
Luteolin Possess Anti-inflammatory Effect on LPS Induced
RAW 264,7 Cell Lines
Ervi Afifah
1a
, Hartini Tiono
2b
, Philips Onggowidjaja
2c
, Selonan Susang Obeng
2d
,
Wahyu Widowati
2,* e
, Cintani Dewi Wahyuni
1f
, Cahyaning Riski Wijayanti
1g
,
Muhammad Aldi Maulana
1h
, Tri Handayani
1i
and Rizal Rizal
l,3 j
1
Aretha Medika Utama, Biomolecular and Biomedical Research Center,
Jl. Babakan Jeruk 2 No. 9, Bandung, West Java, Indonesia
2
Faculty of Medicine, Maranatha Christian University, Jl Prof Drg Surya Sumantri No 65, Bandung, West Java, Indonesia
3
Biomedical Engineering, Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia,
Depok, West Java, Indonesia
*
wahyu_w60@yahoo.com, cintanidewi@gmail.com, cahyaningwidodo@gmail.com, aldimaulana.srl@gmail.com,
mbaktrihandayani@gmail.com, rizal_biotek@yahoo.com
Keywords: Luteolin, Anti-inflammatory, PGE-2, TNF-α and IL-1β.
Abstract: BACKGROUND: Inflammation is a natural human reaction to potentially harmful effects such as tissue
stress, trauma, and microbial infection. Extended inflammation is believed related to several chronic
conditions, involving asthma, rheumatoid arthritis and even cancer. To avoid immune cells from causing more
tissue damage, inflammatory responses must be regulated. Anti-inflammatory agents are particularly
beneficial for these purposes. Luteolin is flavonoid and has potent anti-inflammatory effects. OBJECTIVE:
The study aimed to determine anti-inflammatory effect of luteolin on LPS induced RAW 264,7 cell lines.
METHOD: The MTS assay was used to determine the viability of cells and the nontoxic concentration of cell
lines. The anti-inflammatory activity was assessed with Elisa assay of inflammatory parameters including
PGE-2, TNF-α, and IL-1β using secreted cytokine levels in culture supernatants of RAW 264,7 cell line.
RESULT: The toxic concentration of luteolin was 100 μM/mL, so that the concentration was not used for
treatment. Concentrations of 4 and 20 μM/mL demonstrated high viability (>90%), they were suitable for
treatment. Luteolin 4 µM/mL significantly increased the inhibition of inflammatory cytokines PGE-2, TNF-
α and IL-1β compared to positive control. CONCLUSION: The research reported that Luteolin possesses the
anti-inflammatory effect indicated by properties of inflammatory inhibition toward PGE-2, TNF-α and IL-1β.
1 INTRODUCTION
Inflammation is a critical biological reaction to
damage that is linked to a variety of disorders
including, inflammatory bowel disease, rheumatoid
a
https://orcid.org/0000-0003-4205-2434
b
https://orcid.org/0000-0002-8050-1707
c
https://orcid.org/0000-0002-7161-9762
d
https://orcid.org/0000-0003-0608-3516
e
https://orcid.org/0000-0002-5401-7794
f
https://orcid.org/0000-0002-7764-0482
g
https://orcid.org/0000-0002-3397-099X
h
https://orcid.org/0000-0003-4724-7548
i
https://orcid.org/0000-0001-9186-9841
j
https://orcid.org/0000-0003-2783-0672
*Corresponding author
arthritis, Alzheimer's disease, cancer and
atherosclerosis (Laksmitawati et al., 2016)
The cell line model for inflammation using
macrophage cell (RAW 264.7) which is triggered by
exposure to interferon (IFN), pro-inflammatory
74
Afifah, E., Tiono, H., Onggowidjaja, P., Obeng, S., Widowati, W., Wahyuni, C., Wijayanti, C., Maulana, M., Handayani, T. and Rizal, R.
Luteolin Possess Anti-inflammatory Effect on LPS Induced RAW 264,7 Cell Lines.
DOI: 10.5220/0010744500003113
In Proceedings of the 1st International Conference on Emerging Issues in Technology, Engineering and Science (ICE-TES 2021), pages 74-80
ISBN: 978-989-758-601-9
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

cytokines, or bacterial lipopolysaccharide (LPS)
(Duque & Descoteaux, 2014; Laksmitawati et al.,
2016). LPS has been shown to enhance cytokine
activity as an inflammatory mediator (Widowati et
al., 2016; 2019; 2021). LPS contains pro-
inflammatory glycolipids, which make up the gram
negative bacterial cell wall (Boots et al., 2011). LPS-
activated macrophages and inflammatory processes
are good candidate anti-inflammatory drug
development (Laksmitawati et al., 2016; 2017;
Novilla et al., 2017; Widowati et al., 2016; 2019;
2021)
Activated macrophages produce inflammatory
mediators and pro-inflammatory cytokines including
interleukin (IL-1β) and tumor necrosis factor alpha
(TNF-α) (Duque & Descoteaux, 2014; Widowati et
al., 2016; 2019; 2021). TNF-α activates and controls
the inflammatory mechanism at the multicellular
level through the production of pro-inflammatory
cytokines such as IL-1β and IL-6.(Wang & Tan,
2015). Prolonged inflammation can result in the
overproduction of inflammatory mediators and
cytokines, which can cause cellular and tissue
disruption (Lee and Surh, 2012). Furthermore, IL-1β
and TNF-α can trigger transcriptional factors of
NFKB. Elevated NFKB activation is linked to
increased COX-2 levels, which provide a significant
role in the synthesis of prostaglandin E2 (PGE-2).
PGE-2 upregulation can result in acute inflammatory
and contribute in tumorigenesis (Bustami et al., 2020;
Widowati et al;, 2021).
Anti-inflammatory medications are essential to
treat the risk of persistent inflammation associated
with chronic illness. Over several years, natural
phytochemicals were used therapeutically, leading to
the development of anti-inflammatory medication
including non-steroid anti-inflammatory drugs
(NSAIDs) (Laksmitawati et al., 2016; 2017; Novilla
et al., 2017; Widowati et al., 2016; 2019; 2021;
Girsang et al, 2019)
Plant extracts contain bioactive compounds, the
majority of which have been shown to be free of side
effects (Mehta et al., 2010). These chemical
compounds are often used to treat inflammation.
Flavonoids, which are present in plants, have a high
anti-inflammatory ability ( Novilla et al., 2017).
Luteolin (3’,4’,5,7-tetrahydroxyflavone) is a
flavonoid that is commonly found in edible tropical
fruits such as belimbii and pineapple (Asif et al, 2013;
Vrianty et al., 2019).
The aim of this study is to determine the
antiinflammatory ability of luteolin by measuring
TNF-α, IL-1β, and PGE-2 levels in LPS-induced
murine macrophage cell line (RAW 264.7) model.
2 METHODS
2.1 RAW 264.7 Cells Culture
Mouse macrophage cell line (RAW264.7)
(ATCC
®
TIB-71T
M
) was obtained from Aretha
Medika Utama Biomolecular and Biomedical
Research Center Bandung. The macrophage cells
were cultivated in Dulbecco's Modified Eagle
Medium (DMEM) (Biowest, L0416-500) enriched
with 10% Fetal Bovine serum (FBS) (Biowest,
S1810-500) 1% Antibiotic/antimycotic (ABAM)
(Biowest, L0010100), 1% Nanomycopulitine
(Biowest, L-X16-100), 1% Amphotericin B (Gibco,
1%), 0.1% Gentamicin (Gibco, 15750045). The cells
were incubated at 37
o
C in a humidified atmospheric
incubator of 5% CO
2
until they reached confluence.
The cells were drained, harvested with Trypsin-
EDTA (Biowest, L0931-500), and centrifuged for 4
minutes at 2500 rpm (Laksmitawati et al., 2016;
2017; Novilla et al., 2017; Widowati et al., 2019;
2021).
2.2 Viability Assay
The viability assay was carried out to assess the non -
toxic concentration for the following assay, which
was tested using the 3-(4,5-dimethylthiazol-2-yl)-5-
(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H
tetrazolium (MTS) assay. In brief, 5 x10
3
cells per
well were cultured to 96-well plates in DMEM
combined with 1% pennicilin-streptomcycin 10%
FBS and incubated at 37°c for 24 hours in a
humidified atmosphere incubator with 5% CO
2
. The
medium was then washed and 180 μL of fresh
medium and 20 μL of luteolin in various
concentrations were applied in triplicate to the plate,
which was then incubated for 24 hours. The untreated
cells acted as the control. In a brief, each well
received 20 μL of CellTiter 96® AQueous One
Solution Cell Proliferation Assay (MTS) (Promega,
G3582). For 3 hours, the plate was incubated in a 5%
CO
2
incubator at 37
0
C. A microplate reader was used
to test the absorbance at 490 nm (Laksmitawati et al.,
2016; 2017; Novilla et al., 2017; Widowati et al.,
2016; 2019; 2021).
2.3 Cell Treatment and Induction for
Proinflammatory Activation
Laksmitawati et al (2016) and a modified procedure
is used to induce cells for pro-inflammatory purposes.
The cells were seeded in a 6-well plate at a density of
Luteolin Possess Anti-inflammatory Effect on LPS Induced RAW 264,7 Cell Lines
75
5x10
5
cells per well and incubated for 24 hours at 37ºc
in a humidified atmosphere of 5% CO
2
. The medium
(DMEM combined with 10% FBS and 1% penicillin
streptomycin) was then washed and supplemented
with 1.600 μL growth medium and 200 μL (Luteolin
4 and 20 μM/mL). After around 1-2 hours, the
medium was supplemented with 200 μL LPS (L4516)
and incubated for 24 hours at 37
o
C in a humidified
atmosphere with 5% CO
2
. The RAW 264.7 cells were
incubated with LPS for 24 hours before being tested
(Widowati et al., 2016; 2019; 2021; Sandhiutami et
al., 2017)
2.4 Quantitative Analysis of IL-1β,
TNF α, and PGE-2 Concentrations
The ELISA Kit Elabscience was used to determine
the concentrations of IL-1β (E-EL-M0037), TNF-α
(E-EL-M0049) and PGE-2 (E-EL-0034) in the cell-
free supernatant. Regarding that, 50 μL of stop
solution was applied, and the absorbance was read at
450 nm in a spectrophotometer (Widowati et al.,
2016; 2019; 2021; Laksmitawati et al., 2016; 2017)
2.5 Statistical Analysis
SPSS software (version 20.0) was used for data
analysis . The data where provided in the form of
mean standard deviation. Significant variations
between groups were calculated using the Analysis of
Variance (ANOVA) followed by the Tukey’s Post
Hoc Test, with P < 0.05 found statistically.
3 RESULTS AND DISCUSSION
The preliminary study to assess the effect of Luteolin
on RAW 264.7 cell viability was using MTS assay.
The assay aimed to decide the safe and non-toxic
concentration for the following assay. The MTS assay
was used to determine viability by converting yellow
tetrazolium salt into a purple formazan substance.
The percentage of viable cells was calculated by
comparing the treatment's cell value to the control.
The viability assays revealed that luteolin in the given
concentrations was still accessible for normal RAW
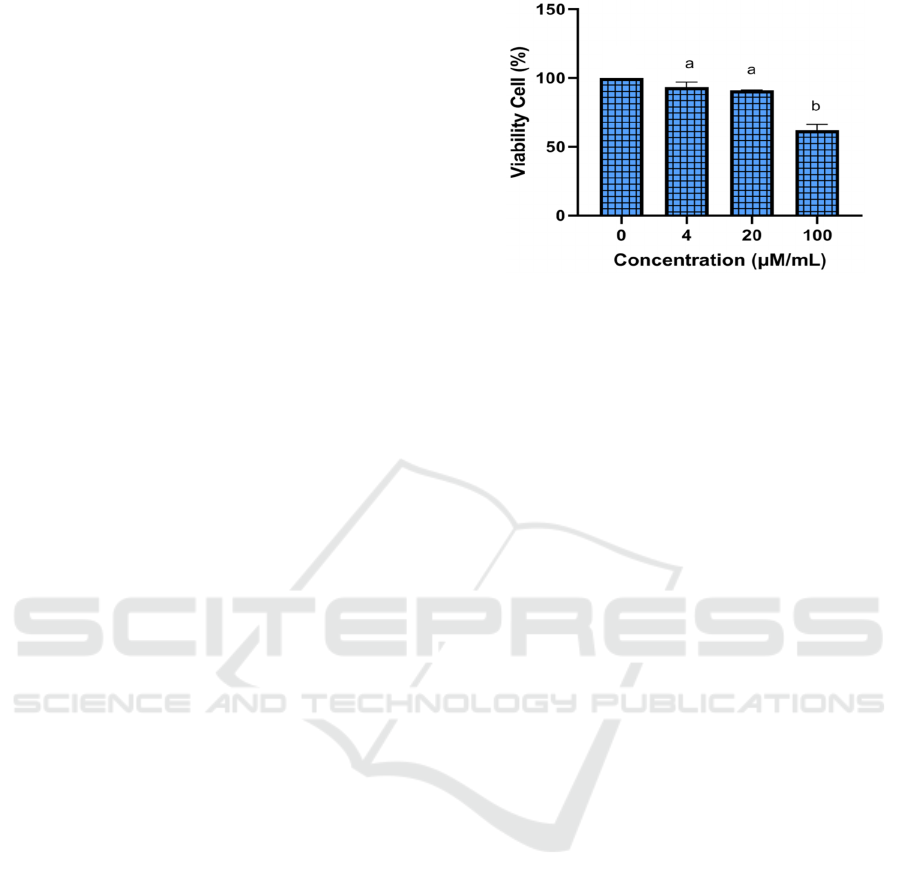
264.7 cells (Figure 1).
*The data was presented as mean ± standard deviation. The
viability of 0 μM/mL was 100%, 4 μM/mL was 93,32%, 20
μM/mL was 91,15% and 100 μM/mL was 62,02 %.
Different letter (a, b) shows significantly differences among
luteolin concentrations (4 µM , 20 µM, 100 µM) based on
Tukey’s HSD post hoc test (p<0.05).
Figure 1: Effect various concentration of Luteolin toward
RAW 264.7 cells viability.
The toxic concentration of luteolin 100 μM/mL
with viability 62,02 % was not used in this treatment.
Luteolin concentrations of 4 μM/mL with viability
93,32% and 20 μM/mL with 91,15% viability
showed good results and also no toxic on RAW
264.7. The non-toxicity of that compound was shown
by the fact that over 90% of cells were viable in
viability test using the MTS assay. The viability test
is a significant feature of pharmacology that deals
with the adverse impact of a bioactive agent on living
organisms before use as a medication or chemical in
clinical use (Jothy et al., 2011; Widowati et al., 2016;
2019; 2021; Laksmitawati et al., 2016; 2017).
LPS is a pro-inflammatory glycolipid part of
Gram-negative bacteria's cell wall that has been
shown to stimulate macrophages and increase the
synthesis of pro-inflammatory mediators such as
nitric oxide (NO), IL-1β, IL-6, and TNF-α.(Saanin et
al, 2020; Widowati et al, 2019; 2021). This condition
was shown in the present research, which showed that
the positive control (RAW 264.7 cells induced by
LPS) had significantly higher TNF-α, IL-1β and
PGE-2 concentrations than the negative control
(RAW 264.7 cells not induced by LPS) showing that
LPS is effective in increasing pro-inflammatory
mediators.
IL-1β is a powerful pro-inflammatory cytokine
released by macrophages during systemic
inflammatory responses that regulate the
inflammatory (Widowati et al., 2021). Inhibiting pro-
inflammatory mediator agent needs to discover for
further inflammatory medication. Luteolin has strong
anti-inflammatory activity.
a
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
76
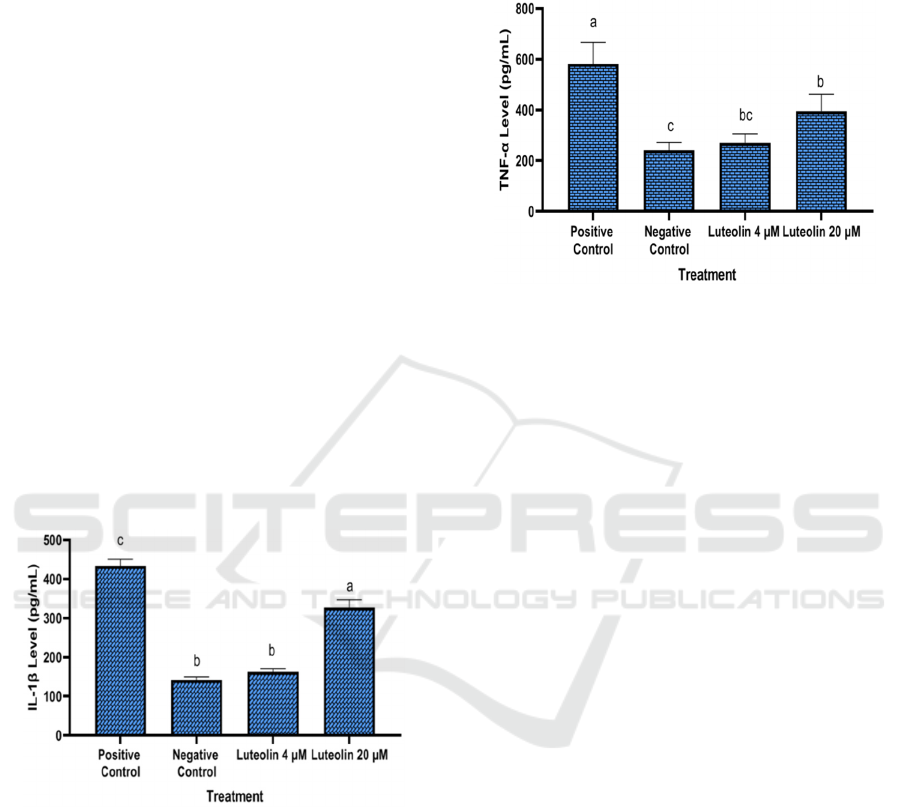
This study indicated that LPS caused
inflammation and elevated IL-1β levels in
RAW264.7, as shown by a high level of IL-1β in the
positive control and a significant difference as
opposed to the negative control. IL-1β levels in the
Luteolin treatment were lower and significantly
different from the positive control. These findings
suggest that luteolin can reduce IL-1β levels in
inflammation-induced cells. Luteolin at
concentration of 4 μM/mL greatly reduced PGE-2
levels and was significantly different from the
positive control (Figure 2).
IL-1β plays a role in homeostatic processes. IL-1β
overproduction results in physiologic changes. IL-1β
is expressed by both immune and non-immune cells
and is involved in inflammation and pain via
Caspase-1 via the inflammasomes. IL-1β may trigger
the release and/or activation of nociceptors molecules
including IL-6, prostaglandins, and MMP-9
(Goldring, et al., 2011) Inhibiting the synthesis of IL-
1β was critical in the discovery of the anti-
inflammatory drug (Widowati et al., 2018)
Based on Lami et al (2015), luteolin blocked IL-
1β mediated phosphorylation of inhibitor of NFᵏB,
unclear transcription factor-B (NFᵏB) p65,
extracellular signal-regulated kinase-1/2, and c-Jun
amino-terminal kinase (Lamy et al, 2015).
*The data was presented as mean ± standard deviation. Different
letter (a, b, c) shows significantly differences among treatment
(positive control, negative control, 4 µM, 10 µM luteloin) based on
Tukey’s HSD post hoc test (p<0.05)
Figure 2: Effect of Luteolin toward IL-1β level in LPS-
induced RAW264.7 cell.
TNF-α is a multipurpose cytokine that has
regulatory and inflammatory effects on a variety of
lymphoid and non-lymphoid cells, as well as tumor
cells (Stamatkina et al, 2011).
Based on a lower concentration of luteolin, it was
shown that luteolin has an inhibitory effect against
TNF-α synthesis as opposed to the positive control
(LPS-stimulated cells free supernatant without
luteolin). The elevated TNF-α inhibitory effect shown
by the negative control was given the low
concentration of TNF-α in the normal cell and used as
a negative control (Figure 3).
*The data was presented as mean ± standard deviation.
Different letter (a, b, bc, c) shows significantly differences
among treatment including positive control, negative
control, 4 µM, 10 µM) based on Tukye’s HSD post hoc test
(p<0.05)
Figure 3: Effect of Luteolin toward TNF-α level in LPS-
induced RAW264.7 cells.
TNF- and IL-1β act as endogenous pyrogens,
causing fever during infection by increasing
inflammatory responses and promoting the
development of chronic diseases (Damte et al, 2011).
Inhibiting TNF-α may have beneficial effects for
further inflammatory medication, according to the
previous study that was conducted by Jinxia et al
(2018), TNF-α was inhibited by luteolin as flavonoid
in RAW 264.7 macrophages. Luteolin inhibits TNF-
α production by blocking the MAPK and InB/NFᵏB
signal pathways.
Prostaglandins (PGE-2) promote cell growth and
tissue regeneration. Pro-inflammatory prostaglandins
contribute to tumor development in a variety of ways,
including cell proliferation, immunosuppression, and
angiogenesis. PGE-2 is commonly regarded as the
main target of NSAID anti-inflammatory action
(Lalier et al., 2011).
In this study, low concentration of luteolin can
inhibit the synthesis of PGE-2. The low luteolin
concentration at 4 μM exhibited the greatest
inhibitory effect, with significant differences
compared to 10 µM and it was comparable with
negative control (Figure 4).
Luteolin Possess Anti-inflammatory Effect on LPS Induced RAW 264,7 Cell Lines
77
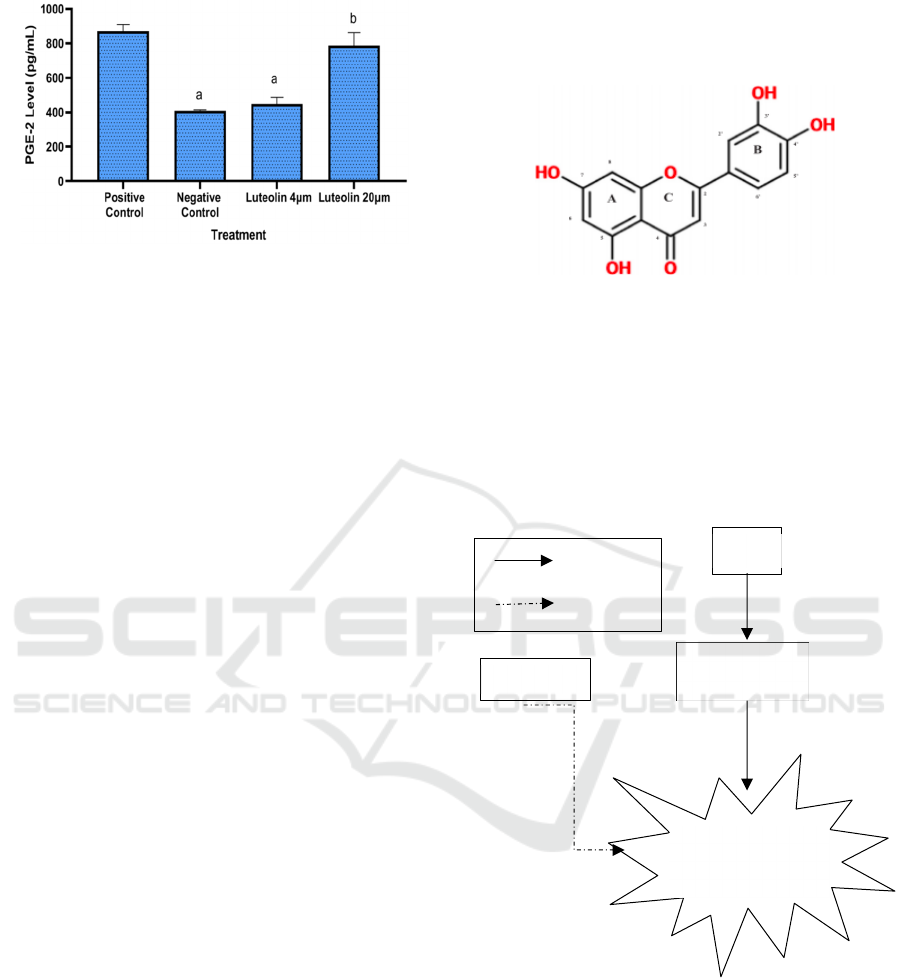
*The data was presented as mean ± standard deviation.
Different letter (a, b) shows significantly differences among
treatment (positive control, negative control 4 µM, 10 µM
luteloin) based on Tukye’s HSD post hoc test (p<0.05)
Figure 4: Effect of Luteolin toward PGE-2 level in LPS-
induced RAW264.7 cells.
PGE-2 was the extremely abundant prostaglandin
found in the human body. PGE-2 is involved in nearly
all inflammatory signals, such as redness, swelling,
and discomfort, during the inflammatory phase.
Reduced PGE-2 activity would minimize
inflammation and promote healing (Ricciotti &
Fitzgerald, 2011). Anti-inflammatory to inhibit PGE-
2 was needed to discover. Jin et al (2017) was
reported that flavonoid significantly reduced
production of PGE-2 in RAW 264.7 cells.
According to recent and previous study, luteolin
has strong anti-inflammatory activity because it is a
flavone compound present in many medicinal plants.
Flavones are a form of flavonoid that is one of the
most prevalent secondary metabolites in plants and is
commonly considered to be involved in a variety of
pharmacological activities (Aziz et al, 2018).
Flavons serve as an anti-inflammatory agent by
modulating the expression of pro-inflammatory genes
such as cyclooxigenase-2 (COX-2) and nitric oxide
synthase (NOS), as well as other cytokines. During
the inflammatory process, cyclooxygenases and
lipooxygenases play essential roles. These enzymes
are involved in the production of arachidonic acid,
which is the first step in the inflammatory process.
Since this activity produces cytokines, inhibiting
these enzymes will decrease the development of
inflammatory metabolites (Masuoka et al., 2011;
Panche et al., 2016 ).
Luteolin has a hydroxyl (-OH) group bound to the
flavone backbone structure at the 5-, 7-, 3′-, and 4’-
places. The existence of a hydroxyl group at the 3′-
position separates this flavone from the long-studied
apigenin. Flavones are distinguished by the presence
of a double bond between C2 and C3, which follows
a ketone at the C-4-position. ring's Flavones are
differentiated from flavonols by the lack of a
hydroxyl group on C3. Article presents the chemical
structure of luteolin in Figure 5 (Aziz et al., 2018).
Figure 5: The chemical structure of luteolin (Aziz et al.,
2018).
These findings contribute to the investigation of
the pharmacological application of luteolin in an in
vitro laboratory model of inflammation. Based on this
study and literature review, we summarized the
mechanism of how luteolin could act as anti-
inflammatory against LPS induced RAW 264,7 Cell
Lines (Figure 6).
Figure 6: The mechanism of luteolin as anti-inflammatory
against LPS induced RAW 264,7 Cell Lines.
4 CONCLUSIONS
This study discovered that Luteolin possesses anti-
inflammatory effects as shown by its inhibitory action
of IL-1β, TNF-α and PGE-2 secretion. The inhibitory
process by luteolin was best against PGE-2 with
62.51% over positive control. Nevertheless,
Luteolin
LPS
RAW 264,7
Cell Lines
Inflammation
(TNF-α, IL-1β, PGE-2)
Activation
b
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
78

additional tests such as clinical and preclinical trials
should be conducted before pharmaceutical
applications.
ACKNOWLEDGEMENTS
We surely appreciate that the work was carried out
successfully with the support of the Biomolecular and
Biomedical Research Center, Aretha Medika Utama,
Bandung, West Java, Indonesia. We are thankful to
Hanna Sari Widya Kusuma, Seila Arumwardana from
the Biomolecular and Biomedical Research Center,
Aretha Medika Utama, Bandung, Indonesia, for their
valuable assistance.
REFERENCES
Asif, M., Khodadi, E. (2013). 'Medicinal uses and
chemistry of flavonoid contents of some common
edible tropical plants'. Journal of Paramedical
Sciences. 4 (3), 119-138.
Aziz, N., Kim, M.Y., & Cho, J.Y. (2018). 'Anti-
inflammatory effects of luteolin: A review of in vitro,
in vivo, and in silico studies'. Journal of
Ethnopharmacology. Elsevier Ireland Ltd.
Boots, A.W., Drent, M., de Boer, V.C.J., Bast, A., Haenen,
G.R.M.M. (2011). 'Quercetin reduces markers of
oxidative stress and inflammation in sarcoidosis'
Clinical Nutrition. 30(4), 506–512.
Bustami, A., Lestari, W. P., Hayuningrum, C. F., Wuyung,
P.E., Wibowo, H., Natadisastra, R.M. (2020). 'The anti-
inflammatory effect of octyl gallate through inhibition
of nuclear factor-κB (NF-κB) pathway in rat
endometriosis model.' Journal of Reproduction and
Infertility. 21(3), 169–175. '
Damte, D., Reza, M.A., Lee, S.J., Jo, W.S., Park, S.C.
(2011). 'Anti-inflammatory activity of dichloromethane
extract of Auricularia auricula-judae in RAW264.7
cells'. Toxicological Research. Korean Society of
Toxicology.
Duque, G.A., Descoteaux, A. (2014). 'Macrophage
cytokines: Involvement in immunity and infectious
diseases.' Frontiers in Immunology. Frontiers Media
S.A.
Girsang, E., Lister, I.N.E., Ginting, C.N., Nasution, S.L.,
Suhartina, S., Munshy, U. Z., Widowati, W. (2020).
'Antioxidant and Anti-inflammatory Activity of Salacca
zalacca (Gaertn.) Voss Peel Ethanolic Extract on Lead
Induced Fibroblast Cells', (ICAMBBE 2019). 68–73.
Jinxia, L., Lu, X., Rui, S., Yifan, Y., Bingjie, G., Xuemei,
Z, (2018). 'Immunomodulatory and anti-inflammatory
effects of total flavonoids of Astragalus by regulating
NF-ΚB and MAPK signalling pathways in RAW 264.7
macrophages'. An International Journal of
Pharmaceutical Sciences. 73(10), 589-593.
Jin, Z.,Yang, Y.Z., Chen J.X., Tang, Y.Z. (2017).
'Inhibition of pro-inflammatory mediators in
RAW264.7 cells by 7-hydroxyflavone and 7,8-
dihydroxyflavone'. Journal of Pharmacy and
Pharmacology. 69(7), 865-874.
Jothy, S. L., Zakaria, Z., Chen, Y., Lau, Y. L., Latha, L. Y.,
Sasidharan, S. (2011). 'Acute oral toxicity of
methanolic seed extract of Cassia fistula in mice.'
Molecules. 16(6), 5268–5282.
Kang, C.H., Choi, Y.H., Choi, I.W., Lee, J.D., & Kim, G.
Y. (2011). 'Inhibition of Lipopolysaccharide-Induced
iNOS, COX-2, and TNF-α Expression by Aqueous
Extract of Orixa Japonica in RAW 264.7 Cells via
Suppression of NF-κB Activity'. Tropical Journal of
Pharmaceutical Research. 10(2), 161–168.
Laksmitawati, D.R., Prasanti, A.P., Larasinta, N., Syauta,
G.A., Hilda, R., Ramadaniati, H.U., Widowati, W.
(2016). 'Anti-inflammatory potential of gandarusa
(Gendarussa vulgaris nees) and soursoup (Annona
muricata L) extracts in LPS stimulated-macrophage
cell (RAW264.7).' Journal of Natural Remedies. 16(2),
73–81.
Laksmitawati, D.R., Widyastuti, A., Karami, N., Afifah, E.,
Rihibiha, D. D., Nufus, H., Widowati, W. (2017). 'Anti-
inflammatory effects of Anredera cordifolia and Piper
crocatum extracts on lipopolysaccharide-stimulated
macrophage cell line'. Bangladesh Journal of
Pharmacology. 12(1), 35- 40.
Lalier, L., Pedelaborde, F., Braud, C., Menanteau, J., M
Vallette, F., Olivier, C. (2011). 'Increase in intracellular
PGE2 induces apoptosis in Bax-expressing colon
cancer cell.' BMC Cancer. 11(1), 1–9.
Lamy, S., Moldovan, P. L., Ben Saad, A., Annabi, B.
(2015). 'Biphasic effects of luteolin on interleukin-1β-
induced cyclooxygenase-2 expression in glioblastoma
cells.' Biochimica et Biophysica Acta - Molecular Cell
Research. 1853(1), 126–135.
Lee, H., Surh, Y.J. (2012). 'Therapeutic potential of
resolvins in the prevention and treatment of
inflammatory disorders'. Biochemical Pharmacology.
84(10), 140-1350.
Masuoka, N., Matsuda, M., Kubob, I. (2012).
'Characterisation of the antioxidant activity of
flavonoids'. Food Chemistry. 131(2), 541-545.
Mehta, R.G., Murillo, G., Naithani, R., Peng, X. (2010).
Cancer chemoprevention by natural products: How far
have we come? Pharmaceutical Research. Pharm Res.
Niu, X. F., Fan, T., Li, W. F., Huang, H. (2012). 'The anti-
inflammatory effects of sanguinarine and its
modulation of inflammatory mediators from peritoneal
macrophages'. European Journal of Pharmacology.
689(1-3), 262-269.
Novilla, A., Djamhuri, D. S., Nurhayati, B., Rihibiha, D. D.,
Afifah, E., Widowati, W. (2017). 'Anti-inflammatory
properties of oolong tea (Camellia sinensis) ethanol
extract and epigallocatechin gallate in LPS-induced
RAW 264.7 cells.' Asian Pacific Journal of Tropical
Biomedicine. 7(11). 1005–1009.
Luteolin Possess Anti-inflammatory Effect on LPS Induced RAW 264,7 Cell Lines
79

Panche, A.N., Diwan, A.D., Chandra, S.R. (2016).
'Flavonoids: an overview'. Journal of Nutritional
Science. 47(5), 1-15.
Goldring, M.B., Otero, M. (2011). 'Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23(5): 471–478.
Ricciotti, E., Fitzgerald, G. A. (2011). 'Prostaglandins and
inflammation.' Arteriosclerosis, Thrombosis, and
Vascular Biology. 31(5), 986–1000.
Rusmana, D., Elisabeth, M., Widowati, W., Fauziah, N., &
Maesaroh, M. (2015). 'Inhibition of inflammatory agent
production by ethanol extract and eugenol of Syzygium
aromaticum (L.) flower bud (Clove) in LPS-stimulated
raw 264.7 cells.' Research Journal of Medicinal Plant.
9(6), 264–274.
Saanin, S., N., Wahyudianingsih, R., Merry, A., Aifah, E.,
Maesaroh, M and Widowati, W. (2020). 'Suppression
of pro-inflammatory cytokines and mediators
production by ginger (Zingiber officinale Roscoe)
ethanolic extract and gingerol in lipopolysaccharide-
induced RAW 264.7 murine macrophage cells.' Indian
Journal of Natural Products and Resources. 11(4), 260-
266.
Sandhiutami N. M. D., Moordiani M., Laksmitawati D. R.,
Fauziah N., Maesaroh M and Widowati, W. (2017). 'In
vitro assesment of anti-inflammatory activities of
coumarin and Indonesian cassia extract in RAW264.7
murine macrophage cell line.' Iran Journal Basic
Medical Sciences, 20 (1), 1-8.
Stamatkina, C. W., Rousseva, R. G., Stout, M., Coulama,
C.B., Trichec, E, Godke, R. A., Barnea, E. R. (2011).
'Preimplantation factor negates embryo toxicity and
promotes embryo development in culture'.
Reproductive BioMedicine Online. 23(4), 517-524.
Wang, W. Y., Tan, M. S., Yu, J. T., Tan, L. (2015). Role
of pro-inflammatory cytokines released from microglia
in Alzheimer’s disease. Annals of Translational
Medicine. AME Publishing Company.
Widowati, W., Darsono, L., Suherman, J., Fauziah, N.,
Maesaroh, M., Erawijantari, P. putu. (2016). 'Anti-
inflammatory effect of mangosteen (Garcinia
mangostana l.) peel extract and its compounds in LPS-
induced RAW 264.7 cells.' Natural Product Sciences.
22(3), 147–153.
Widowati, W., Prahastuti, S., Ekayanti, N.L.W., Munshy,
U.Z., Kusuma, H.S.W., Wibowo, H.S.B., Amalia, A.,
Widodo, W and Rizal R. (2019). 'Anti-Inflammation
Assay of Black Soybean Extract and Its Compounds on
Lipopolysaccharide-Induced RAW 264.7 Cell.' Journal
of Physics: Conference Series. IOP Publishing.
Widowati, W., Jasaputra, D.K., Gunawan, K.Y., Kusuma,
H.S.W., Arumwardana, S., Wahyuni, C., Lister, I.
N.E., Ginting, C.N., Girsang, E., Rizal, R. (2021).
'Turmeric Extract Potential Inhibit Inflammatoory
Marker In Lps-Stimulated Macrophage Cells'
International Journal of Applied Pharmaceutics. 13(3),
7-11.
Zhang, Q., Wan, L., Guo, Y., Cheng, N., Cheng, W., Sun,
Q., Zhu, J. (2009). 'Radiosensitization effect of luteolin
on human gastric cancer SGC-7901 cells.' Journal of
Biological Regulators and Homeostatic Agents. 23(2),
71–78.
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
80
