
Augmenting Machine Learning with Flexible Episodic Memory
Hugo Chateau-Laurent
1,2,3
a
and Fr´ed´eric Alexandre
1,2,3 b
1
Inria Bordeaux Sud-Ouest, 200 Avenue de la Vieille Tour, 33405 Talence, France
2
LaBRI, Universit´e de Bordeaux, Bordeaux INP, CNRS, UMR 5800, Talence, France
3
Institut des Maladies Neurod´eg´en´eratives, Universit´e de Bordeaux, CNRS, UMR 5293, Bordeaux, France
Keywords:
Explicit Memory, Episodic Memory, Prospective Memory, Hippocampus, Prefrontal Cortex.
Abstract:
A major cognitive function is often overlooked in artificial intelligence research: episodic memory. In this
paper, we relate episodic memory to the more general need for explicit memory in intelligent processing. We
describe its main mechanisms and its involvement in a variety of functions, ranging from concept learning
to planning. We set the basis for a computational cognitive neuroscience approach that could result in im-
proved machine learning models. More precisely, we argue that episodic memory mechanisms are crucial for
contextual decision making, generalization through consolidation and prospective memory.
1 INTRODUCTION
Despite recent progress in machine learning, the field
of artificial intelligence has not yet given rise to gen-
erally intelligent and autonomous agents endowed
with autobiographical memory and capable of defin-
ing their own goals and planning their way to reach
them. As a result, machine learning models are
mainly confined to domain-specific processing. Com-
plementary and interacting memories have been ar-
gued to be a key component of autonomous learn-
ing (Alexandre, 2016). We claim that current tech-
niques in artificial intelligence mirror implicit mem-
ory, while topics related to building explicit memory
systems remain largely unaddressed. The view we de-
pict here is mostly consistent with the one proposed
in (Botvinick et al., 2019) in that they both propose to
draw inspiration on both the prefrontal cortex and the
hippocampus to develop more powerful algorithms.
Our work further expands this proposal by consider-
ing additional mechanisms inspired from anatomical
and electrophysiological studies.
We first argue that models need complementary
memories, then set the focus on the organization of
episodic memory in biological organisms. Finally, we
propose a roadmap to implement these mechanisms in
artificial agents.
a
https://orcid.org/0000-0002-2891-0503
b
https://orcid.org/0000-0002-6113-1878
2 THE NEED FOR
COMPLEMENTARY
MEMORIES
Consider a task where you have different colored
shapes displayed on a table and you can press one
among a set of buttons to get rewards. There are hid-
den rules controlling reward delivery that you can dis-
cover by trial and error. An example rule would be
that if a red square is on the left, pressing the left but-
ton is rewarded. This kind of task can be learned very
easily and efficiently with classical machine learn-
ing techniques like reinforcement learning (RL). Sim-
ilarly, the subtask of object identification is prototyp-
ical of gradient-based forward layered architectures.
Through learning, these models build what is called
in cognitive science an implicit memory. This corre-
sponds to a slow and procedural learning of the un-
derlying regularities. If information (like position or
color of the shapes) is encoded in a topographical
way, the model builds an overlapping representation,
which enables it to generalize. In RL, this learning
can be done with a model-free (MF) approach, mean-
ing that the values of configurations are learned with-
out explicit knowledge of the task structure (hence the
term implicit memory).
These computational approaches have been par-
alleled with known principles in neuroscience. For
instance, layered architectures of deep networks have
been compared to the hierarchical structure of the sen-
326
Chateau-Laurent, H. and Alexandre, F.
Augmenting Machine Learning with Flexible Episodic Memory.
DOI: 10.5220/0010674600003063
In Proceedings of the 13th International Joint Conference on Computational Intelligence (IJCCI 2021), pages 326-333
ISBN: 978-989-758-534-0; ISSN: 2184-3236
Copyright © 2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

sory posterior cortex. RL models have been very ben-
eficial to better understand learning principles of the
most ancient regions of the frontal cortex (i.e. the
part of the cortex that is anterior to the central sulcus)
that are also called agranular cortex, corresponding to
the motor, premotor and lateral orbitofrontal cortices
(Domenech and Koechlin, 2015). In association with
the sensory cortex, these regions are involved in deci-
sion making via action selection from perceptual cues
(associated to actions in motor and premotor cortex)
and reward values (learnt in the orbitofrontal cortex).
Loops associating these cortical regions with the basal
ganglia and modulated by dopaminergic projections
carrying reward prediction errors have been carefully
studied, and corresponding models have been com-
pared to MF-RL (Joel et al., 2002). It is also reported
in cognitive neuroscience that, after a long period of
repetition, this reward-based behavior is transformed
into a purely sensorimotor behavior (also called ha-
bitual) simply associating the sensory and motor cor-
tices, with no sensitivity to reward change (Boraud
et al., 2018): after a long practice, you automatically
press the left button when you see a red square on the
left without even thinking of an anticipated reward.
2.1 The Need for Episodic Memory
The functions of these cortical regions are conse-
quently tightly associated with implicit learning, as
performed by the most famous and efficient models
in artificial intelligence today, like deep networks and
MF-RL agents. Nevertheless, these models suffer
from a series of well-identified weaknesses. Layered
architectures with overlapping representations are not
good for learning data without structure or regulari-
ties. They learn very slowly, from global aspects to
details and are sensitive to what is called catastrophic
forgetting: if you first learn a relation (e.g. pressing
the left button when seeing the red square on the left),
then, when learning another one (e.g. pressing the
left button when seeing the blue circle on the left), the
previous association will be forgotten in the absence
of regular recall.
This problem has been clearly identified and ad-
dressed in the framework of the complementarylearn-
ing systems (CLS) (McClelland et al., 1995), intro-
ducing the role of the hippocampus (HPC). This neu-
ronal structure has been originally studied in navi-
gation tasks, demonstrating major learning capabil-
ities and exhibiting place cells that respond to spe-
cific places in the environment (Stachenfeld et al.,
2017). Later on, the HPC has been associated with
more complex and general functions. It is thought to
receive a summary sketch of the current cortical ac-
tivation and perform functions like associative mem-
ory and recall. Thanks to its very sparse coding and
an advanced function of pattern separation (Kassab
and Alexandre, 2018), the HPC is able to learn very
quickly, possibly in one shot, an arbitrary binding
of any distributed cortical representation with a min-
imized risk of interference. Consequently, it can-
not generalize well. However, it also performs pat-
tern completion, namely the ability to rebuild a com-
plete pattern from partial cues, and replay it in (i.e.
send it back to) the cortex. Implemented in attractor
networks, this function is useful for doing reinforce-
ment learning in the real world with high-dimensional
states and noisy observations (Hamid and Braun,
2017). It can consequently complement the cortex by
learning rare patterns very quickly and binding a dis-
tributed cortical representation that would be hard to
associate with a motor response at the cortical level.
At each moment, when the HPC receives a cortical
pattern, it has to decide whether it is novel and must
be learned, or if it is reminiscent of a previouslystored
activation and must be completed (recalled) and sent
back to the cortex. This is done by a clever mech-
anism described in the next section. It must also be
stressed that what is manipulated by the HPC (origi-
nating from current cortical activation)does not repre-
sent a single eventbut rather, thanks to several mecha-
nisms of temporal coding of the cortex evoked below,
a full behavioral episode that can last several seconds.
This faculty of storing and recalling specific episodes
is termed episodic memory. It is a kind of explicit
memory because information can here be explicitly
questioned. In humans, this is often associated with a
conscious declarative process.
We have now discussed principles of these com-
plementary learning systems, with the cortex per-
forming a slow statistical learning using distributed
overlapping representations, and the HPC perform-
ing rapid arbitrary binding of cortical activation to
store new episodes in a sparse associative memory
and recall them later on. Interestingly, the capacity
of the HPC to send back previously stored episodes
to the cortex (
´
Olafsd´ottir et al., 2018) is very use-
ful because it can solve the problem of catastrophic
forgetting. Thanks to the replay of virtual (i.e. not
actually experienced) episodes, the HPC indeed has
the capacity of gradually inserting examples of a new
rule and interleaving them with examples of the previ-
ously stored rules not to forget them. This process of
replay is also very useful for another learning mecha-
nism called consolidation. We have explained above
that it is difficult for the cortex to manipulate and for
example associate by learning a concept which is dis-
tributed in distant regions of its surface. Conversely,
Augmenting Machine Learning with Flexible Episodic Memory
327

it is not possible for combinatorial reasons, to repre-
sent locally in associative regions of the cortex, any
combination of elementary patterns that might con-
stitute the premises of a behavioral rule. By this re-
play mechanism, if it appears that the HPC has to
frequently send back an arbitrary binding of pattern
to answer non tractable situations, the cortex will be
able to slowly learn this combination of activities re-
played by the HPC on its surface and to link units in
an associative area to this distributed activation, thus
forming locally a new concept to be employed in fu-
ture behaviors, without the help of the HPC. Under
this view, it can be said that the HPC is a supervisor
of cortical learning. If the stored episodes represent a
very rare case (or a particular case or an exception), it
can continue and be treated by the HPC.
2.2 The Need for Cognitive Control
Another series of weaknesses associated with implicit
learning in the cortex is linked to the fact that, some-
times, and particularly in a complex and dynamic
world, it is not sufficient for the behavior to be only
reactive, stimulus driven, but it also need to be goal
driven, guided by internal states like motivations, in-
tentions or to obey external instructions providing a
new rule. Consider cases where the rule associat-
ing the red square on the left with the left button
changes for the right button and comes back to the
left button (so-called reversal learning): a reactive
stimulus-driven behavior would slowly unlearn the
previous rule and learn the new one and unlearn it
again, whereas it is rather expected to have a more
flexible behavior including an internal deliberation to
switch rapidly from one rule to the other. You can
also consider cases (like in the Wisconsin Card Sort-
ing Test (Bock and Alexandre, 2019)) where several
rules are proposed but at a given moment only one is
valid or cases where the new rule is given by instruc-
tion from a fellow creature. Here also you should
give up immediately the previously learned rule and
adopt a new one provided by internal process. You
generally wait for errors (difference between antici-
pated and actual reward) to understand that rule has
changed and that you have to find a new one. Some-
times, you can also discover that changes are asso-
ciated with a contextual cue. In this case, you can
learn contextual rules and change your behavior be-
fore making errors (Koechlin, 2014). Consider now
cases where the rule becomes more complex and re-
lies on non visible cues (for example press the left
button if you see a red square on the left and if on the
previous trial the red square was on the right). Here
also a reactive behavior guided by observed cues is
not sufficient and should be completed by the possi-
bility to decide from internal cues corresponding here
to the memory of recently observed cues. Other inter-
nal cues to consider for biasing the decision could be
also related to emotion or motivation associated to the
present situation.
All these cases share common principles (Miller
and Cohen, 2001). We are here in cases where the de-
fault behavior learned by a slow stimulus-driven pro-
cess must be inhibited and replaced with a new behav-
ior adapted to the present context. To ensure flexibil-
ity and reversibility, this process must be explicit and
manipulate knowledge about the world, as in Model
Based approaches in RL. Not only based on the per-
ception of external stimuli, this system must rely on a
internal source of activity to bias the default behavior
suggested by stimuli and impose the new behavior in
a top-down way. At the same time, it must be flexible
but also robust to distractions from external stimuli.
It has been demonstrated by neuroscience that all
these properties are ensured by the prefrontal cortex
(PFC), a more recent (also called granular) region of
the frontal cortex, particularly developed in primates
(Fuster, 1989). This structure, organized according
to the kind of task (Domenech and Koechlin, 2015)
implements contextual rules which can ensure a top-
down control of behavior. It has a modulatory role
and does not replace reactive rules slowly acquired
by implicit learning. Instead, it is able to inhibit the
default (and not adapted here) behavior and trigger
an attentional process to favor task-relevant stimuli
and consequently elicit a previously learnt rule more
adapted to the present context (O’Reilly et al., 2002).
This modulatory role implies that, in case of a lesion,
the behavior is not stopped but comes back to the (non
appropriate) default case, yielding classical deficits
of PFC damage like perseveration. All these mech-
anisms are implemented by the unique mechanism of
working memory, where neuronal populations of the
PFC exhibit a sustained activity to represent, main-
tain and impose this contextual activity. In addition to
its role in learning, the role of the dopamine has been
shown central to regulate the balance between main-
tenance and updating of the sustained activity (Braver
and Cohen, 2000).
In contrast to the classical weight-based learning
(as presented above with the HPC), what we describe
here is an activity based control but if it is frequently
exploited in certain context, it can become a default
behavior by slow implicit learning.
NCTA 2021 - 13th International Conference on Neural Computation Theory and Applications
328
2.3 The Need for Prospective Memory
It is now time to describe what could be called a side
effect of the interactions between these two kinds of
explicit memories, considering that the HPC and the
PFC are interconnected (Simons and Spiers, 2003)
and apply their respective functions one on the other.
In short, the HPC can replay episodes of cortical ac-
tivation, including prefrontal activation; reciprocally,
the PFC is informed about contextual aspects of the
situation by the HPC and can control the generation
of adapted episodes and their storage. These episodes
can be manipulated explicitly by the PFC, the same
way it creates newrules adapted to the situation. Alto-
gether, this gives rise to prospective memory (Schac-
ter et al., 2007), also called memory of the future
(Fuster, 1989), the unique capacity of imagination,
of explicitly anticipating the future from an internal
model of the world and of making a decision informed
by the potential outcomes of possible future scenar-
ios and deliberating on their respective interest. This
is among the most powerful capabilities of human
cognition, also associated to planning and reasoning,
clearly going beyond reactive automatic behavior and
enabled by the combination of the two kinds of ex-
plicit memory evoked above, episodic and working
memory. An alternative computational account of
how these memories interact has been proposed by
(Zilli and Hasselmo, 2008). Yet, this framework is
rather elementary and its limitations have been high-
lighted by (Dagar et al., 2021), who argued that more
complex mechanisms are needed in realistic cases, in
the same vein as what we propose here.
Models for implicit learning are today well mas-
tered and employed in artificial intelligence. Mod-
els for cognitive control by the PFC have been pro-
posed for some time in computational neuroscience
(O’Reilly et al., 2002) and their principles begin to be
transferred to machine learning (Wang et al., 2018).
Episodic learning has also been considered recently
for machine learning (Ritter et al., 2018) but for the
moment, the corresponding models propose a rather
superficial view of this cognitive function and of its
multiple roles in cognitive control, consolidation and
prospective memory, as we have evoked here. In
the forthcoming section, we explain that integrating
information about episodic learning from different
sources in neuroscience can help understand and give
a more precise computational account of this cogni-
tive function in order to define an integrated explicit
learning involving both the HPC and the PFC.
3 ORGANIZATION OF EPISODIC
MEMORY
Hippocampus
Anterior
Posterior
Frontal
cortex
Sensory
cortices
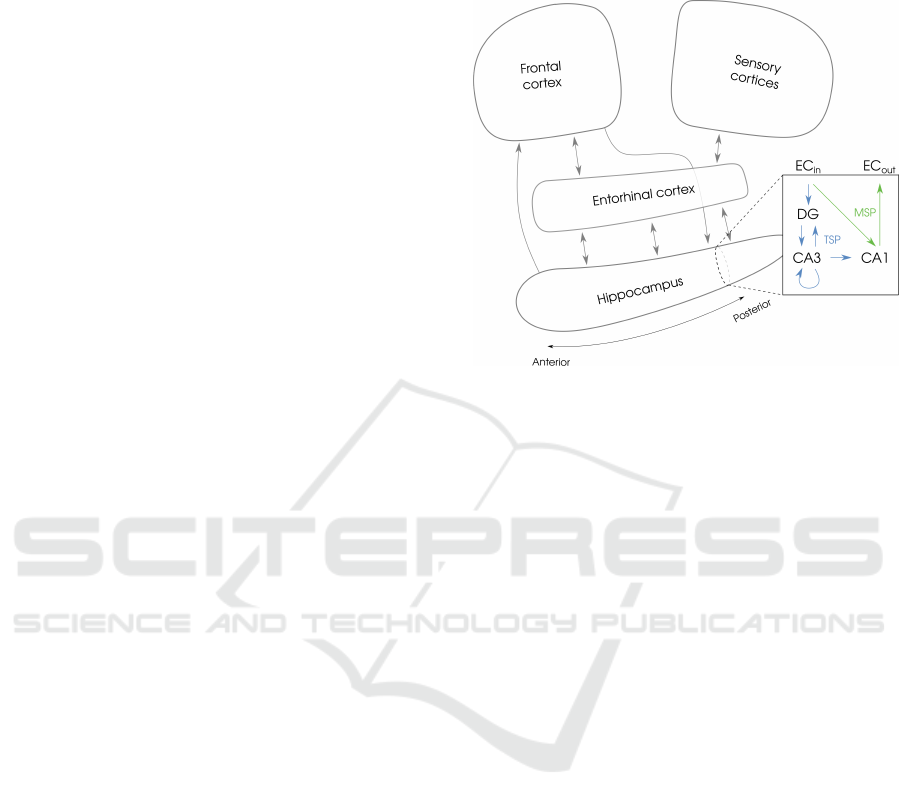
Figure 1: Connectivity within the hippocampus and be-
tween the hippocampus and the frontal, posterior and en-
torhinal cortices. Connections of the trisynaptic pathway
are shown in blue while connections of the monosynaptic
pathway are shown in green.
3.1 Memory Storage in the HPC
As mentioned above, the HPC is able to store and re-
play sequences of experienced states, as well as con-
struct novel ones. These sequences are embedded in
two coupled oscillatory regimes: a theta rhythm (4-
10 Hz) and faster gamma rhythm (20-80 Hz). Ac-
cording to their respective frequency, approximately
seven gamma cycles are nested in each theta cycle.
The entorhinal cortex (EC) constitutes the main in-
put to the HPC by its superficial layers that we call
ECs. They are thought to provide one state per theta
cycle (Jensen and Lisman, 2005). The HPC then ei-
ther engages its gamma regime in a retrospectivecode
supporting short-term memory and episodic storage,
or in a prospective code with which it tries to pre-
dict future states (Lisman and Otmakhova, 2001). In
both cases, sequences are represented in a compressed
way, with one state represented per gamma cycle. In
the retrospective (or learning) code, the sequence of
gamma states reflects the order at which past states
have just been experienced, with the current state be-
ing represented at the end of the theta cycle. The main
computational reason behind having such a buffer is
that the gamma timescale is more appropriate at a bio-
physical level (relating to constants of time of molec-
ular processes) for learning state transitions than the
behavioral timescale at which events naturally occur
(Lisman and Otmakhova, 2001), especially given the
Augmenting Machine Learning with Flexible Episodic Memory
329

fact that the most anterior parts of the HPC learn tran-
sitions between temporally distant events as we will
discuss below. In the prospective (or recall) code, the
item provided by the cortex is followed by one item
per gamma cycle. The order and identity of these sub-
sequent items reflect a prediction of upcoming states,
potentially based on previous learning through ret-
rospective coding. Alternation between these two
mechanisms is presumably based on the ability (or
inability) of the prospective code to accurately pre-
dict future states. Under this view, the HPC begins by
considering the current episode as already known and
attempts to predict the next states until errors indicate
that the current episode is too different from stored
situations and must consequently be considered novel
and learned accordingly. In fact, a crucial part of the
HPC called CA1 is often linked to novelty detection,
namely computation of the mismatch between pre-
dictions and incoming sensory information (Duncan
et al., 2012). When unpredicted sequences of stim-
uli are presented to the HPC, a dopaminergic signal is
used to switch from the prospective code to the learn-
ing retrospective code in order to update the internal
model (Lisman and Otmakhova, 2001).
Retrospective and prospective codes are expanded
with mechanisms of pattern separation and pattern
completion (Kassab and Alexandre, 2018). The HPC
indeed tries to complete each gamma state (whether
being actual or predicted) by relating it to states it
has experienced in the past. This is the task of a re-
current region called CA3. Conversely, if the state
does not match any stored pattern, its representation
is modified in such a way that it becomes as dissim-
ilar (orthogonal) as possible to any other pattern, to
avoid interference in this very fast learning process.
This is performed in the largest region of the HPC, the
Dentate Gyrus (DG), thus encoding what makes each
state unique, with a very sparse coding. In sum, the
HPC performs a succession of auto-associations (en-
coding states) and hetero-associations (encoding their
sequence, whether being retrospective or prospective)
(Lisman and Otmakhova, 2001).
The mechanisms just described are thought to
underlie the learning of individual experienced se-
quences of states (i.e. episodes). They mainly involve
the trisynaptic pathway : ECs −→ DG −→ CA3 −→ CA1.
CA1 also receives information from a more direct
monosynaptic pathway: ECs −→ CA1 −→ ECd. While
fast learning enabling the trisynaptic pathway to learn
to link items represented in successive gamma cycles
is obviously useful for one-shot sequence learning,
slower learning deployed in the monosynaptic path-
way may serve additional roles. We have seen that
what is encoded in DG and CA3 is not the full de-
scription of a state but rather what makes it different
from the others, through orthogonalization processes.
When the HPC sends information back to the cortex
for reasons we will detail in the next part, this partic-
ular encoding cannot be used as is. In fact, the role of
CA1 is to act as a translator between the sparse repre-
sentation of the HPC and the dense overlapping rep-
resentation used in the cortex (Schapiro et al., 2017).
Learning mechanisms in the monosynaptic pathway
ensure CA1 is tuned in such a way that what is re-
constructed in deep layers of EC (ECd) corresponds
to the original information sent by ECs. This tun-
ing can be done at the moment of learning, when
both the original information and its reconstruction
are present. At the moment of prediction, the same
comparison mechanism serves as a novelty proxy for
switching between recall and learning regimes, like
previously mentioned. A side effect of this slower
learning mechanism is the involvement of CA1 in
gathering statistics over multiple episodes (Schapiro
et al., 2017), thus explaining why the activity of some
hippocampal units like place cells reflect statistical
regularities (Stachenfeld et al., 2017). Computer sim-
ulations reveal the importance of such a mechanism
for discovering associations not directly experienced
in for example transitive inference, namely the dis-
covery that if A is associated to B and B is associated
to C, A is indirectly associated to C (Schapiro et al.,
2017). The role of episodic aggregation has also been
described for building a kernel function in the context
of episodic reinforcement learning (Gershman and
Daw, 2017). Finally, these statistics might serve as
an intermediate step for the transfer between episodic
and cortical memory systems (Kumaran et al., 2016).
We explain this in more detail in the next part.
3.2 Interfacing between the HPC and
the Cortex
A constant dialog between the HPC and the cortex is
ensured by multiple pathways, including a major hub:
the entorhinal cortex. This highly integrated cortical
region receives inputs from most regions of the en-
tire cortex in its superficial layers and buffers them
in episodes to be sent to the HPC. Reciprocally, its
deep layers receive replays from the HPC and dis-
tribute them back to the cortex. Other minor paths for
the specific communication with the PFC will also be
evoked below. While the precise description of these
pathways is outside our scope, we would like to high-
light a few key characteristics before addressing the
computational role of this dialog.
Connectivity with the cortex is not homogeneous
throughout the HPC. In humans, the HPC runs along
NCTA 2021 - 13th International Conference on Neural Computation Theory and Applications
330

an anterior-posterior longitudinal axis. Along this
axis, genetic, electrophysiological, and anatomical
differences underlie differences in functionality, and
in particular, decreasing degrees of spatio-temporal
abstraction (see (Strange et al., 2014) for a review). In
the spatial domain, the gradient of abstraction trans-
lates into place cells being sensitive to larger portions
of the environment in the anterior HPC compared to
place cells located in the posterior HPC (Jung et al.,
1994). More generally, it is hypothesized that on the
one hand, more posterior parts of the HPC which are
mostly connected to posterior (and sensory) parts of
the cortex are concerned with specifics (details of a
scene, precise location,...). On the other hand, more
anterior parts which are mostly interacting with an-
terior parts of the cortex (dealing with cognitive and
behavioural control but also emotional and motiva-
tional aspects) represent more abstract and general
information (context, larger scale location, seman-
tic information, mood...). Intermediate portions are
thought to mediate interactions between the two ends
of the longitudinal axis using intermediate represen-
tations (Strange et al., 2014). Organisation in trisy-
naptic and monosynaptic pathways is preserved all
along, although the dentate gyrus is more prominent
in posterior HPC while CA1-3 are more prominent
in its anterior part (Malykhin et al., 2010). Hence,
the same mechanisms for learning detailed percep-
tual state transitions in the posterior HPC are used for
learning transitions between more spatially and tem-
porally distal cognitive cues in the anterior HPC. In-
terestingly, this also constitutes a potential substrate
for transitive inference, since items that are not asso-
ciated at the posterior scale of functioning can poten-
tially be associated at the anterior scale of temporal
abstraction (Strange et al., 2014).
Let us now discuss candidate functions of
hippocampo-corticaldialog, beginning with topics in-
volving communicationthrough the entorhinalcortex.
Firstly, hippocampal pattern completion can be taken
advantage of for the recall of missing information in
the cortex (Rolls, 2013). It is obviously helpful in the
noisy domain of perception, in which objects must be
identified while exhibiting a constantly changing as-
pect, for example dependent on light conditions in the
case of vision. As mentioned before, similar mecha-
nisms are used with more abstract representations in
intermediate and anterior regions of the HPC. Conse-
quently, pattern completion might also be useful for
retrieving more abstract information related to con-
text, emotional states, and even behavioral plans, a
requirement for prospective memory. Secondly, a fur-
ther function of the dialog is to use knowledge about
state transitions stored in the HPC. This has been
shown to be useful for predictive coding in the visual
cortex (Hindy et al., 2016) and cognitive anticipation
(Gershman and Daw, 2017). Thirdly, the complemen-
tary learning systems theory suggests that the HPC
acts as a temporary store for memories to be trans-
ferred to the cortex (McClelland et al., 1995). More
precisely, dense representation and slow learning are
deployed for the cortex to learn high-level generaliza-
tions while avoiding catastrophic forgetting respec-
tively, resembling implicit memory of classical deep
learning models. The other side of the coin is that
the cortex is not able to learn from a single exposure
to a piece of information. Conversely, the HPC de-
ploys fast learning and sparse representation for en-
abling one-shot acquisition. Since sparse encoding is
not suitable for generalization, the HPC alone is not
sufficient either. In fact, it is really the conjunction
and interaction of these two systems that enable hu-
mans to learn fast and exploit acquired knowledge ef-
ficiently. During resting periods, the HPC is thought
to “teach” the cortex by sending recently acquired in-
formation interleaved with more established knowl-
edge. This process is called interleaved learning and
is used to incorporate new information into cortical
knowledge without interference (McClelland et al.,
1995). Note that the aforementioned hypothesis re-
garding the complementarity of the monosynaptic and
trisynaptic pathways was introduced as an analogous
and intermediate step to this consolidation process
(Schapiro et al., 2017). Indeed, intermediate statistics
discovered in the monosynaptic pathway might pref-
erentially be sent to the cortex. There would there-
fore be a trisynaptic-monosynaptic-cortical gradient
of learning abstraction, in the perpendicular direction
of the anterior-posterior axis of representational ab-
straction. To our knowledge, understandingthe differ-
ential contributions of these two axes to functions like
transitive inference remains an open and unaddressed
question. As a concluding remark, the fact that the
monosynaptic pathway might play a more dominant
role in the anterior HPC and the trisynaptic pathway a
more dominant role in the posterior one suggests that
these two axes might not be completely orthogonal.
Concerning specific paths of communication be-
tween the PFC and the HPC, several principles must
be mentioned. In short, a unidirectional loop of com-
munication has been described (Eichenbaum, 2017).
The anterior HPC is hypothesized to send contextual
information about the retrieved episode to the PFC,
which uses it to better select appropriate behavioral
rules. Reciprocally, the PFC controls episodic re-
call and encoding, either directly by modulating ac-
tivity in the posterior HPC (Rajasethupathy et al.,
2015) or indirectly by biasing the activity of the sen-
Augmenting Machine Learning with Flexible Episodic Memory
331

sory and entorhinal cortices. As a result, hippocam-
pal recall and its prospective code are oriented to-
wards task-relevant episodes, whereas encoding and
its retrospective code are modulated to control mem-
ory organization. The PFC would therefore need a
model of what is stored in the HPC, a function that is
sometimes referred to as metamemory. The direction
of these information flows are presumably controlled
by an additional communication pathway linking the
PFC and the HPC through the thalamus, the mecha-
nisms of which are still to be understood in more de-
tails. To better understand the crucial role of these re-
cently discovered and yet to model interactions, let us
consider the example task of deciding how to dress.
While learning to wear warm clothes when perceiv-
ing rain and lighter clothes when perceiving sunlight
is sufficient in some situations, elements of controlled
episodic memory can help us making a better deci-
sion. The HPC might be able to recall and send in-
formation to the PFC concerning a weather forecast
inconsistent with your current perception. The PFC
could then predict that the weather is likely to change
in the near future. It might in turn inhibit the au-
tomatic recall of an alternative but fictional weather
forecast you saw in a movie. This situation illus-
trates how interactions between the HPC and the PFC
harness memory of the past to make decisions in the
present by predicting the future.
4 ROADMAP FOR THE FUTURE
In this paper, we have shown that important limita-
tions in today’s machine learning are due to a lack
of explicit memory and we have reported that, in
the brain, the HPC and the PFC play a fundamental
role in creating and managing this memory. We have
also explained that this form of memory plays a cen-
tral role in concept learning, planning, reasoning and
other major cognitive functions. Although this global
view begins to be understood and exploited in ma-
chine learning, the specific role of episodic memory
and its substrate in these functions is still not well un-
derstood and corresponding working models could be
augmented with additional mechanisms.
We attempted to report results and hypotheses
coming from different domains of neuroscience in or-
der to provide an increasingly precise view of hip-
pocampal circuitry and its relationship with posterior
and prefrontal cortices. On the one hand, we think
that the role of the HPC in supervising the sensory
cortex to allow for consolidation without interference
could be investigated in more details and give rise
to better balanced neural networks capable of form-
ing new conceptual knowledge online. On the other
hand, even if several pieces of information are still
missing, preliminary studies about the involvement of
interactions between the HPC and the PFC in forming
a prospective memory should also be considered for
improving decision making algorithms. Finally, we
believe that explicit cognition is a key ingredient for
creating artificial agents capable of deliberating and
explaining their actions.
REFERENCES
Alexandre, F. (2016). Beyond machine learning: Au-
tonomous learning. In 8th International Confer-
ence on Neural Computation Theory and Applications
(NCTA), pages 97–101.
Bock, P. and Alexandre, F. (2019). [Re] The Wisconsin
Card Sorting Test: Theoretical analysis and modeling
in a neuronal network. ReScience C, 5(3):#3.
Boraud, T., Leblois, A., and Rougier, N. P. (2018). A natural
history of skills. Progress in Neurobiology, 171:114–
124.
Botvinick, M., Ritter, S., Wang, J. X., Kurth-Nelson, Z.,
Blundell, C., and Hassabis, D. (2019). Reinforcement
learning, fast and slow. Trends in cognitive sciences,
23(5):408–422.
Braver, T. S. and Cohen, J. D. (2000). On the control of
control: The role of dopamine in regulating prefrontal
function and working. In MIT Press. Making Working
Memory Work, pages 551–581.
Dagar, S., Alexandre, F., and Rougier, N. (2021). De-
ciphering the contributions of episodic and working
memories in increasingly complex decision tasks. In
Proceedings of the International Joint Conference on
Neural Networks, IJCNN 18-22 July 2021, Shenzhen,
China. IEEE.
Domenech, P. and Koechlin, E. (2015). Executive control
and decision-making in the prefrontal cortex. Current
Opinion in Behavioral Sciences, 1:101–106.
Duncan, K., Ketz, N., Inati, S. J., and Davachi, L. (2012).
Evidence for area ca1 as a match/mismatch detector:
A high-resolution fmri study of the human hippocam-
pus. Hippocampus, 22(3):389–398.
Eichenbaum, H. (2017). Memory: Organization and Con-
trol. Annual Review of Psychology, 68(1):19–45.
Fuster, J. (1989). The prefontal cortex. Anatomy, physiology
and neurophysiology of the frontal lobe. Raven Press,
New-York.
Gershman, S. J. and Daw, N. D. (2017). Reinforcement
learning and episodic memory in humans and animals:
an integrative framework. Annual review of psychol-
ogy, 68:101–128.
Hamid, O. H. and Braun, J. (2017). Reinforcement learn-
ing and attractor neural network models of associative
learning. In International Joint Conference on Com-
putational Intelligence, pages 327–349. Springer.
NCTA 2021 - 13th International Conference on Neural Computation Theory and Applications
332

Hindy, N. C., Ng, F. Y., and Turk-Browne, N. B. (2016).
Linking pattern completion in the hippocampus to
predictive coding in visual cortex. Nature neuro-
science, 19(5):665–667.
Jensen, O. and Lisman, J. E. (2005). Hippocampal
sequence-encoding driven by a cortical multi-item
working memory buffer. Trends in neurosciences,
28(2):67–72.
Joel, D., Niv, Y., and Ruppin, E. (2002). Actor–critic mod-
els of the basal ganglia: new anatomical and compu-
tational perspectives. Neural Networks, 15(4-6):535–
547.
Jung, M. W., Wiener, S. I., and McNaughton, B. L. (1994).
Comparison of spatial firing characteristics of units in
dorsal and ventral hippocampus of the rat. Journal of
Neuroscience, 14(12):7347–7356.
Kassab, R. and Alexandre, F. (2018). Pattern separation
in the hippocampus: distinct circuits under different
conditions. Brain Structure & Function, 223(6):2785–
2808.
Koechlin, E. (2014). An evolutionary computational
theory of prefrontal executive function in decision-
making. Philosophical transactions of the Royal
Society of London. Series B, Biological sciences,
369(1655):20130474+.
Kumaran, D., Hassabis, D., and McClelland, J. L. (2016).
What learning systems do intelligent agents need?
complementary learning systems theory updated.
Trends in cognitive sciences, 20(7):512–534.
´
Olafsd´ottir, H. F., Bush, D., and Barry, C. (2018). The
Role of Hippocampal Replay in Memory and Plan-
ning. Current Biology, 28(1):R37–R50.
Lisman, J. E. and Otmakhova, N. A. (2001). Storage, recall,
and novelty detection of sequences by the hippocam-
pus: elaborating on the socratic model to account for
normal and aberrant effects of dopamine. Hippocam-
pus, 11(5):551–568.
Malykhin, N., Lebel, R. M., Coupland, N., Wilman, A. H.,
and Carter, R. (2010). In vivo quantification of hip-
pocampal subfields using 4.7 t fast spin echo imaging.
Neuroimage, 49(2):1224–1230.
McClelland, J. L., McNaughton, B. L., and O’Reilly, R. C.
(1995). Why there are complementary learning sys-
tems in the hippocampus and neocortex: insights
from the successes and failures of connectionist mod-
els of learning and memory. Psychological review,
102(3):419–457.
Miller, E. K. and Cohen, J. D. (2001). An integrative theory
of prefrontal cortex function. Annu. Rev. Neurosci.,
24:167–202.
O’Reilly, R. C., Noelle, D. C., Braver, T. S., and Cohen,
J. D. (2002). Prefrontal cortex and dynamic catego-
rization tasks: representational organization and neu-
romodulatory control. Cereb Cortex, 12(3):246–257.
Rajasethupathy, P., Sankaran, S., Marshel, J. H., Kim, C. K.,
Ferenczi, E., Lee, S. Y., Berndt, A., Ramakrishnan, C.,
Jaffe, A., Lo, M., et al. (2015). Projections from neo-
cortex mediate top-down control of memory retrieval.
Nature, 526(7575):653–659.
Ritter, S., Wang, J., Kurth-Nelson, Z., and Botvinick, M.
(2018). Episodic Control as Meta-Reinforcement
Learning. preprint, Neuroscience.
Rolls, E. (2013). The mechanisms for pattern completion
and pattern separation in the hippocampus. Frontiers
in systems neuroscience, 7:74.
Schacter, D. L., Addis, D. R., and Buckner, R. L. (2007).
Remembering the past to imagine the future: the
prospective brain. Nature Reviews Neuroscience,
8(9):657–661.
Schapiro, A. C., Turk-Browne, N. B., Botvinick, M. M.,
and Norman, K. A. (2017). Complementary learning
systems within the hippocampus: a neural network
modelling approach to reconciling episodic mem-
ory with statistical learning. Philosophical Trans-
actions of the Royal Society B: Biological Sciences,
372(1711):20160049.
Simons, J. S. and Spiers, H. J. (2003). Prefrontal and me-
dial temporal lobe interactions in long-term memory.
Nature Reviews Neuroscience, 4(8):637–648.
Stachenfeld, K. L., Botvinick, M. M., and Gershman, S. J.
(2017). The hippocampus as a predictive map. Nature
neuroscience, 20(11):1643.
Strange, B. A., Witter, M. P., Lein, E. S., and Moser, E. I.
(2014). Functional organization of the hippocam-
pal longitudinal axis. Nature Reviews Neuroscience,
15(10):655–669.
Wang, J. X., Kurth-Nelson, Z., Kumaran, D., Tirumala, D.,
Soyer, H., Leibo, J. Z., Hassabis, D., and Botvinick,
M. (2018). Prefrontal cortex as a meta-reinforcement
learning system. Nature Neuroscience, 21(6):860–
868. Number: 6 Publisher: Nature Publishing Group.
Zilli, E. A. and Hasselmo, M. E. (2008). Modeling the role
of working memory and episodic memory in behav-
ioral tasks. Hippocampus, 18(2):193–209.
Augmenting Machine Learning with Flexible Episodic Memory
333
