
Antioxidant Properties of Salacca zalacca (Gaertn.) Voss Peel
Ethanolic Extract Compared to Chlorogenic Acid
Ermi Girsang
1,* a
, Chrismis Novalinda Ginting
1b
, I Nyoman Ehrich Lister
1c
,
Cahyaning Riski Wijayanti
2
, Wahyu Widowati
3d
and Rizal Rizal
2,4 e
1
Faculty of Medicine, Universitas Prima Indonesia, Jl. Belanga No. 1 Simp. Ayahanda, Medan 20118, North Sumatera,
Indonesia
2
Biomolecular and Biomedical Research Center, Aretha Medika Utama, Jl. Babakan Jeruk 2 no 9, Bandung 40163,
West Java, Indonesia
3
Faculty of Medicine, Maranatha Christian University, Jl. Surya Sumantri no 65, Bandung 40164, West Java, Indonesia
4
Biomedical Engineering, Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia,
Depok 16426, West Java, Indonesia
wahyu_w60@yahoo.com, rizal_biotek@yahoo.com
Keywords: Antioxidant, Chlorogenic Acid, Flavonoid, Phenolic, Salacca zalacca.
Abstract: Oxidative stress from free radicals can cause a variety of chronic and degenerative diseases. The use of
antioxidants from natural products is one of the breakthroughs. Salacca zalacca (Gaertn.) Voss is one of the
tropical fruits that have biological activities that are important for human health. This study aims to determine
total phenol content (TPC) and flavonoid content (TFC), also the antioxidant activity of Salacca zalacca peel
ethanolic extract (SEE) compared with chlorogenic acid (CGA). METHODS: The total phenolic and
flavonoid content of SEE were measured and followed by 2,2’-azinobis-3-ethylbenzo-thiazoline-6-
sulfonicacid(ABTS), H
2
O
2
, NO, OH
scavenging, and ferric reducing antioxidant power (FRAP) assay to
determine the antioxidant properties. The TPC of SEE value is 6.97 µg GAE/mg extract and the TFC value
is 3.92 µg QE/mg extract. The IC
50
value of ABTS, H
2
O
2
, NO, OH scavenging activity of SEE were 57.71;
103.84; 38.09; 27.77 µg/mL compared to CGA 7.76; 13.07; 27.15; 13.71 µg/mL respectively. The FRAP
activity of SEE, CGA respectively 240.08; 399.21 μm Fe (II)/μg at the highest concentration (50 µg/mL).
SEE and Chlorogenic acid as its compound have antioxidant activity through ABTS, H
2
O
2
, NO, OH
and ferric
reducing antioxidant power (FRAP) scavenging activities.
1 INTRODUCTION
Free radicals can be the cause of oxidative stress,
which leads to a variety of chronic and degenerative
diseases. Oxidative stress can be caused by free
radicals. Free radicals are very reactive and unstable
because the electrons do not pair with the outermost
atomic orbitals. Free radicals react by binding
molecules in cells, which cause the oxidation of
a
https://orcid.org/0000-0003-4313-4941
b
https://orcid.org/0000-0003-2269-2717
c
https://orcid.org/0000-0003-1325-5208
d
https://orcid.org/0000-0002-5401-7794
e
https://orcid.org/0000-0003-2783-0672
*
Corresponding author
nucleic acids, proteins, fats, and DNA (Halliwell &
Gutteridge, 2015).
Sources of free radicals can originate from normal
metabolic processes in the human body or external
exposure (Widowati et al., 2016). The body needs
antioxidants as oxidation inhibitors to overcome the
negative effects of free radicals. Antioxidants work
by reacting to reactive free radicals to form relatively
stable reactive substances. Thus, the antioxidant
supply was needed for the human body to prevent
oxidative stress (Rusmana et al., 2017).
Girsang, E., Ginting, C., Lister, I., Wijayanti, C., Widowati, W. and Rizal, R.
Antioxidant Properties of Salacca zalacca (Gaertn.) Voss Peel Ethanolic Extract Compared to Chlorogenic Acid.
DOI: 10.5220/0010744700003113
In Proceedings of the 1st International Conference on Emerging Issues in Technology, Engineering and Science (ICE-TES 2021), pages 87-94
ISBN: 978-989-758-601-9
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
87

Antioxidants are divided into two types based on
their source, synthetic, and natural antioxidants.
Synthetic antioxidants can be carcinogenic if
consumed continuously. Therefore, natural
antioxidant needs to continue to increase because they
have fewer side effects. In addition to having fewer
side effects, natural antioxidants also protect the body
from damage caused by free radicals and inhibit
degenerative diseases (Xu et al., 2021) (Laintonjam,
2012). Several studies have shown that plant extract
has the potential compound to be active antioxidants.
Among all of the natural compounds, phytochemicals
are best known for their various biological activities,
such as antiaging, antioxidants, and anti-
inflammatory (Widowati et al., 2016)(Girsang et al.,
2020a)(Girsang et al., 2020b).
Secondary metabolites from non-edible fruits can
be source of antioxidants, because they are rich in
polyphenols (Vijayalaxmi et al., 2015). Some tropical
and subtropical fruits have a protective effect on
health. Salak or snake fruit (S. zalacca) is one of the
tropical fruit that has biological activities that are
important for human health. This fruit which has
antioxidant potential is widely cultivated in the
Southeast Asia region (Dembitsky et al., 2011). S.
zalacca has active compounds in the form of
polyphenols, chlorogenic acid, ferulic acid, gallic
acid, and catechins (Hlásná Čepková, et al., 2021).
Previous study stated that S. zalacca fruit has strong
antioxidant activity, which was evaluated by 2,2’-
azinobis-3-ethylbenzo-thiazoline-6-sulfonic acid
(ABTS) and 2,2-diphenyl-1-picrylhydrazil (DPPH)
scavenging assays (Saleh et al., 2018) (Suica-Bunhez
et al., 2016). However, the inhibitory effects of S.
zalacca peel active compounds on specific radical
species have not been widely presented. In the present
study, free radical scavenging activity of S. zalacca
peel ethanolic extract (SEE) compared with its
compounds chlorogenic acid, such as H
2
O
2
, NO, OH,
ABTS scavenging activity, and FRAP activity were
evaluated as well as total phenolic and flavonoid were
measured. Thus, SEE which has been a waste can be
utilized as an antioxidant agent derived from natural
products.
2 METHODS AND MATERIALS
2.1 Plant Material Preparation and
Extraction
Dried salak peels was obtained from Kampung
Rahayu Cicadas, Ciampea, Bogor, West Java,
Indonesia. The phytochemical compound and
chlorogenic acid (CGA) were obtained from Chengdu
Biopurify (Biopurify Phytochemical Ltd, BP0345).
Identification of the salak plants performed by a staff
of herbarium, Department of Biology, School of Life
Sciences and Technology, Bandung Institute of
Technology, Bandung, West Java, Indonesia. The
salak plant was identified as S. zalacca (Gaertn.)
Voss. The extraction method used in this study is the
maceration method using 70% ethanol as the solvent.
The filtrate is collected every 24 hours until the
colorless filtrate. After that, the filtrate is evaporated
using a rotary vacuum evaporator at a temperature of
50
0
C until the extract becomes a paste-shaped extract.
Then, the S. zalacca extract was stored at -20
0
C
(Widowati et al., 2018)(Widowati et al., 2017)(Lister
et al., 2019).
2.2 Total Phenol Assay
Briefly, 15 µl standard gallic acid (Sigma 398225)
solution in 6 concentration level (50.00; 25.00; 12.50;
6.25; 3.13; 1.56 µg/ml) and sample of SEE in
concentration of 2000; 1000; and 500 µg/ml were
prepared for total phenol assay. Each standard and
sample was mixed with 60 µl of Na
2
CO
3
7.5% (Merck
A897992745) and 75 µl Folin- Ciocalteu reagent 10%
(Merck 1.090.010.500) in the microplate.
The solution was incubated at 50
0
C for 10
minutes, then the absorbance was measured at a
wavelength of 760 nm using a microplate reader
(Multiskan Go Reader, Thermo Fisher Scientific
1510). Analysis of the phenol content was carried out
based on the gallic acid (Sigma Aldrich, G7384)
linear regression equations (y = 0.0429x + 0.152)
(Rusmana et al., 2017)(Widowati et al.,
2018)(Nurhayati et al., 2018)(Utami et al., 2019).
2.3 Total Flavonoid Assay
The total flavonoid content was measured with an
AlCl
3
colorimetric assay with minor modification
(15). A 75 µl standard quercetin (Sigma Q4951)
solution in 7 concentration level (500.00; 250.00;
125.00; 62.50; 31.25; 15.60; and 7.80 µg/ml) and
SEE in concentration of 2000 and 1000 µg/ml were
added to microplate and mixed with 75 µl AlCl
3
2%
(Merck 449598). Using a microplate reader
(Multiskan Go Reader, Thermo Fisher Scientific
1510) the absorbance was measured in 415 nm of
wavelength. The linear regression equation
(y=0.0095x+0.037) was created based on the
quercetin standard (Sigma Aldrich, Q4951). The
analysis of the flavonoid content of the sample was
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
88

performed based on each of standard linear regression
equation (Prahastuti et al., 2019).
2.4 ABTS-reducing Activity
Antioxidant capacity of SEE and CGA were
measured using the 2,2’-Azinobis-(3-
ethylbenzothiazoline-6-sulfonic acid) (ABTS
•+
)
(Sigma Aldrich, A1888) diammonium salt-free
radical assay. ABTS
•+
was produced by reacting 14
mM ABTS
•+
and 4.9 mM potassium persulfate
(Merck 1.05091.0250). The final concentration of the
mixture is 7 mM ABTS
•+
in 2.45 mM potassium
persulfate. After that, the mixture was incubated at
the darkroom temperature for 16 h. Using 5.5 mM
PBS (pH 7.4) the ABTS
•+
solution was diluted then
the absorbance of the solution was measured with a
microplate reader at 745 nm, resulting in the
absorbance of 0.70±0.02. Then, a sample about 2 μl
was added of ABTS
•+
solution 198 μl. The solution
was incubated at 30
0
C for 6 min and the absorbance
was measured at a wavelength of 745 nm. The ABTS
radical inhibition percentage (%) was calculated
based on the ratio of ABTS
•+
absorbance reduction of
the sample relative to a negative control (Rusmana et
al., 2017)(Widowati et al., 2018).
2.5 FRAP Activity Assay
Briefly, 10 ml of 300 mM acetate buffer (pH 3.6
adjusted with the addition of acetic acid) was mixed
with 1 mL of 20 mM ferric chloride hexahydrate
(Merck 1.03943.0250) and 1 ml of 10 mM 2,4,6-
Tris(2-pyridyl)-s-triazine (TPTZ) (Sigma-Aldrich,
T1253) to prepare the FRAP reagent. A 142.5 μl
FRAP was mixed with 7.5 μl samples (SEE, CGA) in
a microplate and incubated for 6 min at 37
0
C. Using
a microplate reader the absorbance of the solution
was measured in 593 nm of wavelength (Rusmana et
al., 2017)(Widowati et al., 2018)(Prahastuti et al.,
2019).
2.6 Hydrogen Peroxide (H
2
O
2
)
Scavenging Activity Assay
Hydrogen peroxide scavenging activity was
measured using a method described by Utami et al.
(2017) and Prahastuti et al. (2019) with minor
modifications (Prahastuti et al., 2019)(Utami et al.,
2017). The mixture was made, then transferred into a
microplate and incubated for 5 minutes at room
temperature, then 75 µl 1,10-phenanthroline (Sigma
131377) was added to the mixture and incubate the
mixture for 10 min at room temperature. The
absorbance was measured using a spectrophotometer
at 510 nm. The result was depicted as a scavenging
percentage that calculated using the following
formula:
% scavenging activity = A/C x 100%
when A is sample absorbance and C controls
absorbance.
2.7 Nitrogen Oxide Scavenging Activity
Assay
Sodium nitroprusside (SNP) 10 mM (Sigma Aldrich,
71780) in phosphate buffer saline (PBS) (Gibco,
1740576) was mixed with several concentrations
(2.08-66.67 µg/mL) of SEE and CGA. The mixture
was then incubated for 2 hours at room temperature.
Furthermore, the mixture was added Greiss reagent
containing 1% Sulphanilamide (Sigma Aldrich,
S9251), 2% H3PO4 (Merck, 100573), N-(1-
napththyl) ethylenediamine dihydrochloride (Sigma
Aldrich, N9125). The absorbance was measured at
546 nm wavelength (Multiskan GO Reader, Thermo
Fisher Scientific 1510) (19). The antioxidant activity
of SEE and CGA in the experiment was determined
as follows:
% scavenging activity=(Ac–As)/Ac x 100
Ac: negative control absorbance
As: sample absorbance
2.8 Hydroxyl Radical (OH) Scavenging
Activity Assay
The reaction mixture contained 30 μL of different
concentrations of a sample (0.83 – 26.67 μg/mL), 10
μL of FeCl
3
25 mM-EDTA, 5 μL of 20 mM H
2
O
2
(Merck, 1.08597), 5 μL of 1 mM L-Ascorbic acid
(Sigma Aldrich, K3125), 10 μL of 28 Mm
Deoxyribose (Sigma-Aldrich, 121649), and 70 μL
phosphate buffer. The mixture was incubated at 37 °C
for 30 min and then 25 μL of 5% TCA (Merck,
100807), and 1% TBA (Sigma-Aldrich, T5500) were
added to be further incubated at 80 °C for 30 min. The
absorbance was measured at 532 nm wavelength
using a spectrophotometer (Multiskan GO Reader,
Thermo Fisher Scientific 1510) (Irwan et al., 2020).
The antioxidant activity of SEE and CGA in the
experiment was determined as follows:
% scavenging activity=(Ac–As)/Ac x 100
Ac: negative control absorbance
As: sample absorbance
Antioxidant Properties of Salacca zalacca (Gaertn.) Voss Peel Ethanolic Extract Compared to Chlorogenic Acid
89
2.9 Statistical Analysis
The result data were expressed as mean ± standard
deviation and the data were analyzed using One-way
ANOVA followed by Tukey’s HSD Post-hoc test.
Statistical analysis was performed using SPSS
software (version 20.0), with P < 0.05 as the
significant value of the data.
3 RESULTS AND DISCUSSION
Salak (S. zalacca) is a species of palm tree group
originating from Malaysia and Indonesia. This fruit is
known as the 'snake fruit' because it has skin that is
reddish-brown and scaly. Researchers believe that
residues from plants can still be used because they
have the potential as a source of antioxidants, and
they are rich in polyphenols (Vijayalaxmi et al.,
2015). The previous studies showed that S. zalacca
peel extract had better antioxidant potential through
inhibition of DPPH compared to other tropical fruits
such as Matoa (Pometia pinnata), Papaya (Carica
papaya L.), Soursop (Annona muricata), Chlorine
(Baccaurea racemosa), and Rambai skin and seed
extract (B. motleyana) (Fitri et al., 2016).
Phenol is one of the most contained compounds
in plants and has been widely studied due to its
biological activities such as anti-mutagenic,
anticarcinogenic, anti-aging, and antioxidant (Cetin
et al., 2014). In the present study, the result of total
phenol and total flavonoid SEE has result with a value
of 6.97 ± 0.55 µg GAE/mg and 3.92 ± 0.78 µg QE/mg
extract, respectively (Table 1).
Table 1: The average total phenol and flavonoid level
concentration of S. zalacca peels extract.
Sample
Total Phenolic
Content
(µg GAE/mg
extract)
Total Flavonoid
Content
(µg QE/mg extract)
SEE 6.97 ± 0.55 3.92 ± 0.78
*SEE: S. zalacca peel ethanolic extract
In other study, S. zalacca peel extract has total
flavonoid content is 124.9 ± 0.004 mg/g Catechin and
total phenolic content is 946.61 ± 0.042 mg/g Gallic
acid (Suica-Bunghezet et al., 2016. The value of total
phenol and flavonoids is influenced by the level of
fruit maturity, the young fruit has the highest total
phenol value which is the highest compared to the
ripe fruit (Mokhtar et al., 2014). S. zalacca peel
extract contains phenolic compounds that have an
antioxidant activity such as chlorogenic acid, rutin,
protocatechuic acid, and caffeic acid (Girsang et al.,
2019)(Girsang et al., 2020).
The ABTS-reducing activity assay assesses an
antioxidant’s ability to scavenge the ABTS generated.
The long-wave absorption spectrum is used to
quantify the reduction of blue-green ABTS radical
colored solution by hydrogen-donating antioxidant
(Widowati et al., 2016).
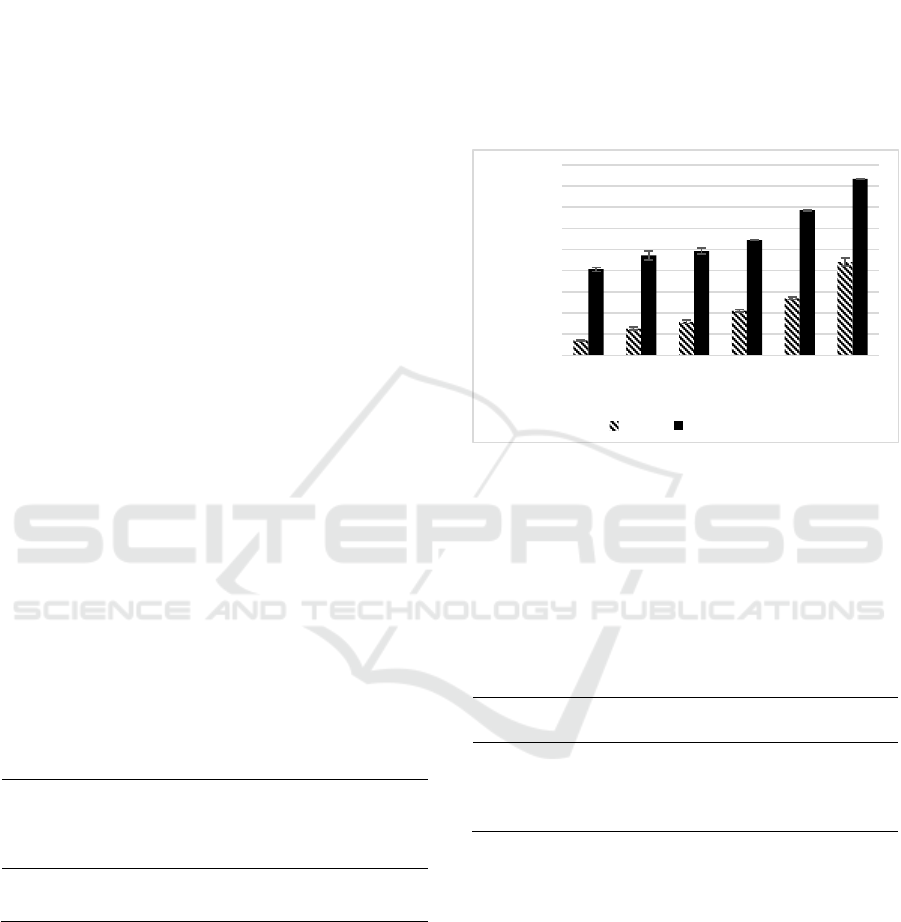
Figure 1: Effect variety concentrations of SEE, CGA
toward ABTS-reducing activity.
*ABTS-reducing activity (%) of SEE, CGA were diluted in DMSO
to reach the final concentration of 1.56; 1.13; 6.25; 12.50; 25.00;
50.00 (µg/mL). Different small letter (a,b,c,d,e,f) shows significant
differences among concentration of SEE and different capital letter
(A,B,C,D,E) among concentration of CGA toward ABTS-reducing
activity based on Tukey HSD post hoc test (p<0.05).
Table 2: IC
50
Value ABTS-reducing Activity of SEE and
CGA.
Sample Linear
Equation
R
2
IC
50
(µM)
IC
50
(µg/ml)
SEE y = 0.6954x +
9.8705
0.97
-
57.71
CGA y = 0.8407x +
43.473
0.97
21.90
7.76
*
Linear equations, coefficient of regression (R
2
), and IC
50
of each
sample were calculated. IC
50
of SEE was presented in μg/ml, while
CGA was presented in μM and μg/ml.
The results showed that both SEE and chlorogenic
acid possess high ABTS-reducing activity, with
chlorogenic acid (CGA) as the highest ABTS-
reducing activity value. The average percentage of
ABTS-reducing activity of chlorogenic acid shown in
Table 2 was higher compared to the ABTS-reducing
activity of SEE. The results of ABTS-reducing
activity of SEE between concentrations 1.56-50
µg/ml was the concentration-dependent manner and
chlorogenic acid compounds between concentrations
a
b
c
d
e
f
A
B
B
C
D
E
0,00
10,00
20,00
30,00
40,00
50,00
60,00
70,00
80,00
90,00
1,56 3,13 6,25 12,50 25,00 50,00
ABTS-Reducing Activity (%)
Concentration (μg/mL)
SEE Chlorogenic Acid
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
90
1.56-50 µg/ml were concentration-independent
manner (Figure 1). The value of IC
50
of SEE and
chlorogenic acid in reducing the ABTS free radical in
Table 2 revealed that SEE has a high value of IC
50
rather than CGA. This assured that CGA exhibited
effective antioxidant activity. CGA is one of the most
available phenolic acid compounds, and it is widely
distributed in plants (Meng et al., 2013). The
antioxidant effects of phenolic acids such as CGA has
been reported in various plant extracts. Extract from
the Hypericum hircinum L., a plant that containing
CGA have been shown to have antioxidant properties
that can inhibit free radicals (Mandrone et al., 2015).
Hereinafter, FRAP reducing power was assessed
to indicate the efficiency of the extract to reduce the
oxidized intermediates of the lipid peroxidation
process.
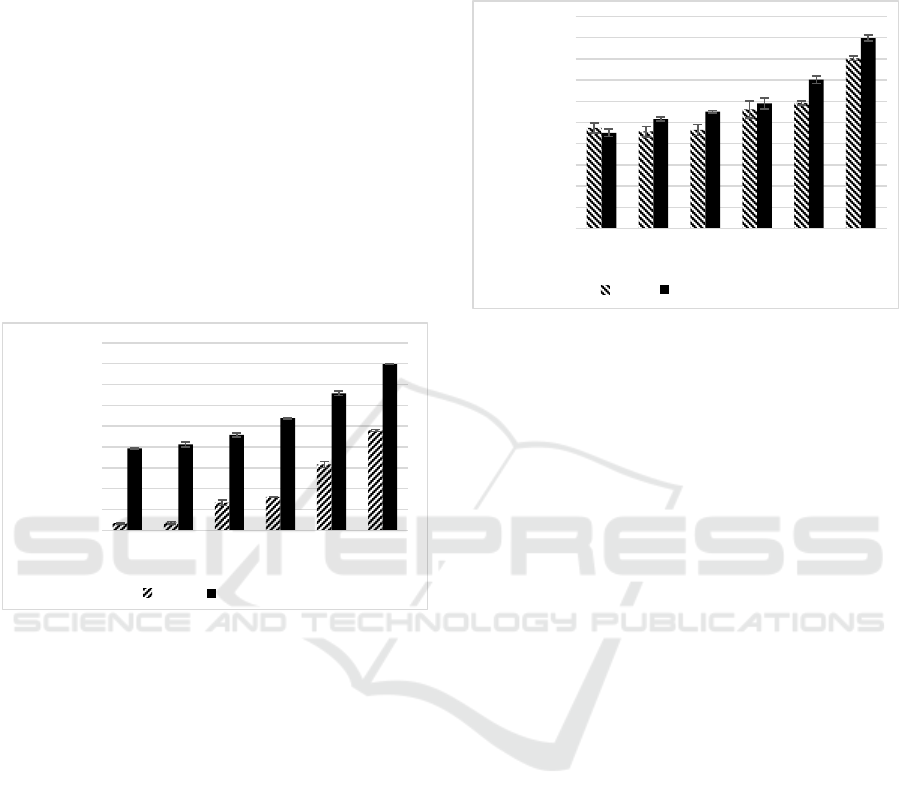
Figure 2: Effect variety concentrations of SEE, CGA
toward FRAP activity.
*FRAP activity (μM Fe (II)/μg sample) of SEE, CGA were were
diluted in DMSO to reach the final concentration of 1.56; 1.13;
6.25; 12.50; 25.00; 50.00 (µg/mL). Different small letter
(a,b,c,d,e,f) shows significant differences among concentration of
SEE and different capital letter (A,B,C,D,E) among concentration
of CGA toward FRAP activity based on Tukey HSD post hoc test
(p<0.05).
The FRAP activity in this study showed that both SEE
and CGA were increased significantly between
concentration 6.25-50 µg/ml (p<0.05) and showed in
a concentration-dependent manner, in which higher
concentration increased FRAP activity (Figure 2).
The CGA indicated high FRAP activity at the highest
concentration (50 µg/ml) with value (399.21 ± 0.59
μM Fe (II)/μg) which indicates high antioxidant
capacity, while SEE shows the lowest activity with
value (240.08 ± 2.65 μM Fe (II)/μg). CGA is
considered as well-known antioxidant agents (Yun et
al., 2012), and also known as antidiabetic, anti-
obesity, anti-hypertension, and anti-inflammatory
(Naveed et al., 2018).
The H
2
O
2
scavenging activity of SEE and CGA of
various concentrations were measured to determine
the antioxidant activity.
Figure 3: Effect variety concentrations of SEE, CGA
toward antioxidant activities.
*H2O2 scavenging activity (%) of SEE, CGA were were diluted in
DMSO to reach the final concentration of 7.81; 15.63; 31.25;
62.50; 125.00; 250.00 (µg/mL). Different small letter (a,b,c) shows
significant differences among concentration of SEE and different
capital letter (A,B,C,D,E) among concentration of CGA toward
H2O2 scavenging activity based on Tukey HSD post hoc test
(p<0.05).
Both SEE and chlorogenic acid expressed high
H
2
O
2
scavenging activity. The highest concentration
(250 µg/mL) of chlorogenic acid was slightly higher
compared to the scavenging activity of SEE, however
at the lowest concentration (7.81 µg/mL) SEE was
slightly higher than CGA. The results of H
2
O
2
scavenging activity of SEE and CGA between
concentrations 7.81-250 µg/mL was concentration-
dependent manner (Figure 3). The IC
50
value of CGA
was lower (13.07 µg/mL) than the IC
50
value
produced by SEE (103.84 µg/mL) (Table 4). These
results showed that the potency of CGA as an H
2
O
2
scavenging agent was better than SEE. Based on in
vivo study, SEE has potential as an antioxidant and
anti-inflammatory activities through suppressed of
intracellular ROS levels and decrease TNF-α, and
increase of IL-10 in lead-induced human fibroblast
cells (Girsang et al., 2020). S. zalacca has
polyphenols are protocatechuic acid and ferulic acid,
both of them have the ability as H
2
O
2
scavenger with
value 42.25 µg/mL and 73.37 µg/mL, respectively
(Girsang et al., 2020). The antioxidant activity of
phenolic compounds in S. zalacca depend on the
amount of hydroxyl group contained in the chemical
structure, that hydroxyl group will react with radical
species such as hydrogen peroxide (H
2
O
2
) (Girsang et
al., 2020).
a
a
b
c
d
e
A
A
B
C
D
E
0,00
50,00
100,00
150,00
200,00
250,00
300,00
350,00
400,00
450,00
1,56 3,13 6,25 12,50 25,00 50,00
FRAP Activity (μM Fe (II)/μg)
Concentration (μg/mL)
SEE Chlorogenic Acid
a
a
a
b
b
c
A
B
B
C
D
E
0,00
10,00
20,00
30,00
40,00
50,00
60,00
70,00
80,00
90,00
100,00
7,81 15,63 31,25 62,50 125,00 250,00
H
2
O
2
Scavenging Activity (%)
Concentration (μg/mL)
SEE Chlorogenic Acid
Antioxidant Properties of Salacca zalacca (Gaertn.) Voss Peel Ethanolic Extract Compared to Chlorogenic Acid
91
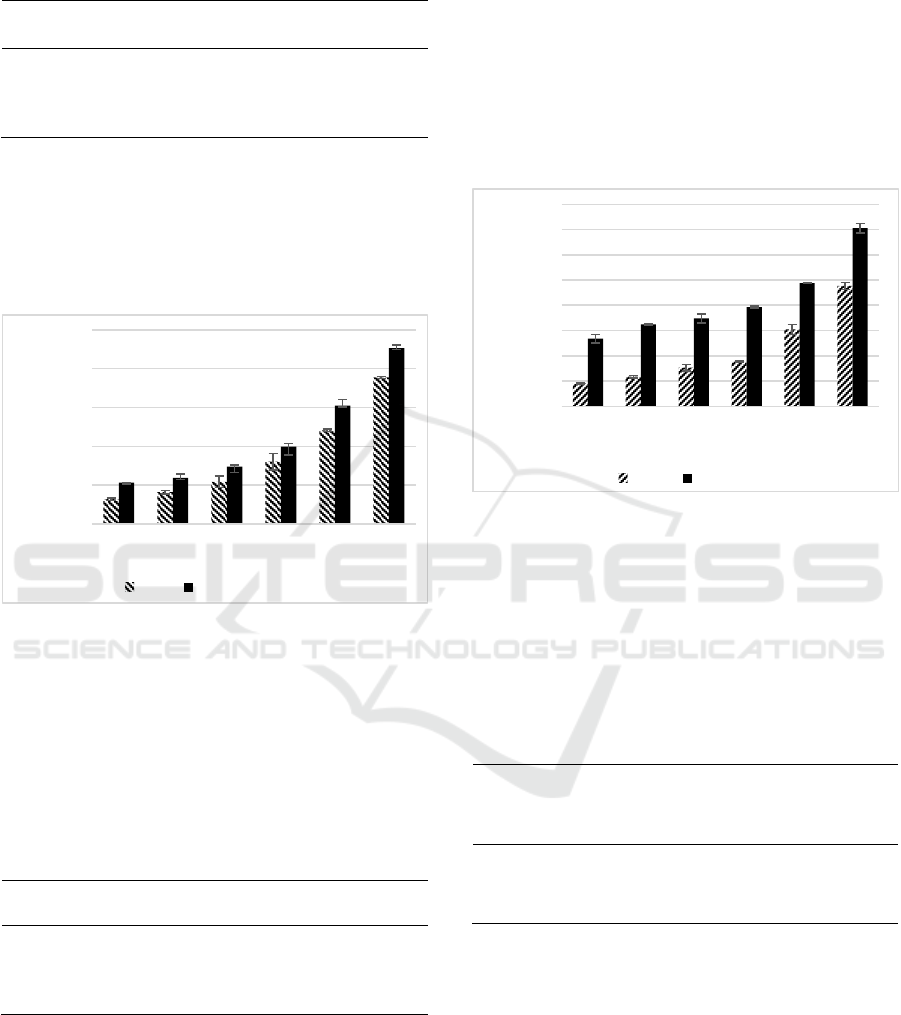
Table 4: IC
50
Value of H
2
O
2
Scavenging Activities of SEE
and CGA.
Sample Linear Equation R
2
IC
50
(µM)
IC
50
(µg/ml)
SEE y = 0.1399x +
44.473
0.97
-
103.84
CGA y = 0.1716x +
47.757
0.98
36.89
13.07
*Linear equations, coefficient of regression (R2), and IC50 of each
sample were calculated. IC50 of SEE was presented in μg/mL,
while CGA was presented in μM and μg/mL
Nitric oxide (NO) is a free radical belonging to
reactive nitrogen species (RNS) (Utami et al., 2018).
The NO
scavenging activity of SEE and CGA can be
seen in Figure 1D.
Figure 4: Effect variety concentrations of SEE, CGA
toward antioxidant activities
.
*NO scavenging activity (%) of SEE, CGA were were diluted in
DMSO to reach the final concentration of 2.08; 4.17; 8.33; 16.67;
33.33; 66.67 (µg/mL). Different small letter (a,b,c,d,e) shows
significant differences among concentration of SEE and different
capital letter (A,AB,B,C,D,E) among concentration of CGA
toward NO scavenging activity based on Tukey HSD post hoc test
(p<0.05)
Table 5: IC
50
Value of NO Scavenging Activities of SEE
and CGA.
Sample Linear Equation R
2
IC
50
(µM)
IC
50
(µg/ml)
SEE y = 1.4726x +
9.1012
0.99
-
27.77
CGA y = 1.5876x +
28.231
0.99
38.66
13.71
*Linear equations, coefficient of regression (R2), and IC50 of each
sample were calculated. IC50 of SEE was presented in μg/mL,
while CGA were presented in μM and μg/mL
SEE and CGA expressed high NO scavenging
activity. The highest concentration (66.67 µg/mL)
CGA was slightly higher compared to SEE. The
results of NO scavenging activity of SEE and CGA
with concentrations 2.08-66.67 µg/mL were in a
concentration-dependent manner (Figure 4). The IC
50
values showed that the IC
50
value of CGA was lower
(27.15 µg/mL) than SEE (38.09 µg/mL) (Table 5),
thus indicated CGA was better than SEE. Based on
another study showed that CGA has antioxidant
activity caused the hydroxyl groups that responsible
as a positive group for its antioxidant properties
(Naveed et al., 2018). OH group molecule has play
role in
•OH radical scavenging mechanism (Treml &
Karelšmejkal, 2016)
,
(Irwan et al., 2020).
Figure 5: Effect variety concentrations of SEE, CGA
toward OH Scavenging Activity.
*OH scavenging activity (%) of SEE, CGA were were diluted in
DMSO to reach the final concentration of 0.83; 1.67; 3.33; 6.67;
13.33; 26.67 (µg/mL). Different small letter (a,ab,bc,c,d,e) shows
significant differences among concentrations of SEE and different
capital letter (A,B,C,D,E,F) among concentration of CGA toward
OH scavenging activity based on Tukey HSD post hoc test
(p<0.05).
Table 6: IC
50
Value of OH Scavenging Activities of SEE
and CGA.
Sample Linear Equation R
2
IC
50
(µM)
IC
50
(µg/ml)
SEE
y = 0.959x + 13.471 0.99
-
38.09
CGA
y = 1.0826x + 20.606 0.99
76.56
27.15
*Linear equations, coefficient of regression (R2), and IC50 of each
sample were calculated. IC50 of SEE was presented in μg/mL,
while CGA were presented in μM and μg/mL
The present study has resulted, SEE and CGA
expressed high OH scavenging activity. The highest
concentration (26.67 µg/mL) CGA was higher
compared to SEE. The results of OH scavenging
activity of SEE and CGA with concentrations 0.83-
6.67 µg/mL was a concentration-dependent manner
(Figure 5). The IC
50
values showed that the IC
50
value
of CGA was lower (13.71 µg/mL) than SEE (27.77
µg/mL) showed that CGA was more active compared
a
a
b
c
d
e
A
AB
B
C
D
E
0,00
20,00
40,00
60,00
80,00
100,00
2,08 4,17 8,33 16,67 33,33 66,67
NO Scavenging Activity (%)
Concentration (μg/mL)
SEE Chlorogenic Acid
a
ab
bc
c
d
e
A
B
C
D
E
F
0,00
10,00
20,00
30,00
40,00
50,00
60,00
70,00
80,00
0,83 1,67 3,33 6,67 13,33 26,67
OH Scavenging Activity (%)
Concentration (μg/mL)
SEE Chlorogenic Acid
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
92

to SEE (Table 6). In Suica-Bunghez et al. (2016)
study was reported that S. zalacca fruit and peel
extract has antioxidant properties through DPPH
scavenging activity with value 82.68% and 73.14%
(Suica-Bunghez et al., 2016). In other side, CGA also
can scavenge free radicals and promote antioxidant
enzymatic activities based on in vivo and in vitro
studies (Shi et al., 2016).
However, all antioxidant potent (ABTS, H
2
O
2
,
NO, OH scavenging activity) of CGA had IC
50
value
< 50 μL categorized as highly active, and SEE had
IC
50
value < 50 μL for OH, NO (13.71 and 27.15
μg/mL) scavenging activities categorized highly
active and the IC
50
value of ABTS scavenging activity
of SEE 57.71 μg/mL was categorized active and H
2
O
2
scavenging activity of SEE 103.84 was categorized
moderate (Marjoni & Zulfisa, 2017). Antioxidant
activity that is strong enough in SEE may be related
to various active compounds in plants, including
flavonoid and phytochemical polyphenols
(Mazumdar et al., 2019). However, the factor that
causes CGA to show more active in free radical
scavenging activities is the number of OH groups
possessed by CGA. The more hydroxyl groups
possessed by active compounds affect the amount of
free radicals that can be scavenged (Mathew et al.,
2015).
4 CONCLUSIONS
In conclusion, S. zalacca peels extract (SEE) contains
phenolic and flavonoid content, which is potential as
an antioxidant. SEE and chlorogenic acid have an
antioxidant-activities against ABTS, FRAP, H
2
O
2
,
NO, and OH in oxidative stress parameters.
Therefore, S. zalacca peel extract and its compound
can be a potential source of antioxidants compound.
ACKNOWLEDGEMENTS
We gratefully acknowledge the financial support
from Ministry of Research and Technology/National
Research and Innovation Agency of Republik
Indonesia (Penelitian Dasar Unggulan Perguruan
Tinggi 2021). We are thankful to Ervi Afifah, Seila
Arumwardana, Hanna Sari Widya Kusuma, Cintani
Dewi Wahyuni, and Muhamad Aldi Maulana from
Biomolecular and Biomedical Research Center,
Aretha Medika Utama, Bandung, West Java,
Indonesia for their valuable assistance.
REFERENCES
Atanassova, M., Georgieva, S. (2011). Total Phenolic and
total flavonoid contents, antioxidant capacity and
biological contaminants in medicinal herbs. J. Univ.
Chem. Technol. Metallurgy. 46, 81–88.
Cetin, E. S., Babalik, Z., Hallac-Turk, F., Gokturk-Baydar,
N. (2014). The effects of cadmium chloride on
secondary metabolite production in Vitis vinifera cv.
cell suspension cultures. Biol. Res. 47(1), 1-6.
Dembitsky, V. M., Poovarodom, S., Leontowicz, H.,
Leontowicz, M., Vearasilp, S., Trakhtenberg, S.,
Gorinstein, S. (2011). The multiple nutrition properties
of some exotic fruits: Biological activity and active
metabolites. Food Res. Int. 44(7), 1671–1701.
Fitri, A., Andriani, M., Sudarman, A., Toharmat, T.,
Yonekura, L., Tamura, H., et al. (2016). Screening of
antioxidant activities and their bioavailability of
tropical fruit by products from Indonesia. Int. J. Pharm.
Pharm. Sci. 8(6), 96-100.
Girsang, E., Lister, I. N. E., Ginting, C. N., Bethasari, M.,
Amalia, A., Widowati, W. (2020). Comparison of
antiaging and antioxidant activities of protocatechuic
and ferulic acids. Mol. Cell. Biomed. Sci. 4(2), 68–75.
Girsang, E., Ginting, C. N., Nyoman, I., Lister, E.,
Widowati, W., Wibowo, S.H.B., et al. (2019). In silico
analysis of phytochemical compound found in snake
fruit (Salacca zalacca) peel as anti-aging agent. Thai J.
Pharm. 43(2), 105–109.
Girsang, E., Lister, I. N. E., Ginting, C. N., Sholihah, I. A.,
Raif, M. A., Kunardi, S., et al. (2020). Antioxidant and
antiaging activity of rutin and caffeic acid.
Pharmaciana, 10(2), 147-156.
Girsang, E, Lister, I. N. E., Ginting, C. N., Nasution, S. L.,
Suhartina, S., Munshy, U.Z., et al. (2020). Antioxidant
and anti-inflammatory activity of Salacca zalacca
(Gaertn.) Voss peel ethanolic extract on lead induced
fibroblast cells. In Proceeding of the 6th ICAMBBE
2019, 68-73.
Halliwell, B., Gutteridge, J. M. C. (2015). Free radicals in
biology and medicine. Oxford University Press, USA.
Hlásná Čepková, P., Jágr, M., Janovská, D., Dvořáček, V.,
Kotrbová Kozak, A., & Viehmannová, I. (2021).
Comprehensive Mass Spectrometric Analysis of Snake
Fruit: Salak (Salacca zalacca). J. Food Qual. 2021.
Irwan, M., Girsang, E., Nasution, A. N., Lister, I. N. E.,
Amalia, A., Widowati, W. (2020). Antioxidant
activities of black soybean extract (Glycine max (L.)
Merr.) and daidzein as hydroxyl and nitric oxide
scavengers. MKB, 52(2), 74–80.
Laitonjam, W. S. (2012). Natural antioxidants (NAO) of
plants acting as scavengers of free radicals. In Studies
in natural products chemistry, Elsevier. 37, 259-275.
Lister, I. N. E., Ginting, C. N., Girsang, E., Armansyah, A.,
Marpaung, H. H., Handayani Rr, A. S. (2019).
Antioxidant properties of red betel (Piper crocatum)
leaf extract and its compounds. J. Nat. Remedies. 19,
199–204.
Mandrone, M., Lorenzi, B., Venditti, A., Guarcini, L.,
Bianco, A., Sanna, C., et al. (2015). Antioxidant and
Antioxidant Properties of Salacca zalacca (Gaertn.) Voss Peel Ethanolic Extract Compared to Chlorogenic Acid
93

anti-collagenase activity of Hypericum hircinum L. Ind.
Crops Prod. 76, 402–408.
Marjoni, M., Zulfisa, A. (2017). Antioxidant activity of
methanol extract/fractions. Pharm. Anal. Acta., 8(8), 1–
6.
Mathew, S., Abraham, T. E., Zakaria, Z. A. (2015).
Reactivity of phenolic compounds towards free radicals
under in vitro conditions. J. Food Sci. Technol. 52(9),
5790-5798.
Mazumdar, P., Pratama, H., Lau, S. E., Teo, C. H.,
Harikrishna, J. A. (2019). Biology, phytochemical
profile and prospects for snake fruit: An antioxidant-
rich fruit of South East Asia. Trends Food Sci. Technol.
91(2019), 147-158.
Meng, S., Cao, J., Feng, Q., Peng, J., Hu, Y. (2013). Roles
of chlorogenic acid on regulating glucose and lipids
metabolism: A review. Evidence-Based Complement.
Altern. Med. 2013, 1–11.
Mokhtar, S., Leong, P., Ven, L., Aziz, N. A. (2014). Total
phenolic contents, antioxidant activities and organic
acids composition of three selected fruit extracts at
different maturity stages. J. Trop. Resour. Sustain. Sci,
2, 40–46.
Naveed, M., Hejazi, V., Abbas, M., Kamboh, A. A., Khan,
G. J., Shumzaid, M., et al. (2018). Chlorogenic acid
(CGA): A pharmacological review and call for further
research. Biomed. Pharmacother. 97(2017), 67–74.
Nurhayati, B., Rahayu, I. G., Rinaldi, S. F., Zaini, W. S.,
Afifah, E., Arumwardana, S., et al. (2018). The
antioxidant and cytotoxic effects of Cosmos caudatus
ethanolic extract on cervical cancer. Indones. Biomed.
J. 10(3), 243–249.
Prahastuti, S., Hidayat, M., Hasianna, S., Widowati, W.,
Amalia, A., Yusepany, D.T. (2019). Antioxidant
potential ethanolic extract of Glycine max (l.) Merr.
Var. Detam and daidzein. J. Phys. Conf. Ser., 1374(1),
012020.
Rusmana, D., Wahyudianingsih, R., Elisabeth, M., Balqis,
B., Maesaroh, M., Widowati, W. (2017). Antioxidant
activity of Phyllanthus niruri extract, rutin and
quercetin. Indones. Biomed. J. 9(2), 84.
Saleh, M. S., Siddiqui, M. J., Mediani, A., Ismail, N. H.,
Ahmed, Q. U., So'ad, S. Z. M., Saidi-Besbes, S. (2018).
Salacca zalacca: A short review of the palm botany,
pharmacological uses and phytochemistry. Asian Pac.
J. Trop. Med. 11(12), 645.
Shi, H., Shi, A., Dong, L., Lu, X., Wang, Y., Zhao, J., et al.
(2016). Chlorogenic acid protects against liver fibrosis
in vivo and in vitro through inhibition of oxidative
stress. Clin. Nutr., 35(6), 1366–1373.
Suica-Bunghez, I., Teodorescu, S., Dulama, I., Voinea, O.,
Ion, R. (2016). Antioxidant activity and phytochemical
compounds of snake fruit (Salacca zalacca). IOP Conf.
Ser. Mater. Sci. Eng. 133(1), 012051.
Treml, J., Karelšmejkal, K. (2016). Flavonoids as potent
scavengers of hydroxyl radicals. Compr. Rev. Food Sci.
Food Saf. 15(4), 720–738.
Utami, S, Endrini, S., Nafik, S., Lestari, I. M., Anindya, D.,
Bakar, E., et al. (2019). In vitro Antioxidant and anti-
obesity activities of freeze-dried Canarium sp.,
Averrhoa bilimbi L. and Malus domestica. Indones.
Biomed. J., 11(3), 225–237.
Utami, S., Adityaningsari, P., Sosiawan, I., Endrini, S.,
Romadhiyani, Q. (2017). Antioxidants and
anticholinesterase activities of the characterized
ethanolic of ripe sesoot (Garcinia picrorrhiza Miq.)
fruit extract (GpKar) and xanthone. Trad. Med. J.
22(2017), 160–165.
Utami, S., Sachrowardi, Q. R., Damayanti, N. A.,
Wardhana, A., Syarif, I., Nafik, S., et al. (2018).
Antioxidants, anticollagenase and antielastase
potentials of ethanolic extract of ripe sesoot (Garcinia
picrorrhiza Miq.) fruit as antiaging. J. HerbMed
Pharmacol. 7(2), 88–93.
Vijayalaxmi, S., Jayalakshmi, S. K., Sreeramulu, K. (2015).
Polyphenols from different agricultural residues:
extraction, identification and their antioxidant
properties. J. Food Sci. Tech. 52(5), 2761-2769.
Widowati, W., B, W. J., Nadya, S., Amalia, A.,
Arumwardana, S., W., Kusuma, H.S.W., Arinta, Y.
(2018). Antioxidant and antiaging activities of
Jasminum sambac extract, and its compounds. J. Rep.
Pharm. Sci. 7(3), 270–285.
Widowati, W., Fauziah, N., Herdiman, H., Afni, M., Afifah,
E., Kusuma, H. S. W., et al. (2016). Antioxidant and
anti aging assays of Oryza sativa extracts, vanillin and
coumaric acid. J. Nat. Remedies, 16(3), 88–99.
Widowati, W., Rani, A. P., Amir Hamzah, R.,
Arumwardana, S., Afifah, E., Kusuma, H. S. W., et al.
(2017). Antioxidant and antiaging assays of Hibiscus
sabdariffa extract and its compounds. Nat. Prod. Sci.
23(3), 192–200.
Xu, X., Liu, A., Hu, S., Ares, I., Martínez-Larrañaga, M. R.,
Wang, X., et al. (2021). Synthetic phenolic
antioxidants: Metabolism, hazards and mechanism of
action. Food Chem. 353, 129488.
Yun, N., Kang, J.-W., Lee, S.-M. (2012). Protective effects
of chlorogenic acid against ischemia/reperfusion injury
in rat liver: molecular evidence of its antioxidant and
anti-inflammatory properties. J. Nutr. Biochem.
23(10), 1249–1255.
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
94
