat. 7(1), 32–4.
Ardiana T, Rizkia Putri Kusuma A, Dian Firdausy M.
(2015). Efektivitas pemberian gel binahong (Anredera
cordifolia) 5% terhadap jumlah sel fibroblast pada soket
pasca pencabutan gigi marmut (Cavia cobaya).
ODONTO Dent. J. 2(1), 64-70.
Mohammadi M, Quan M, Zhang JS, Li X. (2020). FGF
signaling pathway: A key regulator of stem cell
pluripotency. Front. Cell Dev. Biol. 8(12), 1–3.
Etika A, Nurrahayu K, Suhato I. (2017). Pengaruh ekstrak
jahe (Zingiber officinale roscoe) terhadap jumlah sel
fibroblas pada tikus (Rattus Norvegicus). J. Nurs. Care
Biomol. 2(1), 12–3.
Kurahashi T, Fujii J. (2015). Roles of antioxidative
enzymes in wound healing. J. Dev. Biol. 3(2), 57–61.
Jiang X, Liu L, Qiao L, Zhang B, Wang X, Han Y, et al.
(2018). Dracorhodin perchlorate regulates fibroblast
proliferation to promote rat’s wound healing. J.
Pharmacol. Sci. 136(2), 66–72.
Ningrum AP, Kurniawaty E. (2019). The role of
mesenchymal stem cells in repairing lung parenchymal
damage. J. Major. 8(1), 201–4.
Pattarayan D, Sivanantham A, Bethunaickan R,
Palanichamy R, Rajasekaran S. (2018). Tannic acid
modulates fibroblast proliferation and differentiation in
response to pro-fibrotic stimuli. J. Cell Biochem.
119(8), 6732–42.
Aslanturk OS. (2018). In vitro cytotoxicity and cell viability
assays: Principles, advantages, and disadvantages,
Licens IntechOpen J. London 1
st
edition.
Zhang Y. (2018). Cell toxicity mechanism and biomarker.
Clin. Transl. Med. 7(1), 1–4.
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
120

Spindola DG, Hinsberger A, Antunes VM de S, Michelin
LFG, Bincoletto C, Oliveira CR. (2018). In vitro
cytotoxicity of chemical preservatives on human
fibroblast cells. Brazilian J. Pharm. Sci. 54(13), 5–6.
Aykul S, Martinez-Hackert E. (2016). Determination of
half-maximal inhibitory concentration using biosensor-
based protein interaction analysis. Anal. Biochem.
508(16), 97–8.
Sapara TU, Waworuntu O. (2016). Efektivitas antibakteri
ekstrak daun pacar air (Impatiens balsamina L.)
terhadap pertumbuhan porphyromonas gingivalis.
Pharmacon. 5(4), 10–7.
Cushnie TPT, Cushnie B, Lamb AJ. Alkaloids: An
Overview of Their Antibacterial, Antibiotic-Enhancing
and Antivirulence Activities. Int J Antimicrob Agents.
2014;44(5):377–86.
Zeniusa P, Ramadhian MR, Nasution SH, Karima N.
(2019). Uji daya hambat ekstrak etanol teh hijau
terhadap escherichia coli secara in vitro. Majority. 8(2),
136–43.
Liana Y. (2014). Uji aktivitas antibakteri ekstrak daun
gaharu (Aquilarria malaccensis) terhadap
staphylococcus aureus. Nurs J. Indones. 11(7), 96-100.
Septiani, Dewi EN, Wijayanti I. (2017). Aktivitas
antibakteri ekstrak lamun (Cymodocea rotundata)
terhadap bakteri staphylococcus aureus dan escherichia
coli. J. Fish. Sci. Technol. 13(1), 1-4
Hendra H, Moeljopawiro S, Nuringtyas TR. (2016).
Antioxidant and antibacterial activities of agarwood
(Aquilaria malaccensis Lamk.) leaves. AIP Conf Proc.
1755(140004), 1–9.
Begum Y. (2016). Study on agarwood (Aquilaria
malaccensis) to evaluate antibacterial and antioxidant
activities of n-hexane, chloroform and ethyl acetate
extracts. Pharma. Tutor. 4(2), 47–50.
Jihadi NIM, Hashim YZHY, Rahim NA, Kamal KM, Noor
NM, Sani MSA, et al. (2020). Antibacterial activity of
ethanolic leaf extract of aquilaria malaccensis against
multidrug-resistant gram-negative pathogen. Food Res.
4(6), 1962–8.
Balouiri M, Sadiki M, Ibnsouda SK. (2016). Methods for in
vitro evaluating antimicrobial activity: A review. J.
Pharm. Anal. 6(2), 71–9.
APPENDIX
Cytotoxicity Assay
Treatment Results
XA XC Number of Cells %Viability %Inhibition
Cell Control 1,7049 1,2395 17238 100,00 0,00
DMSO 10% 1,6521 1,2337 17154 99,51 0,49
500 µg/mL 0,8455 0,4081 5360 31,10 68,90
250
µg
/mL 1,0178 0,6374 8636 50,10 49,90
125 µg/mL 1,0430 0,6675 9065 52,59 47,41
62.5
µg
/mL 1,4046 1,0277 14211 82,44 17,56
31.3 µg/mL 1,5046 1,1200 15530 90,09 9,91
15.6
µg
/mL 1,6080 1,2064 16765 97,26 2,74
7.81 µg/mL 1,9290 1,5217 21269 123,39 -23,39
XA : Mean of absorbance
XK : Mean of corrective absorbance
ALE : Agarwood leaf extract
Inhibitory Assay
Treatment
Inhibition Zone
Mean
1 2 3
- Control 0,00 0,00 0,00 0,00
1,56% 2,20 2,10 2,25 2,18
3,13% 2,35 2,20 2,50 2,35
6,25% 2,65 2,55 2,70 2,63
12,50% 2,85 3,50 3,35 3,23
25% 3,50 3,70 3,65 3,62
50% 3,85 4,50 4,55 4,30
100% 6,50 6,35 6,80 6,55
+ Control 10,95 12,10 11,80 11,62
The Effect of Agarwood Leaves Ethanol Extract on Porphyromonas gingivalis Growth Inhibition and in Vitro Cytotoxicity Assay on
Fibroblast
121
