
Potential of Black Tea (Camellia Sinensis (L.) O. Kuntze) Extract as
Anti-oxidant and Skin Anti-aging
Wahyu Widowati
1,* a
, Rita Tjokropranoto
1b
, Cindy Damayanti
1c
, Hanna Sari Widya Kusuma
2d
,
Tri Handayani
2e
and Rizal Rizal
2,3 f
1
Faculty of Medicine, Maranatha Christian University, Jl. Surya Sumantri No. 65, Bandung, West Java, Indonesia
2
Biomolecular and Biomedical Research Center, Aretha Medika Utama, Jl Babakan Jeruk II No. 9, Bandung,
West Java, Indonesia
3
Biomedical Engineering, Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia,
Depok, West Java, Indonesia
mbaktrihandayani@gmail.com, rizal_biotek@yahoo.com
Keywords: Black Tea, Anti-oxidant, Anti-aging, Free Radicals, Collagen.
Abstract: Background: Skin aging is characterized by features such as wrinkling, loss of elasticity, laxity, rough
textured appearance, and phenotypic changes in cutaneous cells. Skin aging treatment usually with a
synthetic compound with unknown side effects but with herbal such as black tea, these side effects will be
minimalized. Objective: This research was conducted to evaluate the qualitative phytochemical screening
assay, total phenolic and flavonoid contents, anti-oxidants, and skin anti-aging properties of black tea
extract (BTE). Method: This qualitative phytochemical content using the Farnsworth modified method.
Total phenol content was calculated using gallic acid equivalent (GAE), and total flavonoid content was
calculated using quercetin equivalent (QE). The anti-oxidant properties using 2,2 diphenyl 1 picrylhydrazyl
(DPPH), 2,2′-Azinobis (3- Ethylbenzthiazoline-6-Sulfonate) (ABTS), hydrogen peroxide (H2O2)
scavenging activities. The anti-aging properties were assayed using elastase and collagenase inhibition
activities. Results: BTE contained terpenoids, triterpenoids, phenols, flavonoids, tannins. BTE contained
phenol 52.81 μg GAE/mg, flavonoids 10.96 QE/mg. The IC50 value of DPPH, ABTS, H2O2 scavenging
activities was 15.29; 88.18; 17.21 μg/ml respectively. The IC50 value of elastase and collagenase inhibition
was 31.34; 123.74 μg/ml respectively. Conclusion: BTE has highly active and active anti-oxidant and is also
less active and moderately active in skin anti-aging activities.
a
https://orcid.org/0000-0002-5401-7794
b
https://orcid.org/0000-0002-3077-9057
c
https://orcid.org/0000-0003-4181-5559
d
https://orcid.org/0000-0002-7422-0036
e
https://orcid.org/0000-0001-9186-9841
f
https://orcid.org/0000-0003-2783-0672
*
Corresponding author
1 INTRODUCTION
Aging is a normal physiological process experienced
by all creatures. Skin is the organ that is exposed to
the outer environment so skin suffers from both
intrinsic and extrinsic aging factors. Skin aging can
be characterized by wrinkling, loss of elasticity,
laxity, and rough-textured appearance (Zhang &
Duan, 2018). There are two kinds of factors
inducing skin aging intrinsic and extrinsic factors.
Intrinsic factors are mainly due to genetic and
metabolic factors (Mancini et al., 2014). Collagenase
is the enzyme to cleaving native collagen under the
physiological condition in vivo and in vitro
(Holmbeck & Birkedal Hansen, 2013; Widowati et
al., 2016; 2017; 2018a, 2018b). While elastin plays a
role in maintaining the elasticity of the skin that can
be degraded by enzymes elastase. While the factors
extrinsic including, stress, lifestyles such as smoking
and drinking alcohol, exposure to sun or UV rays.
Premature aging is most often caused by air
pollution and photoaging or UV light. The effects of
Widowati, W., Tjokropranoto, R., Damayanti, C., Kusuma, H., Handayani, T. and Rizal, R.
Potential of Black Tea (Camellia Sinensis (L.) O. Kuntze) Extract as Anti-oxidant and Skin Anti-aging.
DOI: 10.5220/0010744400003113
In Proceedings of the 1st International Conference on Emerging Issues in Technology, Engineering and Science (ICE-TES 2021), pages 65-73
ISBN: 978-989-758-601-9
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
65

photoaging exposure in the long term will result in
an increased risk of premature aging caused by
Reactive Oxygen Species (ROS) which can affect
collagenase and elastase enzymes and levels of anti-
oxidants in the body. That will make the appearance
of wrinkles and dark stains on the skin arise.
(Widowati et al., 2016; 2017; 2018a, 2018b).
To ward off free radicals in the body, humans
can produce anti-oxidant enzymes, e.g., glutathione
peroxidase (GPX), catalase (CAT), and superoxide
dismutase (SOD) (Yadav et al., 2016), but the
amount is still less effective in overcoming oxidative
stress that happens to the body. Many people
consume anti-oxidants and skin anti-aging synthesis
as cosmetics or creams, but over long periods can
cause side effects such as hyperpigmentation or
malignancy of the skin. So it is sought alternatives
from natural ingredients which have anti-oxidant
and skin anti-aging activities such as pineapple
(Jusri, 2019), dragon fruit (Liana, et al., 2019),
jasmine flowers (Widowati et al., 2018a), white rice
(Widowati et al., 2016), rosella flowers (Widowati et
al., 2017), and black tea extract have activity anti-
oxidants (Widowati et al., 2015). Tea has a bioactive
component, such as polyphenols. In general, the
classification of polyphenols exists 2, namely
phenolic acids and flavonoids (Sudaryat et al.,
2015). The function of flavonoids is to protect the
body from damage caused by ROS, inhibit
degenerative diseases, and inhibit the activity of lipid
peroxidase (Sudaryat et al., 2015). Tea also contains
theophylline, tannins, vitamin B complex, C, E, K.12.
The black tea contains bioactive compounds
alkaloids, flavonoids and tannins, phenols, saponins,
and steroids that are thought to have anti-oxidant
activity, anti-collagenase, and anti-elastase (Sudaryat
et al., 2015, Widowati et al., 2015).
This research was conducted to find out the
content of various compounds phytochemicals of
black tea extract obtained commercially
manufacture. The study also measured anti-oxidant
activity including H
2
O
2
, DPPH, and ABTS
scavenging activity, and skin anti-aging activities
including anti-collagenase and anti-elastase of black
tea extract (BTE). This research was conducted for
qualified cosmetic preparation to use certified
extract based on good manufacture practice (GMP).
2 MATERIALS AND METHODS
2.1 Sample Preparation
BTE was obtained from Indesso (SP-766-3). The
BTE using water solvent and followed by the spray
drying process, moisture content 7.0%, turbidity ≤35
FAU, polyphenol content ≥ 15%, soluble in water,
ingredient: BTE and maltodextrin (Certificate of
Analysis by Indesso, Jakarta, Indonesia).
2.2 Phytochemical Analysis
Phytochemical analysis was used to determining the
secondary metabolite content of BTE. The
phytochemical analysis in this research consisted of
flavonoid, saponin, phenolic, tannin, alkaloid,
steroid/triterpenoid, and terpenoid content of BTE.
2.2.1 Flavonoid Content
A 10 mg extract of BTE dissolved in HCl 2 N in the
reaction tube. In the mixture, Mg/Zn was added
sufficiently, then was heated for 5-10 minutes,
cooled down filtered, and added 1 ml of amyl-
alcohol. If red/orange color formed then the sample
contained flavonoid (Widowati et al., 2018a;
Prahastuti et al., 2019; Prahastuti et al., 2020).
2.2.2 Saponin Content
A 10 mg of BTE dissolved in ddH2O in the reaction
tube. The sample heated until boiling for 5 minutes
then filtered and shake strongly before added HCl 1
N. If the bubble still formed and still exist after HCL
1 N was added, then the sample contained saponin
(Widowati et al., 2018a; Prahastuti et al., 2019;
Prahastuti et al., 2020).
2.2.3 Phenolic Content
A 10 mg of BTE dissolved in 5 ml ddH
2
O. Briefly
500 µl FeCl
3
1% added into mixture. If
green/red/purple/blue/black color formed, the
sample contained phenol (Widowati et al., 2018;
Prahastuti et al., 2019; Prahastuti et al., 2020).
2.2.4 Tannin Content
A 10 mg of BTE dissolved in 2 ml HCl 2N in the
reaction tube. The mixture was heated in the water
bath for 30 minutes, cooled down, then 500 µl of
amyl alcohol was added. If there was an orange/red
color in the amyl-alcohol layer, then the sample
contained tannin (Widowati et al., 2018; Prahastuti
et al., 2019; Prahastuti et al., 2020).
2.2.5 Alkaloid Content
A 10 mg of BTE extract dissolved in 5 ml ddH
2
O
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
66

and evaporated in the water bath. The residue from
the evaporation dissolved in 5 ml HCl 2N. Then the
mixture is divided into two reaction tubes. The first
tube added 3 drops of HCl 2N as a blank. The
mixture from the second tube took 1 drop into the
dropping plate, then 3 drops of Dragendorff mixture
were added. If the orange solid formed, then the
sample contained alkaloids (Widowati et al., 2018a;
Prahastuti et al., 2019; Prahastuti et al., 2020).
2.2.6 Steroid/Triterpenoid Content
Glacial acetic acid is added into 10 mg BTE in the
dropping plate, let the mixture for 10-15 minutes. In
the mixture added 1 drop of concentrated H2SO4. If
there was greenish-blue color formed then the
sample contained steroid, but if there was a
purple/red/orange color formed then the sample
contained triterpenoid (Widowati et al., 2018a;
Prahastuti et al., 2019; Prahastuti et al., 2020).
2.2.7 Terpenoid Content
Vanillin was added into 10 mg BTE sufficiently into
the dropping plate. 1 drop of concentrated H2SO4
was added then homogenized. If there was a purple
color formed, then the sample contained terpenoid
(Widowati et al., 2018a; Prahastuti et al., 2019;
Prahastuti et al., 2020).
2.3 Total Phenolic Content
A 15 μl of a sample (BTE and gallic acid) was
loaded into the sample and blank wells. Briefly, 75
μl Folin-Ciocalteu 10% reagent was added into
sample wells. 60 μl Na2CO3 7.5% added to sample
wells. Into the blank well, 150 μl DMSO 10% was
added then incubated for 10 minutes at 50oC.
Absorbance was measured with a microplate reader
at λ = 760 nm. Gallic acid (GA) is used as a standard
to determine the phenolic concentration (Widowati
et al., 2018a; Prahastuti et al., 2019; Prahastuti et al.,
2020).
Linear standard equation: y = ax + b (1)
Total Phenol =
𝑆𝑎𝑚𝑝𝑙𝑒 𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒−𝑏
𝑎
(2)
2.4 Total Flavonoid Content
Briefly, 75 μl of a sample (BTE and Quercetin)
added to the sample and blank wells. Amount 75 μl
AlCl3 2% (in glacial acetic acid 5% and methanol)
was added into sample wells. Into the blank well, 75
μl DMSO was added. Absorbance was measured
with a microplate reader at λ = 415 nm. Total
flavonoid calculated with the standard linear
equation of Quercetin (Widowati et al., 2018a;
Prahastuti et al., 2019; Prahastuti et al., 2020).
Linear standard equation:
y
= ax + b (3)
Total Flavonoid =
𝑆𝑎𝑚𝑝𝑙𝑒 𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒−𝑏
𝑎
(4)
2.5 H2o2 Scavenging Activity
Method to determine the activity of H2O2
scavenging based on the modified method by
Prahastuti et al. (2019 and (2020). Each sample well
was contained 60 μL of BTE, 12 μL of
FeH8N2O8S2 1 mM, and 3 μL of H2O2 5 mM. The
mixture that contains 12 μL of FeH8N2O8S2 and 63
μL of DMSO was used as the negative control, while
the mixture that contains 60 μL of BTE and 90 μL of
DMSO were used as the blank solution. After H2O2
was added, the mixture was incubated in the dark
and room temperature for 5 min. The 75 μL 1,10
phenanthrolines were added into the sample and
control well and incubated again for 10 min in the
dark and room temperature. Sample absorbance was
measured at 510 nm (Prahastuti et al., 2019;
Prahastuti et al., 2020; Girsang et al., 2020).
Percentage of H2O2 scavenging activity
calculated with the equation:
% H
2
O
2
Scavenging Activity =
𝑆𝑎𝑚𝑝𝑙𝑒 𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
x 100
𝐶𝑜𝑛𝑡𝑟𝑜𝑙 𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
(5)
2.6 DPPH Scavenging Activity
Briefly, 200 µl DPPH 0.077 mmol in methanol was
added into 50 µl BTE in a microplate. Mixture
incubated at room temperature for 30 minutes then
absorbance measured at 517 nm with a microplate
reader. Amount 250 µl DPPH used as negative
control and 250 µl absolute DMSO used as blank
(Prahastuti et al., 2019; Girsang et al., 2020;
Prahastuti et al., 2020; Mawarni et al., 2020). Anti-
oxidant activity of the DPPH method (%):
% DPPH Scavenging Activity =
𝐶𝑜𝑛𝑡𝑟𝑜𝑙 𝐴𝑏𝑜𝑟𝑏𝑎𝑛𝑐𝑒−𝑆𝑎𝑚𝑝𝑙𝑒 𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
x100
𝐶𝑜𝑛𝑡𝑟𝑜𝑙 𝐴𝑏 𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
(6)
2.7 ABTS Scavenging Activity
Briefly, 2 µl sample added into 96-well plate and
then 198 µl ABTS working reagent added into the
Potential of Black Tea (Camellia Sinensis (L.) O. Kuntze) Extract as Anti-oxidant and Skin Anti-aging
67

sample well. 200 µl DMSO used as blank and 200 µl
ABTS working reagent used as control. Plate
incubated for 6 minutes at 37oC. The absorbance of
the sample was measured at 745 nm (Prahastuti et
al., 2019; Prahastuti et al., 2020; Girsang et al.,
2020; Mawarni et al., 2020). Anti-oxidant activity of
the ABTS method (%):
% ABTS Reducing Activity =
𝐶𝑜𝑛𝑡𝑟𝑜𝑙 𝐴𝑏−𝑆𝑎𝑚𝑝𝑙𝑒 𝐴𝑏
x 100
𝐶𝑜𝑛𝑡𝑟𝑜𝑙 𝐴𝑏
(7)
2.8 Collagenase Inhibition Activity
Method to measure the inhibition activity of
collagenase based on the method by Sigma Aldrich
with a little modification (Widowati et al., 2018a;
Utami et al., 2018 Girsang et al., 2020). Sample
mixture consist of 30 µl sample (7.81-250 µg/ml),
10 µl Collagenase from Clostridium histolyticum
(0.1 mg/ml, Sigma C8051), and 60 µl tricine buffer
(50 mM Tricine, 10 mM calcium chloride, 400 mM
sodium chloride, pH 7.5) incubated at 37oC for 20
minutes. 10 µl enzyme and 90 µl phosphate buffer
used as control and 10 µl enzyme, 80 µl phosphate
buffer, and 30 µlsample used as blank. 20 µl
FALGPA (1 mM, Sigma F5135) was added to each
well except blank. Sample absorbance was measured
at 335 nm. Inhibition activity calculated with the
equation:
% Collagenase Inhibition =
𝐶𝑜𝑛𝑡𝑟𝑜𝑙 𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒−𝑆𝑎𝑚𝑝𝑙𝑒 𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
x100
𝐶𝑜𝑛𝑡𝑟𝑜𝑙 𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
(8)
2.9 Elastase Inhibition Activity
Method to measure the inhibition activity of elastase
based on the method by Sigma Aldrich and
Widowati et al. (2018a) with a little modification
(Utami et al., 2018; Girsang et al., 2020; Mawarni et
al., 2020). Sample mixture consist of 10 µl sample
(2.08-66.67 µg/ml), 5 µl elastase from porcine
pancreas (0.01 mg/ml, Sigma 45124) and 125 µl Tris
buffer (100 mM, pH 8, Pharmacia Biotech 17-1321-
01) incubated at 25oC for 15 minutes. Briefly 5 µl
enzyme and 135 µl Tris buffer used as control and
130 µl Tris buffer and 10 µl sample used as blank.
Amount 10 µl SucAla3-pNA was added to each well
and incubated at 25oC for 15 minutes. Sample
absorbance was measured at 410 nm. Inhibition
activity calculated with the equation:
% Elastase Inhibition =
𝐶𝑜𝑛𝑡𝑟𝑜𝑙 𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒−𝑆𝑎𝑚𝑝𝑙𝑒 𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
x100
𝐶𝑜𝑛𝑡𝑟𝑜𝑙 𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒
(9)
2.10 Statistical Analysis
All data from H2O2, DPPH, and ABTS scavenging,
collagenase, and elastase inhibition were analyzed
statistically with ANOVA and Tukey HSD Post Hoc
Test (P<0.05). Inhibitory Concentration (IC50) using
linear regression analysis. The column graph was
formed with Graph Pad 7 Prism.
3 RESULT AND DISCUSSION
3.1 Phytochemical Analysis
Phytochemical analysis was done to know what kind
and how many secondary metabolite properties were
from the sample. Even the data obtained as
qualitative data, but data can become the base to
pick the method to measure the total content of the
secondary metabolite of the sample. BTE contains
low content of alkaloid (+), not detected saponin (-),
very high content of phenol (++++), low tannin (+),
not detected steroid (-), high triterpenoid (+++), high
terpenoid (+++), and moderate alkaloid (++).
This result is slightly different from the research
done by Widowati et al. (2015) that showed black
tea extract contains medium content on flavonoid,
terpenoid, and phenol, low content on terpenoid,
tannin, and saponin, and negative content on
alkaloid and steroid. During fermentation, catechin
is oxidized and polymerized into theaflavins (TF)
and thearubigins (TR) or degraded to another form
(Das and Datta, 2019). The difference of secondary
metabolites of BTE is caused by different
environmental and management factors (Ahmed et
al., 2019) including plant genotype (Cherotich et al.,
2013; Chen et al., 2018; Mu et al., 2018), shade
(Sano et al., 2018), elevation (Han et al., 2017;
Kfoury et al., 2018), drought (Scott et al., 2019),
precipitation (Ahmed et al., 2014; Kowalsick et al.,
2014), temperature (Ahmed et al., 2019), model of
agricultural production (Ahmed et al., 2013; Han et
al., 2018), microbes (Ahmed et al., 2019), and
numerous pest insects (Scott and Orians, 2018).
Because the source of used BTE in this research was
different from previous research resulted different
secondary metabolites (Ahmed et al., 2019). The
different extraction solvents resulted in different
compound content (Yusnawan, 2013). The different
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
68
extraction solvents resulted from different
bioactivity (Ngo et al., 2017).
3.2 Total Phenolic and Flavonoid
Content
Total phenol activity was used to know how many
phenol contained in the sample by measure the
sample + reagent with spectrophotometry. The
phenol content of BTE is 52.81±1.38 µg QE/mg
sample. Total flavonoid activity was used to know
how many flavonoids were contained in the sample
by measure the sample + reagent with
spectrophotometry. The flavonoid content of BTE is
10.96±12.46 µg QE/mg sample.
Phenol total activity of BTE is 52.81 µg
GAE/mg extract indicated that flavonoid is the most
secondary metabolites in black tea. But the
flavonoid total activity is 11.73 µg QE/mg extract,
this result was in line with previous research that
BTE has phenol standard catechin (14.33 μg
Catechin/mg), kaempferol (4.33 μg kaempferol/mg),
myricetin (4.17 μg myricetin/mg) dan quercetin
(4.30 μg quercetin/mg) (Widowati et al., 2015).
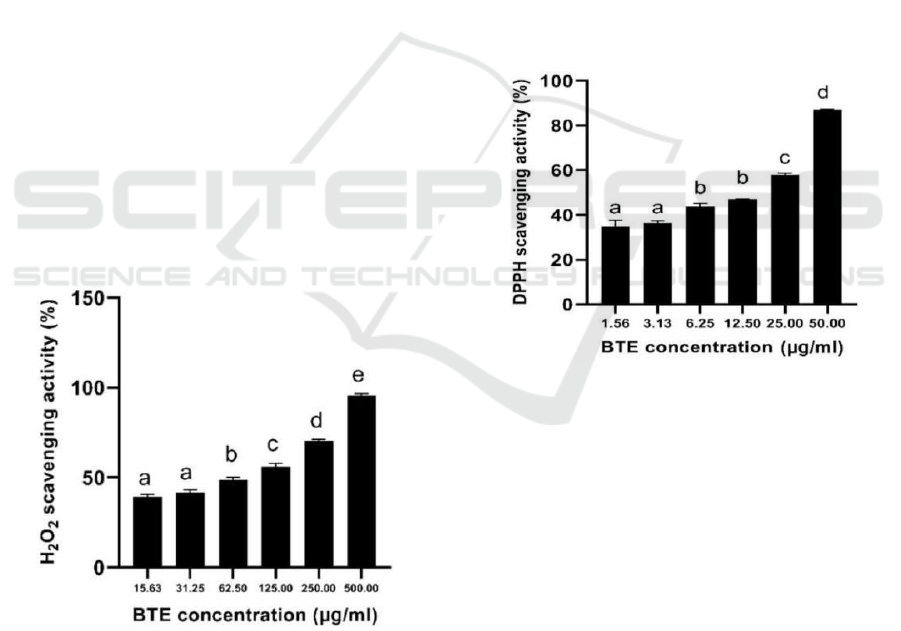
3.3 H2O2 Scavenging Activity
BTE has anti-oxidant activity in scavenging H2O2
with a higher concentration of BTE increased the
H2O2 scavenging activity (Figure 1).
Figure 1: Anti-oxidant activity of BTE at various
concentrations against H2O2 scavenging activity.
*The data was presented as mean ± SD. The assay was
done in triplicate for each concentration. The different
letter (a.b.c.d.e) showed significant difference among BTE
concentration (P<0.05).
The IC50 value of H2O2 scavenging activity was
88.18 µg/ml, according to Marjoni and Zulfisa
(2017), BTE was categorized as active when the
IC50 value <100 µg/ml. The research was done by
Fernando and Soysa (2015) the BTE has Effective
Concentration 50 (EC50) at 91.96 µg/ml. The EC50
means the half maximal effective concentration
extract to scavenge the H2O2 that same with IC50.
The IC50 of BTE is nearly the same that means the
result is valid.
3.4 DPPH Scavenging Activity
BTE has anti-oxidant activity in scavenging DPPH
with higher concentration BTE increased the DPPH
scavenging activity (Figure 2). The IC50 value of
DPPH scavenging activity of BTE is 15.29 µg/ml
which means with 15.29 µg/ml extract can inhibit
50% of DPPH become lost its radical properties, it
was categorized highly active anti-oxidant (Marjoni
and Zulfisa, 2017).
Figure 2: Anti-oxidant activity of BTE at various
concentrations against DPPH scavenging activity.
*The data was presented as mean ± SD. The assay was
done in triplicate for each concentration. The different
letter (a.b.c.d) showed significant difference among
BTE concentration (P<0.05).
Research by Leslie & Gunawan (2019), the
DPPH scavenging activity of BTE has IC50 137.60
µg/ml. The difference in the value of IC50 means
that the BTE has the differences between each
research. The differences included the genotype, the
method of extraction, the source of black tea, etc.
The DPPH scavenging activity of BTE was lower
than previous research (0.48 µg/ml) because, in this
research using filler mannitol, lactose, and starch 10-
50% which added in extract, it will lower the active
compound of BTE and decrease the anti-oxidant
activity.
Potential of Black Tea (Camellia Sinensis (L.) O. Kuntze) Extract as Anti-oxidant and Skin Anti-aging
69
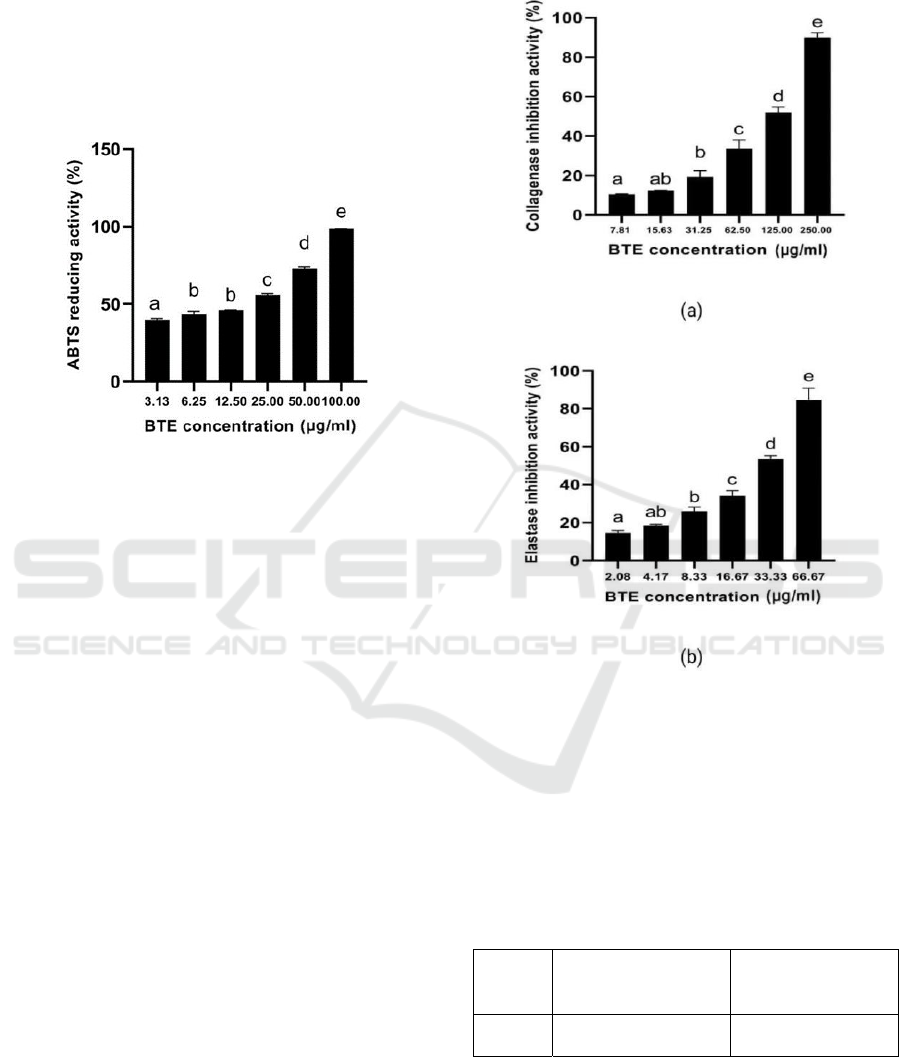
3.5 ABTS Reducing Activity
BTE has anti-oxidant activity in reducing ABTS
with higher concentration BTE increase the ABTS
reducing activity (Figure 3). The IC50 value ABTS
reducing the activity of BTE was 17.21 μg/ml, it was
categorized highly active anti-oxidant (Marjoni and
Zulfisa, 2017).
Figure 3: Anti-oxidant activity of BTE at various
concentrations against ABTS scavenging activity.
*The data was presented as mean ± SD. The assay was
done in triplicate for each concentration. The different
letter (a.b.c.d.e) showed significant difference among BTE
concentration (P<0.05).
The IC50 value of ABTS scavenging activity of
BTE was 17.68 µg/ml which means with 15.29
µg/ml, it was categorized as a very strong activity
(Marjoni and Zulfisa, 2017). This result was similar
to previous research that BTE had active anti-
oxidant because it contained high secondary
metabolites namely epigallocatechin gallate
(EGCG)> epigallocatechin (EGC)>epicatechin (EC)
= catechin. EGCG is the most effective anti-oxidant
polyphenol against free radicals (He et al., 2018).
C. sinensis tea contains high polyphenol (30%) with
EGCG consists of 9% of that total polyphenol
(Crozier et al., 2012).
3.6 Skin Anti-aging Activity
The result of the skin anti-aging activity is shown in
Figure 4 and Table 1. Based on the data (Figure 4,
Table 1) showed that a higher concentration of BTE
increased inhibition activity toward elastase and
collagenase inhibition. Based on the result, the IC50
value of collagenase inhibition activity of BTE was
categorized as less active in skin anti-aging activity
when the IC50 value > 100.00 µg/ml (Vijayakumar
et al., 2017)., and BTE has categorized moderate
active elastase inhibition activity (IC50: 15.01-50.00
µg/ml) (Vijayakumar et al., 2017).
Figure 4: Skin anti-aging activity of BTE at various
concentrations against collagenase and elastase inhibition
activity.
*The data was presented as mean ± SD. The assay was
done in triplicate for each concentration. The different
letter (a.ab,bc.d.e) for collagenase and elastase inhibition
showed significant difference among BTE concentration
(P<0.05).
Table 1: The value of IC50 for collagenase and elastase
inhibition activity of BTE.
Sample
IC
50
of Collagenase
Inhibition Activity
(µg/ml)
IC
50
of Elastase
Inhibition Activity
(µg/ml)
BTE 123.72 ± 2.44 31.34 ± 1.70
*The data was presented as mean ±SD. The IC
50
value
was
calculated according to regression linear y=a+bx
BTE has collagenase and elastase inhibition
activity with IC50 values is 123.72 µg/ml and 31.34
µg/ml, respectively. From the IC50 value, BTE has
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
70

higher elastase inhibition activity than collagenase.
The anti-aging properties of black tea are because of
the presence of high polyphenol content. The
polyphenol that is responsible for anti-aging
properties is catechin (Khanna & Maurya, 2012).
The hydroxyl groups of polyphenol and flavonoid
chemicals are effective at forming bonds with the
carboxyl groups of the serine amino acid at the
elastase enzyme's active site, altering the enzyme's
mechanism of action. As a result, elastase is unable
to cleave peptide bonds, which considerably aids in
the prevention of skin elasticity loss and wrinkle
formation (Vijayakumar et al., 2017).
Collagenase is a zinc-containing
metalloproteinase, catechins are known as metal
chelators that may bind to the Zn2+ ion within
collagenase that prevent it from binding with the
substrate (Zeng et al., 2019; Voos et al., 2021).
Therefore, polyphenol content may bind to the Zn
ionactive site, preventing the substrate from
digesting the enzyme, and this mechanism could
contribute to the collagenase inhibition activity of
BTE (Pientaweeratch et al., 2016).
4 CONCLUSIONS
Black tea extract contains phenolic compound
52.81µg QE /mg sample and total flavonoid 10.96
µg QE /mg sample. The IC50 of black tea extract in
H2O2 scavenging activity 88.17±1.69 μg/ml, DPPH
scavenging activity 15.29±0.31 μg/ml and ABTS
17.21±1.22 μg/ml. Black tea extract has active to
highly active anti-oxidant properties. Anti-aging
activity namely collagenase inhibition with IC50
123.72±2.44 μg/ml as less activity and elastase
inhibition with IC50 31.34±1.70 μg/ml as moderate
active activity show that black tea extract has less
and moderately active in anti-aging properties.
ACKNOWLEDGEMENTS
This study was supported by the Grants-in-Aid from
Aretha Medika Utama Biomolecular and Biomedical
Research Center, Bandung, Indonesia. The author is
also thankful to Ervi Afifah, Muhammad Aldi
Maulana, Cintani Dewi Wahyuni, and Cahyaning
Riski Wijayanti from Biomolecular and Biomedical
Research Center, Aretha Medika Utama, Bandung,
Indonesia for their valuable assistance.
REFERENCES
Adnyana, I. K., Abuzaid, A. S., Iskandar, E. Y. and
Kurniati, N. F. (2016). ‘Pancreatic lipase and α-
amylase inhibitory potential of mangosteen (Garcinia
mangostana Linn.) pericarp extract’. Int. J. Med. Res.
Health Sci. 5(1), 23- 28.
Ahmed, S., Griffin, T.S., Kraner, D., Schaffner, M.K.,
Sharma, D., Hazel, M., Leitch, A.R., Orians, C.M.,
Han, W., Stepp, J.R. and Robbat, A. (2019).
‘Environmental factors variably impact tea secondary
metabolites in the context of climate change’. Front
Plant Sci. 10(939), 1- 16.
Ahmed, S., Peters, C.M., Chunlin, L., Meyer, R.,
Unachukwu, U., Litt, A., Kennelly, E., Stepp, J.R.
(2013). ‘Biodiversity and phytochemical quality in
indigenous and state-supported tea management
systems of Yunnan, China’. Conserv. Lett. 6, 28–36.
Ahmed, S., Stepp, J.R., Orians, C., Griffin, T., Matyas, C.,
Robbat, A., Cash, S., Xue, D., Long, C., Unachukwu,
U., Buckley, S. (2014). ‘Effects of extreme climate
events on tea (Camellia sinensis) functional quality
validate indigenous farmer knowledge and sensory
preferences in tropical China’. PLoS One.
9(10),e109126.
Asan, T., Lister, I.N.E., Fachrial, E., Widowati, W.,
Samin, B., Liena, L. (2019). ‘Potency of black
soybean (Glycine max (L.) Merr) extracted and
daidzein as anti-oxidant and antihyaluronidase’. Trad.
Med. J. 24(1), 52-58.
Bera, T. K., Chatterjee, K., Ghosh, D. B. G. M. (2015). ‘In
vitro anti-oxidant properties of the hydro-methanol
extract of the seeds of Swienia mahagoni (L.) Jacq’.
Biomark. Genom. Med. 7, 18-24.
Chen, S., Li, M., Zheng, G., Wang, T., Lin, J., Wang, S.,
Wang, X., Chao, Q., Cao, S., Yang, Z., Yu, X. (2018).
‘Metabolite profiling of 14 wuyi rock tea cultivars
using UPLC-QTOF MS and UPLC-QqQ MS
combined with chemometrics’. Molecules. 23(2), 104-
121.
Cherotich, L., Kamunya, S.M., Alakonya, A., Msomba,
S.W., Uwimana, M.A., Wanyoko, J.K., Owuor, P.O.
(2013). ‘Variation in catechin composition of
popularly cultivated tea clones in East Africa
(Kenya)’. Am. J. Plant Sci. 4(3), 628-640.
Crozier, A., Clifford, M. N., & Del Rio, D. (2012).
Bioavailability of dietary monomeric and polymeric
flavan-3-ols. Bioavailability and function of
flavonoids: oxidative stress and disease, 30, 45-78.
Das, S., Samanta, T., Datta, A.K. (2019). ‘Analysis and
modeling of major polyphenols during oxidation in
production of black tea’. J. Food Process.
Preserv. 43(12), e14283.
Eric R.S., Xin L., Ji-Peng W., Nicole K., Joshua M.,
Ming- Ming G., Amma A., Albert R.Jr., Selena A.,
Sean B.C., Timothy S.G., John R. S., Wen-Yan H.,
Colin M. O. (2020). ‘Changes in tea plant secondary
metabolite profiles as a function of leafhopper density
and damage. Front. Plant. Sci. 11(636), 1-15.
Potential of Black Tea (Camellia Sinensis (L.) O. Kuntze) Extract as Anti-oxidant and Skin Anti-aging
71

Fernando, C.D., Soysa, P. (2015). ‘Optimized enzymatic
colorimetric assay for determination of hydrogen
peroxide (H2O2) scavenging activity of plant
extracts’. Methods X. 2(2015), 283-291.
Girsang, E., Lister, I.N.E., Ginting, C.N., Sholihah, I. A.,
Raif, M., A., Kurniadi, S., Million, H., Widowati, W.
(2020). ‘Anti-oxidant and skin anti-aging activity of
rutin and caffeic acid.’ Pharmaciana. 10(2), 147-156.
Girsang, E., Lister, I.N.E., Ginting, C.N., Khu, A., Samin,
B., Widowati, W., Wibowo, S., Rizal, R. (2019).
‘Chemical constituents of snake fruit (Salacca zalacca
(Gaert.) Voss) peel and in silico anti-aging analysis’.
Mol. Cell. Biomed. Sci. 3(2), 122-128.
Han, W.Y., Huang, J.G., Li, X., Li, Z.X., Ahammed, G.J.,
Yan, P.. Stepp, J.R. (2017). ‘Altitudinal effects on the
quality of green tea in east China: a climate change
perspective’. Eur. Food Res. Technol. 243(2), 323–
330
Han, W.-Y., Wang, D.-H., Fu, S.-W., Ahmed, S. (2018).
‘Tea from organic production has higher functional
quality characteristics compared with tea from
conventional management systems in China.’ Biol.
Agric. Hortic. 34, 120–131
He J., Lei X., Le Y., Xiaofeng W. (2018).
‘Epigallocatechin gallate is the most effective catechin
against anti-oxidant stress via hydrogen peroxide and
radical scavenging activity’. Med. Sci. Monit. 24,
8198–8206.
Holmbeck H., Birkedal-Hansen H. (2013). ‘Collagenases.
encyclopedia of biological chemistry’. Academic Press
Jusri, R., Widodo, W. S., Widowati, W., Sormin, D. E.,
Irmansyah, A., Fachrial, E., et al. (2019) ‘Comparison
of anti-oxidant and anti-hyaluronidase potentials of
pineapple core extract (Ananas comosus (L.) Merr.)
and luteolin.’ MKB. 51(2), 63–69.
Kfoury, N., Morimoto, J., Kern, A., Scott, E. R., Orians,
C. M., Ahmed, S., et al. (2018). ‘Striking changes in
tea metabolites due to elevational effects’. Food
Chem. 264, 334–341.
Khanna, A., Maurya, P.K. (2012). ‘Role of tea catechins in
prevention of aging and age-related disorders’.
CellMed. 2(1), 2-1.
Kowalsick, A., Kfoury, N., Robbat Jr, A., Ahmed, S.,
Orians, C., Griffin, T., Cash, S.B., Stepp, J.R. (2014).
‘Metabolite profiling of Camellia sinensis by
automated sequential, multidimensional gas
chromatography/mass spectrometry reveals strong
monsoon effects on tea constituents’. J. Chromatogr. A
1370, 230–239
Leslie, P.J., Gunawan, S. (2019). ‘Uji fitokimia dan
perbandingan efek antioksidan pada daun teh hijau, teh
hitam, dan teh putih (Camellia sinensis) dengan
metode DPPH (2,2-difenil-1- pikrilhidrazil)’.
Tarumanagara Medical Journal 1(2).
Liana, L., Rizal, R., Widowati, W., Fioni, F., Akbar, K.,
Fachrial, E., Lister, I.N.E. (2019). ‘Anti-oxidant and
anti-hyaluronidase activities of dragon fruit peel
extract and kaempferol-3-o-rutinoside’. J. Kedokt.
Brawijaya. 30(4), 247-252.
Mancini M., Anna M.L., Gaelle S., Christian M., Nicola
D.D., Gerry M., and Eleonora C. (2014). ‘MicroRNAs
in human skin ageing’. Ageing Res. Rev. 17, 9-15.
Marjoni, M.R. Zulfisa, A. (2017). ‘Anti-oxidant activity of
methanol extract/fractions of Senggani leaves
(Melastoma candidum D. Don)’. Pharm. Analytic.
Acta. 8(8):1-6.
Mawarni, E., Ginting, C. N., Chiuman, L., Girsang, E.,
Handayani, R. A. S., Widowati, W. (2020). ‘Anti-
oxidant and elastase inhibitor potential of petals and
receptacle of rose flower (Rosa damascena)’. Pharm.
Sci. Res. 7(2), 105-113.
Mu, B., Zhu, Y., Lv, H.-P., Yan, H., Peng, Q.-H., Lin, Z.
(2018). ‘The enantiomeric distributions of volatile
constituents in different tea cultivars’. Food Chem.
265, 329–336.
Mukhopadhyay, D., Dasgupta, P., Roy, D. S.,
Palchoudhuri, S., Chatterjee, I., Ali, S., Dastidar, S. G.
(2016). ‘A Sensitive in vitro spectrophotometric
hydrogen peroxide scavenging assay using 1, 10-
phenanthroline.’ Free Radicals & Anti-oxidants. 6(1),
124-132.
Ngo, T.V., Scarlett, C.J., Bowyer, M.C., Ngo, P.D.,
Vuong, Q.V. (2017) ‘Impact of different extraction
solvents on bioactive compounds and antioxidant
capacity from the root of Salacia chinensis L’. J. Food
Quality. ID 9305047 :1-8
Pientaweeratch, S., Panapisal, V., Tansirikongkol, A.
(2016). ‘Antioxidant, anti-collagenase and anti-
elastase activities of Phyllanthus emblica, Manilkara
zapota and silymarin: An in vitro comparative study
for anti- aging applications’. Pharm. Biol. 54(9), 1865-
1872.
Prahastuti, S., Hidayat, M., Hasianna, S.T., Widowati, W.,
Amalia, A., Yusepany, D.T., Rizal, R., and Kusuma,
H.S.W. (2019). ‘Anti-oxidant potential ethanolic
extract of Glycine max (l.) Merr. Var. Detam and
daidzein.’ J. Physics: Conf. Series. 1374, 1-12.
Prahastuti, S., Hidayat, M., Hasiana, S.T., Widowati, W.,
Widodo, W.S., Handayani, A.S., Rizal, R., Kusuma,
H. S. W. (2020). ‘The ethanol extract of the bastard
cedar (Guazuma ulmifolia L.) as anti-oxidants’.
Pharmaciana. 10(1), 77-88.
Sano, T., Horie, H., Matsunaga, A., and Hirono, Y.
(2018). ‘Effect of shading intensity on morphological
and color traits and on chemical components of new
tea (Camellia sinensis L.) shoots under direct covering
cultivation.’ J. Sci. Food Agric. 98, 5666–5676.
Scott, E. R., Li, X., Kfoury, N., Morimoto, J., Han, W.-Y.,
Ahmed, S., et al. (2019). ‘Interactive effects of
drought severity and simulated herbivory on tea
(Camellia sinensis) volatile and non-volatile
metabolites.’ Environ. Exp. Bot. 157, 283–292
Scott, E. R., and Orians, C. M. (2018). ‘Differential
changes in tea quality as influenced by insect
herbivory.’ in Stress physiology of tea in the face of
climate change, eds W.-
Y. Han, X. Li, G. J. Ahammed (Singapore: Springer),
217–240 Siregar, I.D., Kusuma, H.S.W., Widowati,
W., Marpaung, H.H., Ferdinand, S., Fachrial, E.,
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
72

Lister, I.N.E., 2019. ‘Anti-oxidant and antityrosinase
activities of ethanolic Pachyrhizus erosus peel and
tuber extract’. MKB. 51(2), 75-81.
Sudaryat, Y., Kusmiyati, M., Pelangi, C. R., Rustamsyah,
A. 2015. ‘Aktivitas antioksidan seduhan sepuluh jenis
mutu teh hitam (Camellia sinensis (L.) O. Kuntze)
Indonesia Anti-oxidant activity of ten grades of
Indonesia black tea’. Jurnal Penelitian Teh dan Kina.
18(2), 95–100.
Utami S, Sachrowardi QR, Damayanti NA, Wardhana A,
Syarif I, Nafik, S Arrahmani BC, Kusuma HSW,
Widowati W. (2018). ‘Anti-oxidants, anticollagenase
and antielastase potentials of ethanolic extract of ripe
sesoot (Garcinia picrorrhiza Miq.) fruit as skin anti-
aging’. J. Herbmed. Pharmacol. 7(2), 88-93.
Vijayakumar, R. A. M. Y. A., Gani, S., Mokhtar, N.
(2017). ‘Anti-elastase, anti-collagenase and
antimicrobial activities of the underutilized red pitaya
peel: An in vitro study for anti-aging applications’.
Asian J. Pharm. Clin. Res. 10(8), 251-255.
Voos, K., Schönauer, E., Alhayek, A., Haupenthal, J.,
Andreas, A., Müller, R., Hartmann, R.W.,
Brandstetter, H., Hirsch, A.K.H., Ducho, C. (2021).
‘Phosphonate as a stable zinc‐binding group for
“pathoblocker” inhibitors of clostridial collagenase H
(ColH)’. ChemMedChem. 16(8), 1257-1267.
Vrianty, D., Qodariah, R.L., Widowati, W., Sinaga,
A.P.F., Fibrina, D., Fachrial, E., Lister, I.N.E. (2019).
‘Comparison of anti-oxidant and anti-tyrosinase
activities of pineapple (Ananas comosus) core extract
and luteolin compound’. J. Kedok. Brawijaya. 30(4),
240-246.
Widowati, W., Herlina, T., Ratnawati, H., Constantia, G.,
Deva, I.D.G S, Maesaroh, M. (2015). ‘Anti-oxidant
potential of black, green and oolong tea methanol
extracts.’ Biol. Med. Nat. Prod. Chem.4(2), 38-43.
Widowati, W., Fauziah, N., Herdiman, H., Afni, M.,
Afifah, E., Kusuma, H. S. W., Nufus, H.,
Arumwardana, S,Rihibiha, D.D. (2016). ‘Anti-oxidant
and anti-aging assays of Oryza sativa extracts, vanillin
and coumaric acid’. J. Nat. Remed. 16(3), 88-99.
Widowati, W., Rani, A. P., Hamzah, R. A., Arumwardana,
S., Afifah, E., Kusuma, H. S. W., Rihibiha, D. D.,
Nufus, H., Amalia, A. (2017). ‘Anti-oxidant and skin
anti-aging assays of Hibiscus sabdariffa extract and its
compounds’. Nat. Prod. Sci. 23(3), 192-200
Widowati, W., Janeva, W. B., Nadya, S., Amalia, A.,
Arumwardana, S., Kusuma, H. S. W., Arinta, Y.
(2018a). ‘Anti-oxidant and skin anti-aging activities of
Jasminum sambac extract, and its compounds.’ J. Rep.
Pharm. Sci. 7(3), 270-285
Widowati W, Noverina R, Ayuningtyas W. Kurniawan D,
Faried A, Laksmitawati DR, Rihibiha DD, Rizal R,
Suciati T, Sumitro SB. Recative oxygen species and
aging mechanism (2018b). In Wilkerson S ed.,
Reactive oxygen species (ROS) mechanism and role in
health and disease. Nova Science Publishers, New
York, p 101-134 Wittenauer, J., Mackle, S., Submann,
D., Schweiggert- Weisz, U., Carle, R. (2015).
‘Inhibitory effects of polyphenols from grape pomace
extract on collagenase and elastase activity’.
Fitoterapia. 101, 179 – 187.
Yadav, A., Rewa, K., Ashwani, Y., Mishra, J.P., Seweta,
S., Shashi, P. (2016). ‘Anti-oxidants and its functions
in human body - a review’. Res. Environ. Life Sci.
9(11), 1328-1331.
Yusnawan, E. (2013). ‘The effectiveness of polar and non-
polar fractions of Ageratum conyzoides L. to control
peanut rust disease and phytochemical screenings of
secondary metabolites’. J. HPT Tropika, 13(2):159-
166.
Zeng, X., Du, Z., Xu, Y., Sheng, Z., Jiang, W. (2019).
‘Characterization of the interactions between apple
condensed tannins and biologically important metal
ions [Fe2+ (3d6), Cu2+ (3d9) and Zn2+ (3d10)].’
LWT. 114(2019), 108384.
Zhang S., Duan E. (2018). ‘Fighting against skin aging the
way from bench to bedside. Cell Transplant. 27(5),
729– 738.
Potential of Black Tea (Camellia Sinensis (L.) O. Kuntze) Extract as Anti-oxidant and Skin Anti-aging
73
