
The Optimization of the Adsorption of the Tannic Acid on the
Fluorite Mineral Surface using a Response Surface Methodology
Mariam Tangarfa
1 a
, Naoual Semlali Aouragh Hassani
1,2 b
1
Engineering Mohammadia School, Mohamed V University in Rabat, Department of Industrial Process, B.P 765, 10090
Agdal Rabat, Morocco
2
Engineering Mohammadia School, Mohamed V University in Rabat, Department of Industrial Process and Civil
Engineering, B.P 765, 10090 Agdal Rabat, Morocco
Keywords: Response surface methodology, central composite design, optimization, fluorite, tannic acid, adsorption.
Abstract: The separation flotation of fluorite containing calcite by depression using the tannic acid is complex due to
similar surface properties of calcium minerals. The improvement of this process requires an essential
evaluation of the adsorption process. Therefore, the objective of this study is the optimization of the adsorption
of the tannic acid onto fluorite. We conducted this experimental study by Central Composite Design of
response surface methodology. We used experimental results obtained to develop a statistical model at a
confidence level of 95 % using Statgraphics centurium software 18. This model revealed that the initial tannic
acid concentration and the solution pH were the most significative parameters. Therefore, we exploited the
obtained model to reach optimal conditions, allowing to achieve a maximum adsorbed tannic acid amount.
1 INTRODUCTION
Fluorite is an essential industrial mineral used to
produce essentially hydrofluoric acid (Zhang and
Song 2003; Gao et al. 2018; Zhang et al. 2018b). It
usually coexists with other calcium-containing
minerals, including calcite (Chen et al. 2019; Gao et
al. 2019). Generally, physicochemical properties of
calcium minerals such as fluorite and calcite are
similar. Both interact similarly with anionic reagents
(Liu et al. 2016). Thus, the valorisation of fluorite
containing calcite as a gangue mineral is a very
industrial problem and remains a challenging to
overcome (Ren et al. 2017a; Gao et al. 2019).
In general, flotation is the physicochemical
technique that permits to separation between fluorite
and calcite minerals (Rutledge and Anderson 2015;
Liu et al. 2016; Chen et al. 2017; Zhang et al. 2018a).
The principle of this technique is the tannic acid
adsorption on the fluorite mineral surface (Zhang et
al. 2018c). To improve the separation process
between calcium minerals, it is so important to
evaluate this primary step before reaching flotation.
To our knowledge, there are few published studies
a
https://orcid.org/0000-0003-4303-4166
b
https://orcid.org/0000-0001-5969-9459
about the evaluation of the tannic acid adsorption
process on fluorite surface. Most of them showed a
strong interaction between phenolic tannin groups
and ion calcium onto fluorite using optimization
conventionnel method of adsorption (Ren et al.
2017b; Wei et al. 2017; Gao et al. 2018; Zhang et al.
2018a). Thus, the use of an efficient method to design
and optimize the adsorption process is necessary.
In this work, we optimized the tannic acid
adsorption process on the fluorite surface by a
response surface methodology using Statgraphics
centurium 18 software. This methodology permit to
obtain a statistical model at a confidence level of 95
%. This model has been then exploited to determine
the effect of reactional parameters (initial tannic acid
concentration, solution pH and temperature) on the
studied adsorption process. Finally, we used three-
dimensional response surface methodology to
determine optimal conditions of adsorption
investigated.
Tangarfa, M. and Semlali Aouragh Hassani, N.
The Optimization of the Adsorption of the Tannic Acid on the Fluorite Mineral Surface using a Response Surface Methodology.
DOI: 10.5220/0010732600003101
In Proceedings of the 2nd International Conference on Big Data, Modelling and Machine Lear ning (BML 2021), pages 285-288
ISBN: 978-989-758-559-3
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
285
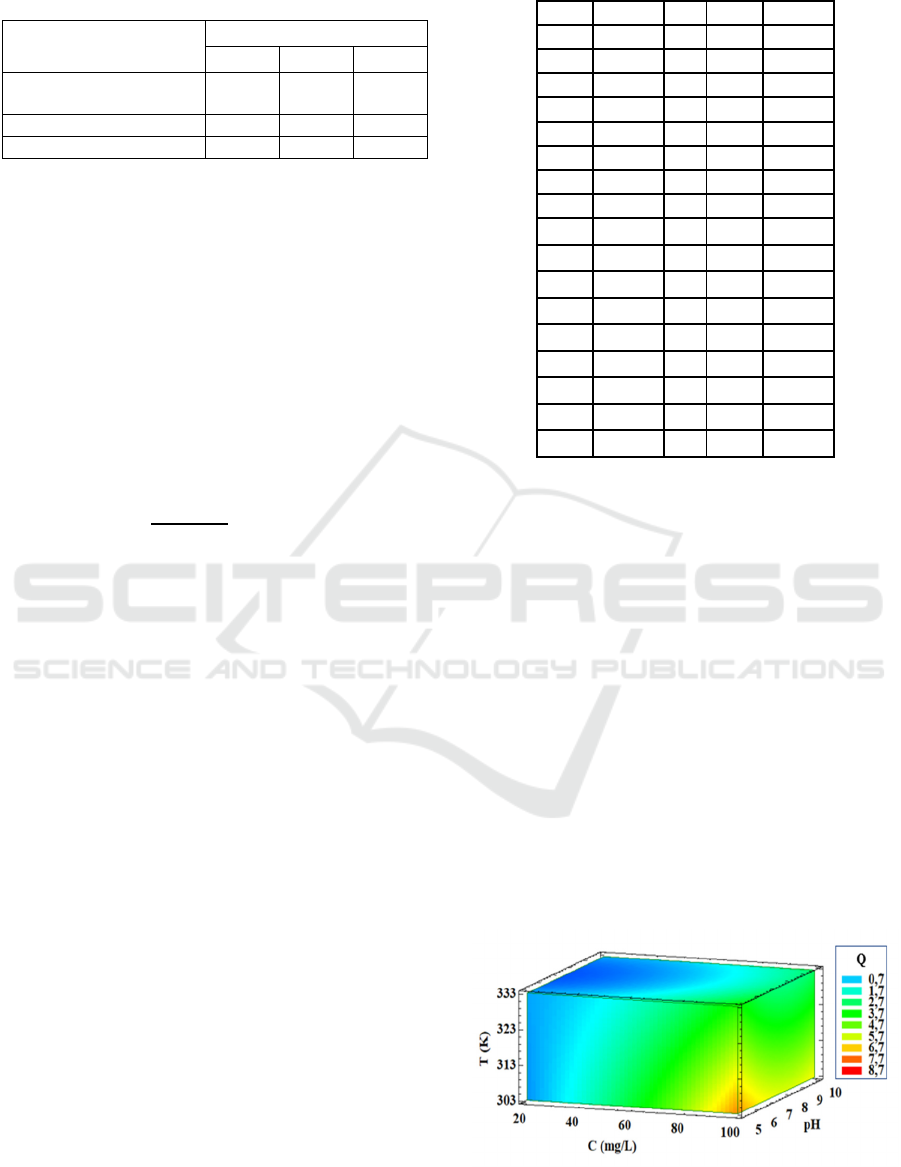
Table 1: Experimental range of independent variables and
their real and coded values.
Variables
Levels and ranges
-1 0 1
C: Initial tannic acid
concentration (mg/L)
20 60 100
p
H: Solution
p
H 5 7,5 10
T: Tem
p
erature
(
K
)
303 318 333
2 SETTINGS: ADSORPTION
EXPERIMENTAL DESIGN
we conducted adsorption experiments by mixing 1 g
of fluorite and 100 mL of distilled water using a
thermostatic water bath equiped with an electric
shaker under different conditions of the initial tannic
acid concentration, the pH and the temperature. The
mixture was analysed after equilibrium by the
spectrophotometer Ultra Violet Visible to obtain the
adsorbed tannic acid amount using the following
expression:
Q
C
C
m
∗V
(1
)
Where Ci and Ce (mg/L) are the initial and the
equilibrium tannic acid concentration, respectively. V
(L) is the solution volume, and m (g) is the adsorbent
mass.
We selected operating parameters, including
initial the tannic acid concentration, the solution pH
and the temperature to evaluate their effect on the
adsorption capacity. We chose the experimental range
of each operating parameter based on preliminary
experiments. We coded real factor values as -1, 0 and
1. Table 1 presented the experimental range, real and
coded values of these parameters.
We conducted experimental tests under Central
Composite Design CCD. We calculated the number
of CDD experiments by the following formula:
N2
2∗
k
N
(2)
Where k is the variable number, and N0 is the
replicate number of the central value of the
experimental range. We replicated this value three
times (N
0
=3) to estimate the experimental error and
data reproducibility. Using thus the equation (2) and
knowing that the variable number is 3 (k=3), we
obtained 17 tests. Table 2 summarized CDD with its
experimental results. We performed this experimental
design using Statgraphics Centurium 18.
Table 2: CDD and experimental results.
Row C
(
m
g
/L
)
p
H T
(
K
)
Q
(
m
g
/
g)
1 20 5 303 0,035
2 100 5 303 7,56413
3 20 10 303 0,0337
4 100 10 303 6,2756
5 20 5 333 0,02865
6 100 5 333 3,9976
7 20 10 333 0,016203
8 100 10 333 2,6506
9 20 7,5 318 0,01024
10 100 7,5 318 3,62935
11 60 5 318 2,31678
12 60 10 318 1,94653
13 60 7,5 303 1,76912
14 60 7,5 333 0,85021
15 60 7,5 318 1,20021
16 60 7,5 318 1,1984
17 60 7,5 318 1,1996
3 RESULTS AND DISCUSSIONS
3.1 Statistical Modelling
In the present research, experimental results of Table
2 were exploited to obtain a statistical model using
Box-Cox procedure (Box P 1964). We studied this
model by analysis of variance ANOVA using
Statgraphics Centurium 18. Table 3 summarized
obtained results of this analysis.
Based on the high correlation coefficient (0,9994)
and the low mean absolute error (0,027), the Box-Cox
model provides a good description of tannic acid
adsorption onto fluorite. In addition, ANOVA results
indicate that all parameters are significantly based on
the low P-Value (less than 0,05) except the second
term of temperature.
Figure 1:
Contours of the estimated response surface
.
BML 2021 - INTERNATIONAL CONFERENCE ON BIG DATA, MODELLING AND MACHINE LEARNING (BML’21)
286
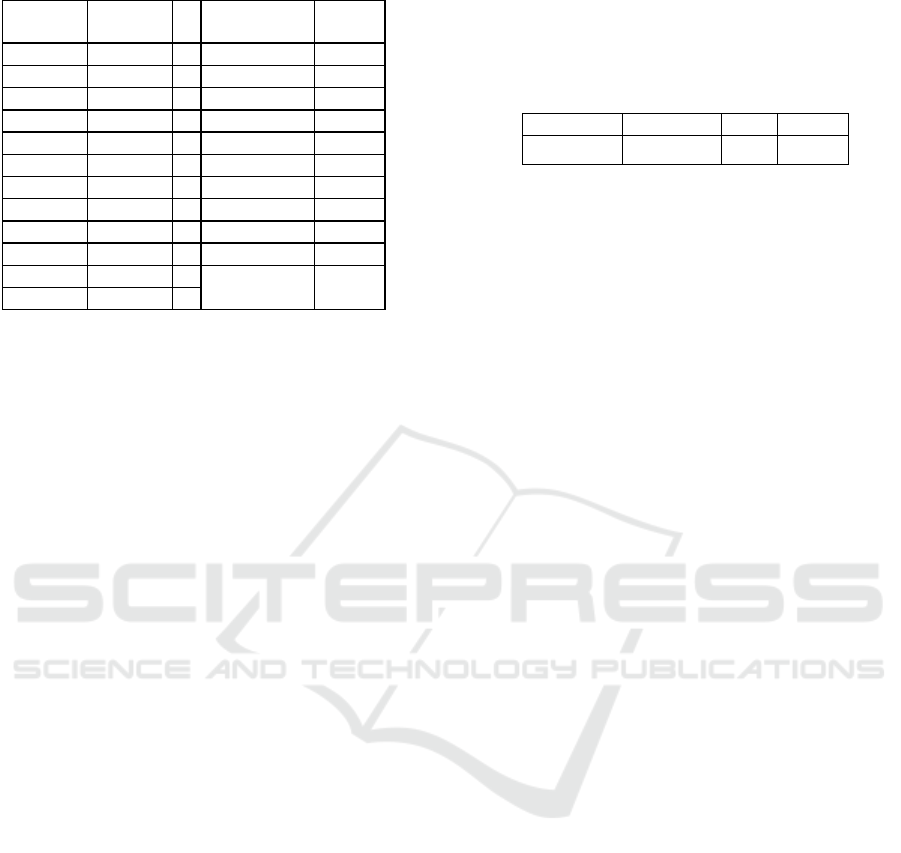
Table 3: ANOVA analysis.
Source
Sum of
S
q
uares
Df Mean Square P-Value
C 28,9465 1 28,9465 0,0000
p
H 4,97127 1 4,97127 0,0000
T 1,09445 1 1,09445 0,0000
C*C 1,52303 1 1,52303 0,0000
C*
p
H 0,0307715 1 0,0307715 0,0071
C*T 0,192934 1 0,192934 0,0000
p
H*
p
H 0,33356 1 0,33356 0,0000
p
H*T 0,0150994 1 0,0150994 0,0361
T*T 0,0032496 1 0,0032496 0,2768
Model 37,1108 9 4,12343 0,0000
Residual 0,019088 8 0,002386
Total 37,1299 17
The obtained statistical model is expressed by:
Q = 0,16*C - 0,46*pH - 0,004*T -
0,0005*C*C - 0,0004*C*pH - 0,0002*C*T
+ 0,05*pH*pH - 0,001*pH*T +
0,00001*T*T
(3)
3.2 Optimization
To optimize the tannic acid adsorption process onto
fluorite, we used a three-dimensional (3D) response
surface methodology. Figure 1 illustrates the contours
of estimated response surface. Table 4 summarize
obtained optimal conditions of the studied process.
The optimization results show that we reach a
maximum tannic acid adsorption onto fluorite using
an initial tannic acid concentration of 100 mg/L, a pH
of 5 and a temperature of 30 °C. Thus, we can
compare these results with those obtained in our
previous work (Tangarfa et al. 2021) of the
optimization of the tannic acid adsorption onto
calcite. We obtained a maximum adsorbed tannic acid
amount onto calcite under an initial tannic acid
concentration of 175 mg/L and a pH of 8. This means
that the obtained optimal conditions of the adsorption
corresponding to fluorite and calcite minerals are so
different and selective. Therefore, we can use these
results in our next works to separate between fluorite
and calcite by flotation.
4 CONCLUSIONS
The experimental study of the tannic acid adsorption
onto fluorite as a function of initial tannic acid
concentration, pH and temperature was carried out
and optimized by CDD of response surface
methodology. We considered obtained results to get a
validated statistical model checked by ANOVA
analysis. Based on the high correlation coefficient
Table 4: Optimal conditions of the tannic acid adsorption
on the fluorite surface.
Q (mg/g) C (mg/L)
p
H T (K)
6,99 100 5 303
and the low mean absolute error, we indicated that the
Box-Cox model described well the tannic acid
adsorption onto fluorite. Furthermore, we revealed
based on the low P-Value (less than 5%) that the
initial tannic acid concentration and the solution pH
influenced the studied adsorption. Optimization
results using 3D response surface methodology
showed that we reached a maximum adsorbed tannic
acid amount of about 7 mg/g using an initial tannic
acid concentration of 100 mg/L, a pH of 5 and a
temperature of 30 °C. The all above finding provides
valuable results in mineral processing, allowing to
enhance the fluorite valorisation by flotation using the
tannic acid as a depressant.
REFERENCES
Box P CR (1964) An analysis of
transformation_boxcox_1964. Journal of the Royal
Statistical Society 26:211–252
Chen W, Chen Y, Bu X, Long T, Zhang G, Chen F, Liu R,
Jia K, Song Y (2019) Rheological investigations on the
hetero-coagulation between the fine fluorite and quartz
under fluorite flotation-related conditions. Powder
Technology 354:423–431 .
https://doi.org/10.1016/j.powtec.2019.06.019
Chen W, Feng Q, Zhang G, Yang Q, Zhang C (2017) The
effect of sodium alginate on the flotation separation of
scheelite from calcite and fluorite. Minerals
Engineering 113:1–7 .
https://doi.org/10.1016/j.mineng.2017.07.016
Gao J, Hu Y, Sun W, Liu R, Gao Z, Han H, Lyu F, Jiang W
(2019) Enhanced separation of fl uorite from calcite in
acidic condition. 133:103–105 .
https://doi.org/10.1016/j.mineng.2019.01.013
Gao J, Sun W, Hu Y, Wang L, Gao Z, Chen P, Tang H,
Jiang W, Lyu F (2018) Propyl gallate : A novel
collector for flotation separation of fluorite from
calcite. Chemical Engineering Science.
https://doi.org/10.1016/j.ces.2018.09.017
Liu C, Feng Q, Zhang G, Chen W, Chen Y (2016) Effect of
depressants in the selective flotation of scheelite and
calcite using oxidized paraffin soap as collector.
International Journal of Mineral Processing 157:210–
215 . https://doi.org/10.1016/j.minpro.2016.11.011
The Optimization of the Adsorption of the Tannic Acid on the Fluorite Mineral Surface using a Response Surface Methodology
287

Ren Z, Yu F, Gao H, Chen Z, Peng Y, Liu L (2017a)
Selective separation of fluorite, barite and calcite with
valonea extract and sodium fluosilicate as depressants.
Minerals 7: . https://doi.org/10.3390/min7020024
Ren Z, Yu F, Gao H, Chen Z, Peng Y, Liu L (2017b)
Selective separation of fluorite, barite and calcite with
valonea extract and sodium fluosilicate as depressants.
Minerals 7:24 . https://doi.org/10.3390/min7020024
Rutledge J, Anderson CG (2015) Tannins in mineral
processing and extractive metallurgy. Metals 5:1520–
1542 . https://doi.org/10.3390/met5031520
Tangarfa M, Semlali N, Hassani A, Alaoui A (2021)
Preparation and characterization of calcite for tannic
acid adsorption : Optimization by response surface
methodology coupled by Box-Cox transformation
procedure. 36–45 .
https://doi.org/10.37190/ppmp/134242
Wei S, Tang H, Yin Z, Zhang C, Guan Q, Hu Y, Gao J
(2017) Selective adsorption of tannic acid on calcite and
implications for separation of fluorite minerals. Journal
of Colloid and Interface Science 512:55–63 .
https://doi.org/10.1016/j.jcis.2017.10.043
Zhang C, Sun W, Hu Y, Tang H, Yin Z, Guan Q, Gao J
(2018a) Investigation of two-stage depressing by using
hydrophilic polymer to improve the process of fluorite
flotation. Journal of Cleaner Production 193:228–235 .
https://doi.org/10.1016/j.jclepro.2018.05.055
Zhang C, Wei S, Hu Y, Tang H, Gao J, Yin Z, Guan Q
(2018b) Selective adsorption of tannic acid on calcite
and implications for separation of fluorite minerals.
Journal of Colloid and Interface Science 512:55–63 .
https://doi.org/10.1016/j.jcis.2017.10.043
Zhang C, Wei S, Hu Y, Tang H, Gao J, Yin Z, Guan Q
(2018c) Selective adsorption of tannic acid on calcite
and implications for separation of fluorite minerals.
Journal of Colloid and Interface Science 512:55–63 .
https://doi.org/10.1016/j.jcis.2017.10.043
Zhang Y, Song S (2003) Beneficiation of fluorite by
flotation in a new chemical scheme. Minerals
Engineering 16:597–600 .
https://doi.org/10.1016/S0892-6875(03)00136-5
BML 2021 - INTERNATIONAL CONFERENCE ON BIG DATA, MODELLING AND MACHINE LEARNING (BML’21)
288
