
A Methodology based on Formal Methods for Thermal Ablation Area
Detection
Luca Brunese, Francesco Mercaldo, Antonella Santone and Giuseppe Peter Vanoli
Department of Medicine and Health Sciences “Vincenzo Tiberio”, University of Molise, Campobasso, Italy
Keywords:
Formal Methods, Thermal Ablation, Model Checking.
Abstract:
Thermal ablation is the process related to the destruction of tissue by elevated tissue temperatures or depressed
tissue temperatures. The machine exploited to perform for this process is named thermal ablator, requiring
in input the area of the tissue to be subjected to treatment. In this proposal, with the aim to assist doctors
in the process of the detection of the area targeted by the thermal ablator, we propose a methodology based
on formal methods considering the representation of medical images in terms of formal and mathematical
representations for the detection of the area.
1 INTRODUCTION AND
BACKGROUND
The thermal ablation is defined as a needle-based
treatment finalized to destroy cancerous or not nor-
mal tissue. There are two main ablation methods i.e.,
the extreme cold (also known as cryoablation) and the
extreme heat (also known as radiofrequency or mi-
crowave ablation) (Choi and Jung, 2020).
The focus of thermal ablation is the cancer tis-
sue destruction considering the generation of cyto-
toxic temperatures for a really short time-window in
a not invasive way, clearly without damaging vital
structures adjacent to the cancerous area. Typically
considered techniques to perform the thermal ablation
procedure for destroying tissue by elevating the tissue
temperature above 55
◦
C in the cancerous area include
radiofrequency, microwave, ultrasound, and also laser
ablation (Heged
¨
us et al., 2020). The cryoablation,
from the other side, considers subzero temperatures
to selectively freeze with the aim to destroy (only) the
cancerous tissue. The innovation represents by both
these ablative procedures is that they provide a mini-
mal (e.g. percutaneously or laparoscopically) or non-
invasive approach to the tumour therapy (Zhang et al.,
2020).
The damage of the tissue can be controlled in an
accurate way by considering a range of focused ultra-
sound transducers with different sonication sizes. In
this context, medical images (for instance, magnetic
resonance and computed tomography) allows the ex-
perts to continuously monitor the temperature rise in
real time, allowing also in real-time the quantification
of the dose of the therapy.
From the other side, ultrasound imaging and tech-
nique for the characterization of the tissue (for in-
stance, elastography) can be exploited for monitoring
the treatment relating to several clinical applications.
Depending on the equipment and parameters consid-
ered, the volume of focused ultrasound lesions can be
as small as a grain of rice (i.e., 10 cubic millimeters)
(Song et al., 2013). This allows for an extremely lo-
calized treatment and a sharp border between treated
and untreated areas.
For treatment of larger structures, as for instance
tumors, multiple lesions can be combined in order to
contain the full volume (Uchida et al., 2012; Song
et al., 2013) of the cancerous area. A cooling pe-
riod between different sonications is typically con-
sidered with the aim to reduce hte possibility of un-
wanted heating of surrounding tissue. This is the rea-
son way, the treatment of really large tissue struc-
tures can be time-consuming. Anyway, optimized
scanning algorithms, the injection of microbubbles
aimed to increase the absorption of acoustic energy,
and the adoption of spiral sonications are techniques
currently exploited ofr the reduction of the treatments
time (Brunese et al., 2019b).
The treatments exploiting thermal ablation are ob-
taining an increasing attention, as a matter of fact
are considered an alternative to classic invasive surgi-
cal therapies, with particular regards to patients with
contraindications or those who refuse open surgery
(De Baere and Deschamps, 2014).
Brunese, L., Mercaldo, F., Santone, A. and Vanoli, G.
A Methodology based on Formal Methods for Thermal Ablation Area Detection.
DOI: 10.5220/0010394801910194
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 3: BIOINFORMATICS, pages 191-194
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
191

Today, thermal ablation is exploited in clinical
applications with particular regard for kidney treat-
ing, prostate and others non-operable liver tumors.
In this direction there is also an increasing applica-
tion of thermal ablation techniques related to other or-
gan sites including the brain (Brunese et al., 2020c),
prostate, breast, lung, pancreas, thyroid and bone,
symptomatic uterine fibroids; tumors in the prostate
(Blana et al., 2004; Brunese et al., 2020f), breast, low
back pain and brain disorders such as essential tremor,
disease of Parkinson and neuropathic pain. The po-
tential benefits of thermal ablation therapy are includ-
ing reduced morbidity but also mortality in compari-
son with standard surgical resection and the ability to
treat patients who are not surgical candidates (Thanos
et al., 2004).
Even researchers have demonstrated that thermal
ablation represents a successful technique for reduce
tumours surface with minimal thermal damage to sur-
rounding healthy tissue(Sajjadi et al., 2011), this tech-
nique requires expert pathologist and radiologist to
localise the cancer area target of the thermal abla-
tion (Baisi et al., 2013; Morgan et al., 2010; Raveglia
et al., ).
Starting from these considerations, in this paper
we introduce a proposal for a methodology based on
formal methods for the automatic detection of the
cancer area subjected to thermal ablation.
2 MODEL CHECKING FOR
THERMAL ABLATION AREA
DETECTION
In Figure 1 we show the flowchart related to our pro-
posal.
We start from the analysis of medical images,
that we convert into numerical values by exploiting
radiomics i.e., a method that extracts a large num-
ber of features from radiographic medical images us-
ing data-characterisation algorithms (Brunese et al.,
2020d; Brunese et al., 2020b; Iadarola et al., 2020;
Brunese et al., 2020a).
We consider radiomics since it has been shown
that it is able to exhibit disease characteristics that the
naked eye fails (Brunese et al., 2020a).
In detail, we exploit different radiomic features
belonging to five different categories (Van Griethuy-
sen et al., 2017):
• First Order: this category describes the distribu-
tion of voxel intensities within the ROI (i.e., the
region of interest, in this study related to the areas
in the MRI interested by the cancer);
• Shape: this feature category includes descrip-
tors of the three-dimensional size and shape of
the ROI. These features are independent from the
gray level intensity distribution in the ROI and are
therefore only calculated on the non-derived im-
age and mask;
• Gray Level Co-occurrence Matrix (GLCM): this
category considers the spatial relationship of pix-
els is the gray-level co-occurrence matrix i.e., the
gray-level spatial dependence matrix. The GLCM
functions characterize the texture of an image by
computing how often pairs of pixel with specific
values and in a specified spatial relationship occur
in an image and then extracting statistical mea-
sures from this matrix;
• Gray Level Run Length Matrix (GLRLM): the
grey-level run length matrix (GLRLM) gives the
size of homogeneous runs for each grey level. It
quantifies gray level runs, which are defined as the
length in number of pixels, of consecutive pixels
that have the same gray level value;
• Gray Level Size Zone Matrix (GLSZM): the fea-
tures belonging to this category quantify gray
level zones in an image. A gray level zone is de-
fined as the number of connected voxels that share
the same gray level intensity. A voxel is consid-
ered connected if the distance is 1 according to the
infinity norm.
The radiomic feature set obtained from each med-
ical image related to the patient under analysis, is then
translated into a formal model by exploiting the Cal-
culus of Communicating Systems (Milner, 1989). To
detect the area of the thermal ablation on the formal
model (Casolare et al., 2019; Casolare et al., 2020),
we need a set of properties expressed in a tempo-
ral logic, for instance, in the mu-calculus logic (Stir-
ling, 1989), describing the cancerous area subjected
to thermal ablation. The properties are formulated
with the knowledge of expert radiologists and pathol-
ogists. In details, our proposal considers several prop-
erties, each one related to a particular area of the med-
ical image. For example, if we ideally divide the
medical image into four equal parts, we will define
four properties, each one relating to one of the four
areas. Once obtained the formal model and the re-
lated properties, we invoke a formal verification envi-
ronment (for instance, the CWB-NC
1
) to verify if the
thermal ablation area properties are satisfied by the
formal model obtained from the medical images.
When the formal verification environment outputs
TRUE on a certain property, the formal model will
1
https://www3.cs.stonybrook.edu/
∼
cwb/
BIOINFORMATICS 2021 - 12th International Conference on Bioinformatics Models, Methods and Algorithms
192
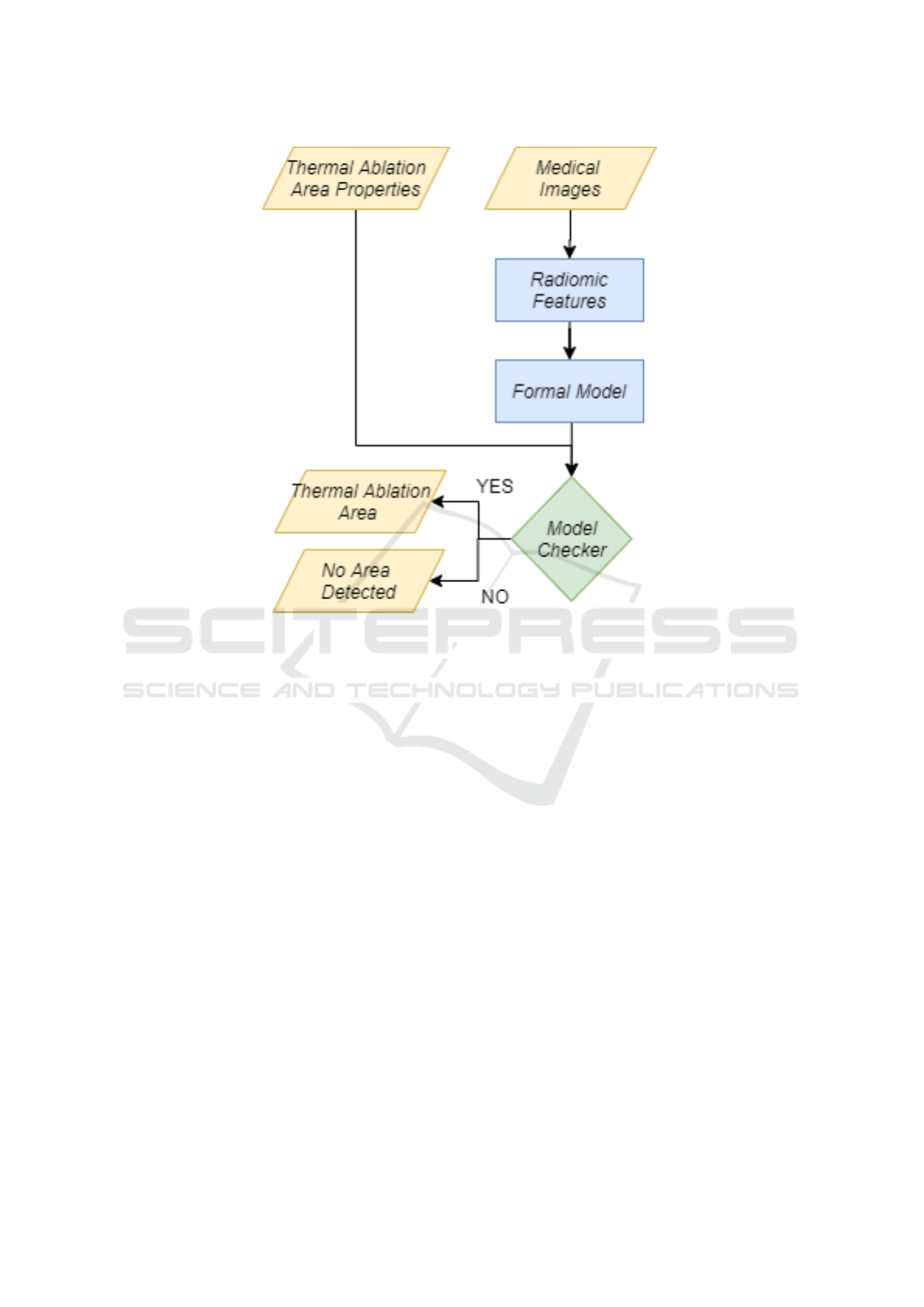
Figure 1: The flowchart.
exhibit the area subjected to thermal ablation in the
zone identified by the formula. Otherwise, the formal
verification environment outputs FALSE meaning that
the formal model will not exhibit an area subjected to
thermal ablation in the zone identified by the formula
(Iadarola et al., 2019; Brunese et al., 2020e; Brunese
et al., 2019a).
3 CONCLUSION AND FUTURE
WORK
The thermal ablation technique is typically used for
patients with unresectable and borderline resectable
disease, which may be due to the size, number or lo-
cation of the tumors, or for patients judged inoperable
due to the poor helath of the patient. This technique
requires the aid of radiologists and pathologists to ex-
actly localise the tumour area object of the thermal ab-
lation. In this paper, we propose a method to localise
the cancerous area subjected to the thermal ablation
therapy by exploiting medical image analysis. In de-
tails, we consider radiomics features to obtain numer-
ical values from medical images, and model checking,
to automatically detect the area for the thermal abla-
tion application. As future works, we plan to specify
the temporal logic properties for the automatic area
detection. Moreover, it will be of interest to under-
stand whether the automatic area detection properties
rightly work on different type on organ. Moreover,
we plan to apply also deep techniques in order to un-
derstand the results. We plan to apply an architecture
based on Convolution Neural Network, typically ex-
ploited for processing visual data and 2D data. Basi-
cally, a CNN is made up of one or more convolutional
layers with fully connected upward layers. It also
consider common weights and layers (i.e., pooling
layers). In particular, ”max-pooling” is often used in
Fukushima’s convolutional architecture (Deng et al.,
2014), allowing CNNs to take advantage of 2D input
structures. As a matter of fact, they are particularly ef-
fective in the area of images and speech recognition.
REFERENCES
Baisi, A., De Simone, M., Raveglia, F., and Cioffi, U.
(2013). Thermal ablation in the treatment of lung can-
cer: present and future. European Journal of Cardio-
Thoracic Surgery, 43(4):683–686.
Blana, A., Walter, B., Rogenhofer, S., and Wieland, W. F.
(2004). High-intensity focused ultrasound for the
treatment of localized prostate cancer: 5-year expe-
A Methodology based on Formal Methods for Thermal Ablation Area Detection
193

rience. Urology, 63(2):297–300.
Brunese, L., Martinelli, F., Mercaldo, F., and Santone, A.
(2020a). Deep learning for heart disease detection
through cardiac sounds. Procedia Computer Science,
176:2202–2211.
Brunese, L., Martinelli, F., Mercaldo, F., and Santone, A.
(2020b). Machine learning for coronavirus covid-19
detection from chest x-rays. Procedia Computer Sci-
ence, 176:2212–2221.
Brunese, L., Mercaldo, F., Reginelli, A., and Santone, A.
(2019a). Formal methods for prostate cancer glea-
son score and treatment prediction using radiomic
biomarkers. Magnetic resonance imaging.
Brunese, L., Mercaldo, F., Reginelli, A., and Santone,
A. (2019b). Radiomic features for medical images
tamper detection by equivalence checking. Procedia
Computer Science, 159:1795–1802.
Brunese, L., Mercaldo, F., Reginelli, A., and Santone,
A. (2020c). An ensemble learning approach for
brain cancer detection exploiting radiomic features.
Computer methods and programs in biomedicine,
185:105134.
Brunese, L., Mercaldo, F., Reginelli, A., and Santone,
A. (2020d). Explainable deep learning for pul-
monary disease and coronavirus covid-19 detection
from x-rays. Computer Methods and Programs in
Biomedicine, 196:105608.
Brunese, L., Mercaldo, F., Reginelli, A., and Santone, A.
(2020e). Formal methods for prostate cancer glea-
son score and treatment prediction using radiomic
biomarkers. Magnetic resonance imaging, 66:165–
175.
Brunese, L., Mercaldo, F., Reginelli, A., and Santone,
A. (2020f). Radiomics for gleason score detection
through deep learning. Sensors, 20(18):5411.
Casolare, R., Martinelli, F., Mercaldo, F., and Santone, A.
(2019). A model checking based proposal for mo-
bile colluding attack detection. In 2019 IEEE Inter-
national Conference on Big Data (Big Data), pages
5998–6000. IEEE.
Casolare, R., Martinelli, F., Mercaldo, F., and Santone, A.
(2020). Malicious collusion detection in mobile en-
vironment by means of model checking. In 2020
International Joint Conference on Neural Networks
(IJCNN), pages 1–6. IEEE.
Choi, Y. and Jung, S.-L. (2020). Efficacy and safety of
thermal ablation techniques for the treatment of pri-
mary papillary thyroid microcarcinoma: a systematic
review and meta-analysis. Thyroid, 30(5):720–731.
De Baere, T. and Deschamps, F. (2014). New tumor abla-
tion techniques for cancer treatment (microwave, elec-
troporation). Diagnostic and interventional imaging,
95(7-8):677–682.
Deng, L., Yu, D., et al. (2014). Deep learning: methods
and applications. Foundations and Trends
R
in Signal
Processing, 7(3–4):197–387.
Heged
¨
us, L., Miyauchi, A., and Tuttle, R. M. (2020). Non-
surgical thermal ablation of thyroid nodules: Not if,
but why, when, and how? Thyroid.
Iadarola, G., Martinelli, F., Mercaldo, F., and Santone, A.
(2019). Formal methods for android banking malware
analysis and detection. In 2019 Sixth International
Conference on Internet of Things: Systems, Manage-
ment and Security (IOTSMS), pages 331–336. IEEE.
Iadarola, G., Martinelli, F., Mercaldo, F., and Santone, A.
(2020). Image-based malware family detection: An
assessment between feature extraction and classifica-
tion techniques. In IoTBDS, pages 499–506.
Milner, R. (1989). Communication and concurrency. PHI
Series in computer science. Prentice Hall.
Morgan, G. J., Clarke, K., Caldarone, C., and Benson,
L. N. (2010). Radiolucent retractor for angiographic
analysis during hybrid congenital cardiac procedures.
The Journal of thoracic and cardiovascular surgery,
140(5):1195–1196.
Raveglia, F., Rizzi, A., De Simone, M., Cioffi, U., Sacrini,
A., and Baisi, A. State of the art in alternative treat-
ments for lung cancer: Thermal ablation therapy.
Sajjadi, A. Y., Mitra, K., and Grace, M. (2011). Ablation
of subsurface tumors using an ultra-short pulse laser.
Optics and Lasers in Engineering, 49(3):451–456.
Song, J. H., Yoo, Y., Song, T.-K., and Chang, J. H. (2013).
Real-time monitoring of hifu treatment using pulse in-
version. Physics in Medicine & Biology, 58(15):5333.
Stirling, C. (1989). An introduction to modal and temporal
logics for ccs. In Yonezawa, A. and Ito, T., editors,
Concurrency: Theory, Language, And Architecture,
volume 491 of LNCS, pages 2–20. Springer.
Thanos, L., Mylona, S., Pomoni, M., Kalioras, V., Zoganas,
L., and Batakis, N. (2004). Primary lung cancer: treat-
ment with radio-frequency thermal ablation. Euro-
pean radiology, 14(5):897–901.
Uchida, T., Nakano, M., Hongo, S., Shoji, S., Nagata, Y.,
Satoh, T., Baba, S., Usui, Y., and Terachi, T. (2012).
High-intensity focused ultrasound therapy for prostate
cancer. International Journal of Urology, 19(3):187–
201.
Van Griethuysen, J. J., Fedorov, A., Parmar, C., Hosny, A.,
Aucoin, N., Narayan, V., Beets-Tan, R. G., Fillion-
Robin, J.-C., Pieper, S., and Aerts, H. J. (2017).
Computational radiomics system to decode the radio-
graphic phenotype. Cancer research, 77(21):e104–
e107.
Zhang, T., Liang, W., Song, Y., Wang, Z., and Zhang, D.
(2020). Us-ct fusion image-guided microwave abla-
tion of lung cancer—-a new mode of image guidance
in lung cancer ablation. ADVANCED ULTRASOUND
IN DIAGNOSIS AND THERAPY, 4(4):343–348.
BIOINFORMATICS 2021 - 12th International Conference on Bioinformatics Models, Methods and Algorithms
194
