
Cardio: An Edge-enabled Wearable ECG Vest for Office Worker’s Heart
Condition Monitoring
Dimitrios Amaxilatis
1 a
, Athanasios Antoniou
1
and Ioannis Chatzigiannakis
2 b
1
SparkWorks ITC Ltd, Derbyshire DE11 8HS, U.K.
2
Department of Computer, Control, and Management Engineering “Antonio Ruberti”, Sapienza University of Rome,
00185 Rome, Italy
Keywords:
Electrocardiogram, Wearable Device, Feature Extraction.
Abstract:
Heart conditions are one of the most common health problems for people aged above 50 years, with the
percentage of people suffering from chronic heart diseases increasing year by year. These problems are more
common in modern western societies, where sedentary life and stressful lifestyles are the norms. People at
these ages are in the final steps of their professional careers and need to balance the effect of their work on
their health while staying safe and productive to achieve the best future quality of life for themselves and their
families. In this work, we present a novel wearable ECG Vest that can help them monitor in real-time their
known heart conditions while they work, reducing stress and fear. Its operation is simple enough for the device
to be worn, as a normal jacket without the need to know where exactly to connect electrodes. Its operation is
also controlled with a single button without the need for any further configuration.
1 INTRODUCTION
Health monitoring is an extremely active research
field, especially after the recent COVID-19 pandemic
crisis that altered the lives of billions of people on
the planet. Visits to hospitals have been significantly
reduced, due to the COVID-19 focused operation
of hospitals or even the people’s fear of coming in
contact with the virus. Physicians, clinics and gov-
ernments have been trying to find alternative ways
to provide their patients and citizens with effective
telemedicine and home monitoring and home care so-
lutions, to reduce the stress in hospitals and medi-
cal personnel. Detecting potentially dangerous health
conditions quickly and with minimal effect on the
people’s routines and ensuring them that they are safe
during their everyday interactions is now of utmost
importance to everyone, as societies start to emerge
from prolonged lockdowns and people start to return
to their ”normal” daily routines.
SmartWork is an EU-funded research project that
intends to provide older workers (aged 55+) with ser-
vices that help them stay safe and more productive in
their professional life. Such services can offer them
a
https://orcid.org/0000-0001-9938-6211
b
https://orcid.org/0000-0001-8955-9270
and their loved ones reassurances that their health
conditions are not deteriorated by their work. Smart-
Work uses an Internet of Things (IoT) powered un-
obtrusive sensor network to understand and observe
the older worker’s health conditions, their work en-
vironments, and their productivity in real-time. This
information is then used to provide suggestions for
behaviors and habits that have negative effects on
the health conditions of workers and their work ef-
ficiency. SmartWork achieves all that by using robust
and proven in real world trials solutions for collecting,
storing and processing of all the needed information
using resource-constrained devices that are integrated
into the final system (Chiang and Zhang, 2016).
Cardiovascular diseases, especially after the pan-
demic, are of extreme importance and require imme-
diate care, as the stress and anxiety cause by stay
at home guidelines can have serious negative effects.
Additionally diseases of the circulatory system cause
more than 1.68 million deaths every year (based on
data from 2016) (cde, ), with more than 10 million
patients that needed hospital care in 2018, depicting
the importance of early diagnosis and proper monitor-
ing. Having access to tools and methods to effectively
monitor heart conditions can give doctors the chance
to save thousands of lives every year (O’Connor et al.,
2015). The most common way to detect and iden-
420
Amaxilatis, D., Antoniou, A. and Chatzigiannakis, I.
Cardio: An Edge-enabled Wearable ECG Vest for Office Worker’s Heart Condition Monitoring.
DOI: 10.5220/0010724700003063
In Proceedings of the 13th International Joint Conference on Computational Intelligence (IJCCI 2021), pages 420-426
ISBN: 978-989-758-534-0; ISSN: 2184-3236
Copyright © 2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

tify these heart conditions is the use of an Electro-
cardiography monitoring (ECG) device. This device
can monitor the electrical activity of the heart, and
its electrical impulses generated by the polarization
and depolarization of cardiac tissue through properly
placed electrodes. ECG devices range in both sizes
and accuracy from stationary hospital-grade equip-
ment to miniature devices with lower accuracy but
higher portability and comfort for the users.
The Cardio ECG Vest is one of the SmartWork IoT
devices used to gather cardiological-health related in-
formation from office workers. It is a miniature ECG
device designed as a wearable vest that can be used
during office-related or other activities while collect-
ing ECG data in real-time and with minimal discom-
fort to the office workers. It is capable of producing
high quality, hospital grade, 12-channel ECG record-
ing with additional features such as on-board data
processing, real-time notifications while being com-
fortable to wear and easy to use. This is a huge benefit
in terms of accuracy and data quality, when compared
to other portable ECG devices that rely on a limited
number of leads with limited accuracy, such as the
AliveCor Heart Monitor
1
, the HeartCheck Pen
2
or the
Polar H10 Heart Rate Sensor
3
. The produced electro-
cardiogram (ECG) and the extracted analysis can be
used in decision-support systems to assist physicians
and cardiologists evaluate irregular heart rhythm, po-
tentially diagnose cardiac abnormalities, and predict
critical clinical states (da S. Luz et al., 2016).
A huge problem with existing solutions is the high
noise added to the ECG recording by nearby elec-
tronic devices (e.g., mobile phones, electrical wires,
appliances) or by the muscular movement of the
wearer that can severely affect the quality of the data
collected (Luo et al., 2017). The Cardio ECG Vest is
equipped with specially designed noise reduction cir-
cuits that can help reduce such interference to a min-
imum. It can also operate for long times, at least 8
hours (the full duration of a normal workday) without
the need for recharging. The data produced, are pro-
cessed to a very large degree on the device itself, elim-
inating the need to send unnecessary personal data to
a nearby smartphone or any cloud service.
Transmitting sensor data to cloud services for pro-
cessing and analysis creates several security issues
that need to be addressed as they are directly related to
the privacy of the users (Angeletti et al., 2018; Chatzi-
giannakis et al., 2011) Existing portable solutions are
cloud-centric: all personal data collected are stored
1
http://www.alivecor.com/
2
http://www.theheartcheck.com/
3
https://www.polar.com/en/products/accessories/H10 h
eart rate sensor
on the cloud and, in most cases, users have reduced
control over the data they produce, although certain
legislative actions have given users much more power
over their personal data (e.g., the EU General Data
Protection Regulation, GDPR (Commission, 2018)).
This cloud-focused architecture severely limits the
ability of the user to maintain control of personal data.
Now, more than ever, there is a need for privacy-
preserving applications where users remain always in
control of their sensitive data. (Angeletti et al., 2018;
Angeletti et al., 2017).
The goal of this paper is to present the usage of
this novel miniature ECG device and its wearable vest
design in SmartWork powered office environments to
provide workers with reassurance over their chronic
health conditions. This is achieved using lightweight
algorithms for the analysis and interpretation of ECG
sensor data that can be executed in the embedded pro-
cessor of the wearable device.
In this context, the wearable device becomes re-
sponsible for the extraction of features from the col-
lected sensor data and providing actionable alerts
without any dependence on cloud services. It is an
evolution from the traditional cloud-based or offline
(Holter ECG devices) solutions following the highly
promoted wearable approach. By following this path,
we do not only achieve a much more energy and pro-
cessing power-efficient solution but also manage to
respect users’ privacy.
The rest of the paper is structured as follows: In
Section 2 we present the miniature ECG device and
the Vest used. Section 3 describes some basic points
regarding the ECG data analysis and the steps per-
formed to extract characterizations for the worker’s
heartbeats. In Section 4 and 5 we showcase the ar-
chitecture of our system and the connectivity options
available for integrating the ECG device with a com-
panion smartphone application and the cloud. An
evaluation of the operation and the data analysis of the
Cardio ECG Vest is available in Section 6. Finally, in
Section 7 we present our conclusions and next steps.
2 THE ECG DEVICE
The wearable hardware device consists of a small
main board responsible for the core processing func-
tions, communication with a smart device and ten (10)
ECG sensor pads which are attached via a ribbon ca-
ble to the main board.
The main board features the ultra low power sys-
tem on chip (SoC) nRF52840, with Low Energy Blue-
tooth capabilities, and a number of peripheral mod-
ules, such as an Real-Time Clock, an Inertial Mea-
Cardio: An Edge-enabled Wearable ECG Vest for Office Worker’s Heart Condition Monitoring
421

Figure 1: The main board of the Cardio ECG device.
surement Unit, an external Flash Memory module,
a LED, a multi-functional push button and a power
and battery charging circuit. The nRF52840 is built
around a 32-bit ARM Cortex M4F CPU which, as
the name indicates, has a dedicated hardware floating-
point unit (FPU). It also has a Bluetooth 5, IEEE
802.15.4-2006, 2.4 GHz transceiver, which is back-
wards compatible with the BLE 4 communication
protocol ensuring compatibility with a wide range of
smart devices in the market. A MCP79411 RTC mod-
ule provides timestamps for the ECG sampling ses-
sions and can also be used to set alarms for the de-
vice to wake up at specific time or intervals in or-
der to further reduce power consumption. The Iner-
tial Measurement Unit is the LSM6DSRXTR module
which features a 3D accelerometer and gyroscope and
can provide 16 bit samples at a configurable rate and
scale, based on the required sensitivity of the mea-
surements by the application. This module also has
a few embedded functions that can provide interrupts
to the main SoC for events such as significant move-
ment, or taps, which can be utilized to detect inac-
tivity periods to put the device to sleep mode, or as
wake-up signals for the device. The (W25Q128JVSIQ
flash memory chip is a NOR Flash memory with a 16
MBs storage capacity, which can store up to about 46
minutes of 12bit ECG samples from 8 analogue leads
sampled at a rate of 500Hz. The memory can also be
used for storing user profiles and application settings,
as well as pre-compiled machine learning models for
the classification of the sampled data. The ECG sen-
sor pads have electrodes that provide the eight (8) ana-
log input signals to the internal 12bit ADC module of
the nRF52840 SoC. These eight signals are used to
produce the 12-lead ECG of the subject patient.
With the support of Nordic Semiconductor’s pre-
compiled library, SoftDevice S140, which is special-
Figure 2: The Cardio ECG Vest.
ized for the nRF52 SoC series, implementing the
Bluetooth protocol stack and providing an easy to use
API to configure Bluetooth connectivity, the applica-
tion firmware can construct a custom high level com-
munication protocol to facilitate the interactions with
a client Bluetooth enabled smart device. Our protocol
exposes a custom Bluetooth Service, and a number of
child BLE characteristics which are used to receive
commands, stream sampled or processed data and
notify for device status changes and special events.
Specifically, there are characteristics that handle is-
sued commands and their execution status. Such com-
mands include starting and stopping the sampling of
ECG and IMU data, setting and reading the RTC time,
resetting the device, formatting the external mem-
ory, and resetting to factory defaults. The ECG data
are streamed in a dedicated BLE characteristic, with
packet sizes that are set based on whether the device
operates in BLE4 or BLE5 mode. BLE5 mode al-
lows for a high throughput of up to 2Mbps, facili-
tating greater sampling rates. Similarly, the sampled
data from the IMU are streamed from a separate char-
acteristic and independently of the ECG sampling.
Another import BLE characteristic is used to notify
the calculated extracted features on the sampled data,
which can be used to either form training vectors for
our machine learning models or used for the classifi-
cation of the active ECG data stream. Finally, aux-
iliary exposed characteristics include one providing
information on the firmware and configuration of the
device, an indicator of the device’s charging status,
and one notifying periodically of the current battery
power level.
The whole system is powered by a high capacity
402030 3.7V 500mAh Lithium-ion battery cell. It also
supports battery charging via a micro-USB port and
has an onboard extension port for wireless charging.
SmartWork 2021 - 2nd International Workshop on Smart, Personalized and Age-Friendly Working Environments
422

3 ECG DATA PROCESSING
The analysis of an ECG recording can be performed
by many available methods in an embedded device.
Most of these techniques offer low accuracy lev-
els and result in a high number of alerts (Shah and
Rubin, 2007). Machine learning techniques (Saini
et al., 2013) and deep neural networks (DNN) (Ka-
plan Berkaya et al., 2018) have been tested and
showed that can achieve higher levels of accuracy in
diagnosing heart conditions using appropriate mod-
els and relying on high-performance computing in-
frastructures. To use them in low-power embedded
hardware with limitations in memory and processing
power, proper transformations are needed, using hard-
ware ((Du et al., 2017; Gokhale et al., 2017)) and
algorithmic solutions ((Luo et al., 2017; Qin et al.,
2020; for the Advancement of Medical Instrumenta-
tion, 2013)).
Our processing over the sampled data on the main
ECG device tries to follow the best outcomes from
the above approaches to get the best accuracy with
the lowest penalties on alert numbers and power con-
sumption. Our aim is to produce a dataset of extracted
features from the ECG signals recorded. These fea-
tures can the be used to provide descriptive vectors
on consecutive segments of an actively sampled ECG
that is cross-checked and validated by a separate pro-
cessing module on the client smart device or even on
the ECG device itself.
In the current implementation, we use a single 12
bit lead signals as the input to extract descriptive fea-
tures from. The feature extraction process takes place
over ten (10) second intervals of the input signal, and
employs a basic implementation of the Pan-Tompkins
algorithm (Pan and Tompkins, 1985) for QRS com-
plex detection. The extracted features include:
• the average R-R interval in milliseconds,
• the count of R peaks,
• the average R-amplitude,
• the average S-trough amplitude,
• the average R-S peak-to-peak amplitude and
• a history of the 5 most recently calculated R-R
intervals
The implementation uses variables to adapt for
various sample rates on the input ECG signal and
noisy segments of data. The whole feature vector can
be used to classify this 10-second segment in one of
3 generic groups, normal ECG recording, noisy ECG
recording, marked ECG recording. The marked ECG
recordings are those that display characteristics that
need to be reviewed by the medical practitioner in or-
der to understand if they are actually showing a phys-
iological condition that needs to be further checked.
4 DEVICE TO SMARTPHONE
CONNECTION
The ECG device itself has limited interaction with the
user. It is equipped with an LED light for simple bat-
tery charging and operation indications and a single
button used to power on the device from its deep sleep
state. Starting and stopping ECG recordings is done
using a companion application in the user’s smart-
phone, with which the device is communicating over
Bluetooth-LE.
While the phone is in the vicinity of the ECG de-
vice it can receive in real time both the full ECG
recording, IMU data, results from the on-board fea-
ture extraction, battery levels and notifications (ECG
or device related). Each one of these sources is a sep-
arate BLE characteristic that the smartphone can sub-
scribe to, in order to receive it form the ECG device
according to the BLE5 specification. In order to fit all
these data in the BLE notification packets and avoid
hardware related restrictions, we attempted to limit as
much the number of notifications generated by the de-
vice, increasing the amount of data we send in batches
during each notification. For this, each ECG data noti-
fication contains a total of 19 ECG samples with each
sample consisting of 8 12-bit values, while each IMU
data notification contains 19 full samples with each
sample containing 6 values of 2 bytes. In this con-
figuration, we manage to keep a constant flow of data
from the ECG device to the smartphone with minimal
packets lost. For example, in a 3 minute recording
window, the number of packets lost less than 300 out
of a total of 90000 (around 0.003%). The same be-
haviour is observed with the IMU data packets, where
in the same period, around 50 packets are lost out of a
total of 18000 (again around 0.003%). Similar num-
bers are achieved even when only one of the notifica-
tions is enabled, letting us believe that this behavior
is not related to the actual BLE medium but to the
handling of the packets on the smartphone side or the
generation of the packets in the ECG device’s side.
As this was observed using multiple phones with dif-
ferent specifications, the most probable cause is the
later.
The smartphone application itself does minimal
processing on the received data. It displays the re-
ceived ECG in a simple graph for the user to see that
everything is operating normally, the battery level of
the ECG device, and some of the features provided by
Cardio: An Edge-enabled Wearable ECG Vest for Office Worker’s Heart Condition Monitoring
423
the device. The most important task is the storage of
the real-time ECG recording on the phone’s storage so
that it can then be shared with a medical practitioner
as needed. The file is stored in a properly encrypted
format to avoid any usage without the direct consent
of the worker.
5 SMARTPHONE TO CLOUD
CONNECTION
To provide the data collected on the phone to their
medical practitioner, users have two options. The first
one is the most simple, by physically giving their
phone to their doctor to review the data collected.
This is no a very sophisticated approach but simpli-
fies all the actions needed to safeguard personal data,
as no information leaves the user’s phone ever. The
second option is to properly share the recordings with
the medical practitioners via our cloud services. In
this flow, the user uploads the recording to the Car-
dio service and shares it with the doctor’s account.
The doctor by accepting the recording receives the
keys to access it and sends a data access receipt to the
worker/patient. During the whole flow, the data are
encrypted, and the worker has the option to revoke
access at any time.
This whole flow is implemented using Amazon
Web Services
4
. In more detail we are using Amazon
Cognito & IAM to manage users and their permis-
sions, Amazon S3 to store and share recordings, AWS
SNS for sending notifications to users, and Amazon
Lambda to implement our serverless API for sharing
and getting access to ECG recordings and user infor-
mation (such as access receipts).
6 EVALUATION
To prove our system’s operation we performed a se-
ries of tests on the ECG devices using both ECG sim-
ulators and in-lab test patients. As soon as the sys-
tem’s operation is proven, we will extend our testing
during the SmartWork’s trials, where workers will use
the ECG vest to perform a number of recordings of
variable intervals to help us understand better (1) the
ease of use of the ECG Vest itself, (2) the ease of use
of the Cardio application interface, (3) the quality of
the data recorded on various body types, (4) the qual-
ity of the feature extraction algorithms with real world
data.
4
https://aws.amazon.com/
From the performed in-lab tests we performed, we
hereby present some results that depict the key fea-
tures of the operation of our system.
Figure 3: Recorde ECG pulses - L1 channel.
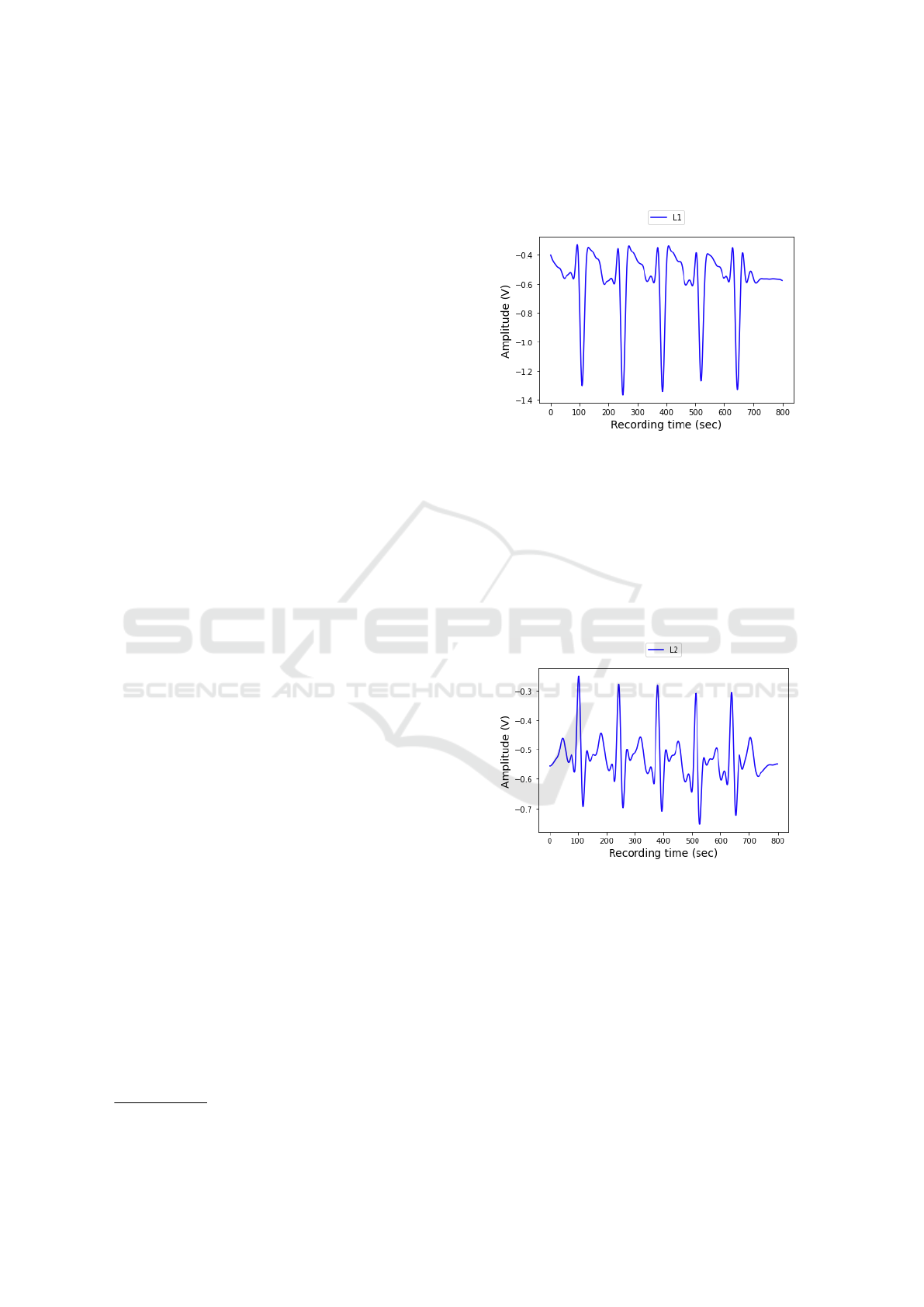
Fig. 3 and Fig. 4 show 5 consecutive recorded
heartbeats recorded from an in-lab test patient dur-
ing a larger recording. These figures give us a better
understanding of how heartbeats are depicted in dif-
ferent channels and how their PQRST characteristics
look like. Based on this view we are able to extract
the needed characteristics (as we described in Sec-
tion 3), like R-R interval, the R-amplitude or the R
peak count.
Figure 4: Recorde ECG pulses - L2 channel.
For each one of the features, we present here the
calculated values from the onboard algorithm. We
start with the R-R interval, which gives us an esti-
mate of the heart rate of the patient and its variance
during each interval. Fig. 5 shows the calculated R-R
interval from the ECG device (tumbling R-R) and the
calculated value using a sliding window (sliding R-R)
instead of a tumbling one as they are calculated on the
smartphone application. As we see, values using the
tumbling window are more smooth than the sliding
one, as they are not so much affected by noise in the
ECG signal.
SmartWork 2021 - 2nd International Workshop on Smart, Personalized and Age-Friendly Working Environments
424
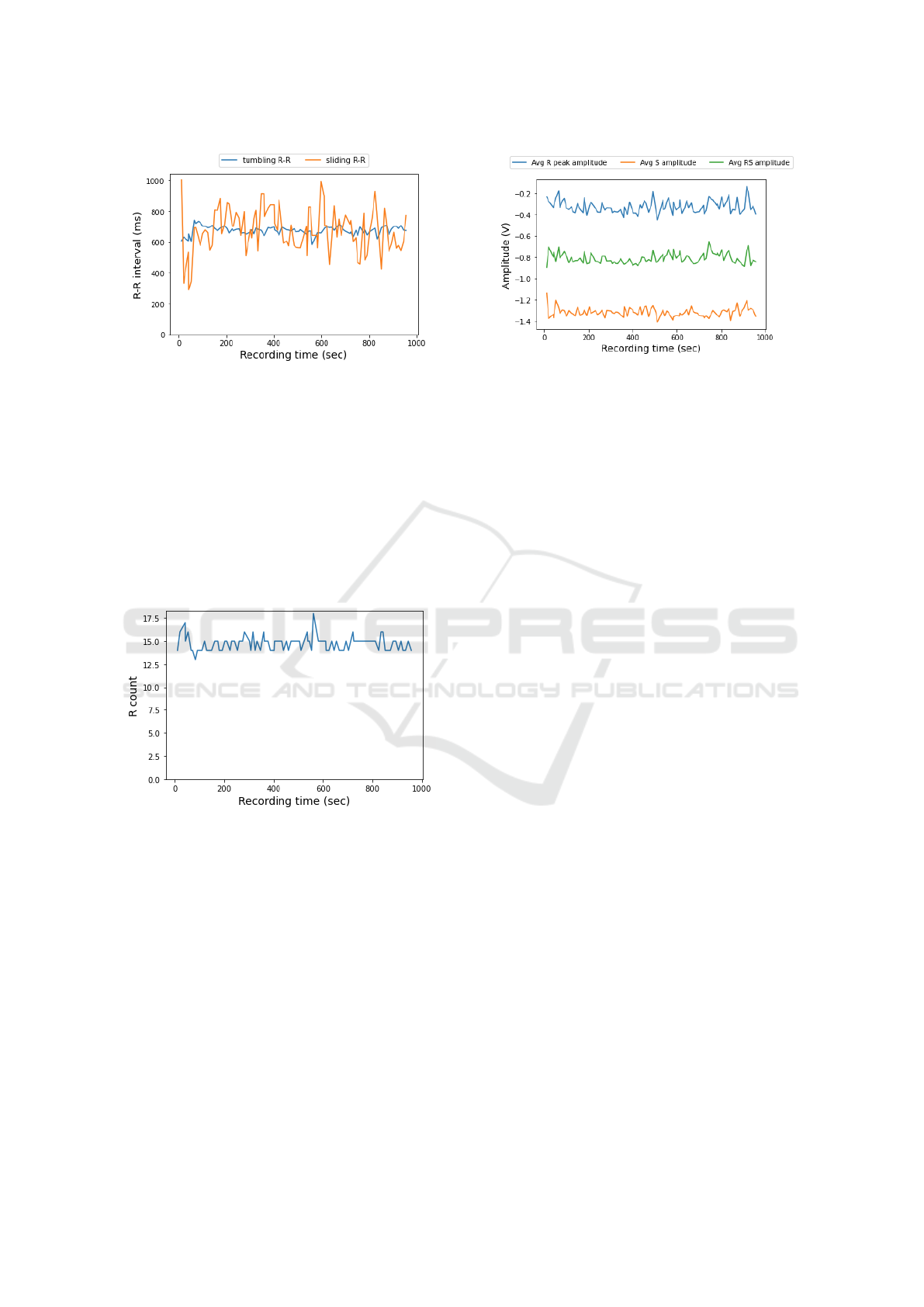
Figure 5: R-R interval using tumbling and sliding windows
of 10 seconds.
Fig. 6 shows the number of R peaks detected from
our algorithm in each 10 second window the ECG de-
vice detects. These values do help us detect both the
number of heartbeats and the location of these heart-
beats inside the window, helping find the exact start
and end locations of each heartbeat, information that
will help us in the future of our research. Using the
R counts we can calculate the heartrate of the wearer
( 10-sec-R-count ×6 = heartrate), so in our test, this
results in a heart rate of around 90 beats per minute.
Figure 6: R count in each 10 second tumbling window.
Finally, Fig 7 depicts the average R peak am-
plitude, S amplitude, and RS amplitude for each of
the tumbling windows. As we see, these values are
quite stable, as expected, during the whole recording
given that this recording is from a test patient with no
chronic heart condition diagnosed.
7 CONCLUSIONS
In this work, we showed how a wearable ECG mon-
itoring device is used in the context of SmartWork to
provide information and feedback on office workers
with chronic heart conditions regarding the effects of
their works in their everyday lives and their diagnosed
Figure 7: R, S and RS amplitudes in each 10 second tum-
bling window.
conditions. We presented how the device operates in
order to generate such conclusions based on the ECG
input signals and how the resulting events are trans-
ferred to SmartWork to properly notify the worker’s
doctors. We showcased the resulting data from the
ECG recordings and the results of the analysis done
on the ECG device.
As our next steps, we plan to further increase the
analysis of the ECG traces on the device itself, provid-
ing more accurate detection and heartbeat character-
ization by identifying the possible problems in each
heartbeat, instead of a general alert characterization.
We also plan to test our system with real users, during
the SmartWork’s trials that are currently being exe-
cuted to gather as much user feedback regarding the
usability of our system and the quality of the whole
experience.
ACKNOWLEDGEMENTS
This work has been partially supported by the Smart-
Work project (GA 826343), EU H2020, SC1-DTH-
03-2018 - Adaptive smart working and living envi-
ronments supporting active and healthy ageing.
REFERENCES
Cardiovascular diseases statistics.
Angeletti, F., Chatzigiannakis, I., and Vitaletti, A. (2017).
The role of blockchain and iot in recruiting partic-
ipants for digital clinical trials. In 2017 25th In-
ternational Conference on Software, Telecommunica-
tions and Computer Networks (SoftCOM), pages 1–5.
IEEE.
Angeletti, F., Chatzigiannakis, I., and Vitaletti, A. (2018).
Towards an architecture to guarantee both data privacy
and utility in the first phases of digital clinical trials.
Sensors, 18(12).
Cardio: An Edge-enabled Wearable ECG Vest for Office Worker’s Heart Condition Monitoring
425

Chatzigiannakis, I., Pyrgelis, A., Spirakis, P. G., and Stama-
tiou, Y. C. (2011). Elliptic curve based zero knowl-
edge proofs and their applicability on resource con-
strained devices. In 2011 IEEE Eighth International
Conference on Mobile Ad-Hoc and Sensor Systems,
pages 715–720.
Chiang, M. and Zhang, T. (2016). Fog and iot: An overview
of research opportunities. IEEE Internet of things
journal, 3(6):854–864.
Commission, E. (2018). 2018 reform of EU data protection
rules. https://ec.europa.eu/commission/sites/beta-
political/files/data-protection-factsheet-
changes en.pdf.
da S. Luz, E. J., Schwartz, W. R., C
´
amara-Ch
´
avez, G., and
Menotti, D. (2016). Ecg-based heartbeat classification
for arrhythmia detection: A survey. Computer Meth-
ods and Programs in Biomedicine, 127:144–164.
Du, L., Du, Y., Li, Y., Su, J., Kuan, Y.-C., Liu, C.-C., and
Chang, M.-C. F. (2017). A reconfigurable streaming
deep convolutional neural network accelerator for in-
ternet of things. IEEE Transactions on Circuits and
Systems I: Regular Papers, 65(1):198–208.
for the Advancement of Medical Instrumentation, A.
(2013). Testing and reporting performance re-
sults of cardiac rhythm and st segment measure-
ment algorithms. American National Standard 2013,
ANSI/AAMI EC57:2012.
Gokhale, V., Zaidy, A., Chang, A. X. M., and Culurciello,
E. (2017). Snowflake: An efficient hardware acceler-
ator for convolutional neural networks. In 2017 IEEE
International Symposium on Circuits and Systems (IS-
CAS), pages 1–4. IEEE.
Kaplan Berkaya, S., Uysal, A. K., Sora Gunal, E., Ergin, S.,
Gunal, S., and Gulmezoglu, M. B. (2018). A survey
on ecg analysis. Biomedical Signal Processing and
Control, 43:216–235.
Luo, J.-H., Wu, J., and Lin, W. (2017). Thinet: A filter level
pruning method for deep neural network compression.
In Proceedings of the IEEE international conference
on computer vision, pages 5058–5066.
O’Connor, R. E., Al Ali, A. S., Brady, W. J., Ghaem-
maghami, C. A., Menon, V., Welsford, M., and Shus-
ter, M. (2015). Part 9: acute coronary syndromes:
2015 american heart association guidelines update for
cardiopulmonary resuscitation and emergency cardio-
vascular care. Circulation, 132(18 suppl 2):S483–
S500.
Pan, J. and Tompkins, W. J. (1985). A real-time qrs de-
tection algorithm. IEEE Transactions on Biomedical
Engineering, BME-32(3):230–236.
Qin, H., Gong, R., Liu, X., Bai, X., Song, J., and Sebe, N.
(2020). Binary neural networks: A survey. Pattern
Recognition, 105:107281.
Saini, I., Singh, D., and Khosla, A. (2013). Qrs detection
using k-nearest neighbor algorithm (knn) and evalua-
tion on standard ecg databases. Journal of Advanced
Research, 4(4):331 – 344.
Shah, A. P. and Rubin, S. A. (2007). Errors in the com-
puterized electrocardiogram interpretation of cardiac
rhythm. Journal of Electrocardiology, 40(5):385 –
390.
SmartWork 2021 - 2nd International Workshop on Smart, Personalized and Age-Friendly Working Environments
426
