
Exloration of High Risk Medical Devices Methodologies for
Optimized Evaluations
Thomas Lihoreau
1a
, Chrystelle Vidal
1b
, Tianguang Wang
2c
and Lionel Pazart
1d
1
CHU Besançon, INSERM CIC 1431, Centre d'Investigation Clinique, Besançon, France
2
Institut Supérieur d'Ingénieurs de Franche-Comté, University of Franche-Comté, Besançon, France
Keywords: High Risk Medical Devices, Evaluation, Methodology.
Abstract: Medical devices are developed by manufacturers that need to provide proofs of safety, efficacy, efficiency.
In the same time they could be specialists in the technologies, they could not be necessary experts for the
targeted medical field and need to be surrounded to build the correct clinical evaluation strategy. Skills
required are specific to these particular instruments, and need to be optimized and innovative, as there is as
much different devices than the start-ups in the arena. Even if works are performed on the methodological
aspects since years, we propose to state a snap of the situation thanks to clinical trials databases exploration,
with the aim to extract typical cases for future help and support for the actors. The current article offer to
present our strategy of work as well as first quantitative results.
1 INTRODUCTION
In Clinmed special session of Biostec 2020 in Malta,
we discussed the adapted methodologies for medical
devices field (Vidal, 2020), which is characterized
with specificities well documented, on the subjects of
randomization, comparator, blinding, acceptance, or
endpoints selection… French Haute Autorité de santé
(2013: https://www.has-sante.fr/jcms/c_1696842/en/
methodological-choices-for-the-clinical-developmen
t-of-medical-devices), as well as American Food and
Drug Administration (2016, https://www.fda.gov/
media/92671/download) for example underlined
these points since years.
In Europe, the European commission adopted in 2017
an updated regulation on medical devices EU MDR
2017/745, and on in vitro diagnostic medical devices
EU IVDR 2017/746, repealing previous directives
(https://ec.europa.eu/health/md_sector/overview_en).
Guidance documents are developed to help actors for
implementation of these directives, previously
Meddevs, going onto updated Medical Device
Coordination Group: https://ec.europa.eu/health/
md_sector/new_regulations/guidance_en.
a
https://orcid.org/0000-0001-8417-6609
b
https://orcid.org/0000-0002-0882-5299
c
https://orcid.org/0000-0002-3652-5461
d
https://orcid.org/0000-0002-9104-0862
The general context have been related in previous
Clinmed sessions -and will be also debated in other
articles of this session. In a synthetic approach, we
can observe an updated framework around medical
devices requiring more clinical evidences, through
clinical investigations conceived, realized and
analysed with independent medical and clinicians
experts, high risk medical devices being the main
impacted by these considerations. The way a
technological innovation needs to be evaluated being
different that the historical well-known ones drugs.
We propose then to:
- formalize an analysis of the registered studies
mixing high risk medical devices and interesting
methodologies,
- discuss the quantitative results,
- analyse the studies retained in our approach,
- ultimately, we will try to sort out and propose
some recommendations for the actors.
In the present paper and to match with the
pedagogical objective of the Clinmed session we will
focus on the strategy of research and present the first
quantitative results, the final report being planned for
2021.
Lihoreau, T., Vidal, C., Wang, T. and Pazart, L.
Exloration of High Risk Medical Devices Methodologies for Optimized Evaluations.
DOI: 10.5220/0010386502850289
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 1: BIODEVICES, pages 285-289
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
285
2 EXPLORATION STRATEGY
In this aim to explore the methodologies adapted to
medical devices, we follow a the work from Vidal
2020, and Pruniaux 2021.
2.1 High Risk Medical Devices
The definition of high risk medical devices join the
classical criteria defining the classification linked to
the level of risk of each device (from I, IIa, IIb, to III),
but can not be only reduced to.
European commission even recently open a call in the
Horizon 2020 framework, named “Developing
methodological approaches for improved clinical
investigation and evaluation of high-risk medical
devices” (https://ec.europa.eu/info/funding-tenders/
opportunities/portal/screen/opportunities/topic-
details/sc1-hco-18-2020), without using namely the
“class” reference to continental regulations.
Brunotte (2020) add notions of delicate targeted
anatomical area, implantability character, or novelty
in the technology or the material used.
Following a work performed by Pruniaux et al. in
summer 2020 that defined an algorithm (Matlab and
Scilab softwares) allowing to search occurences of
keywords in databases, we use the same key-words
mixing the aspects of implantability, risk in
morbidity/mortality, adverse event and misuse risks.
2.2 Methodology
In the same way and following the work of Vidal
2020, we extend her research focused on adaptive
methodologies, with the concepts of : Zelen
randomization (Zelen et al. 1990), adaptive design
(response adaptive randomization, Jiang, F, et al.,
2013, or adaptive enrichment, Simon et al., 2013, Lai
TL et al., 2019), cross-over, flexible design,
sequential trial (Hamilton et al., 2012), treatment
switching, sequential multiple assignment
randomized trial (SMART) (Tamura et al., 2016, Wei
et al., 2018, Meurer et al., 2017), multi-arm multi-
stage trial (Simon et al., 1985), stepwise multiple
arms, cluster trial, tracker study (for fast technology
evolution, Lilford, et al., 2000), Bayesian approaches
(Pennello et al., 2008, Campbell et al., 2011,
Campbell et al., 2016), sample size reassessment (re-
estimation/adjustment) (Magirr et al., 2016), or trial
without informed consent or within cohort (Kim,
Weijner 2018)…
3 RESULTS
Our first researches focused on https://www.clinical
trials.gov/ database. The explorations allowed to
detected the defined keywords in brief titles, official
titles or brief summaries/detailed descriptions.
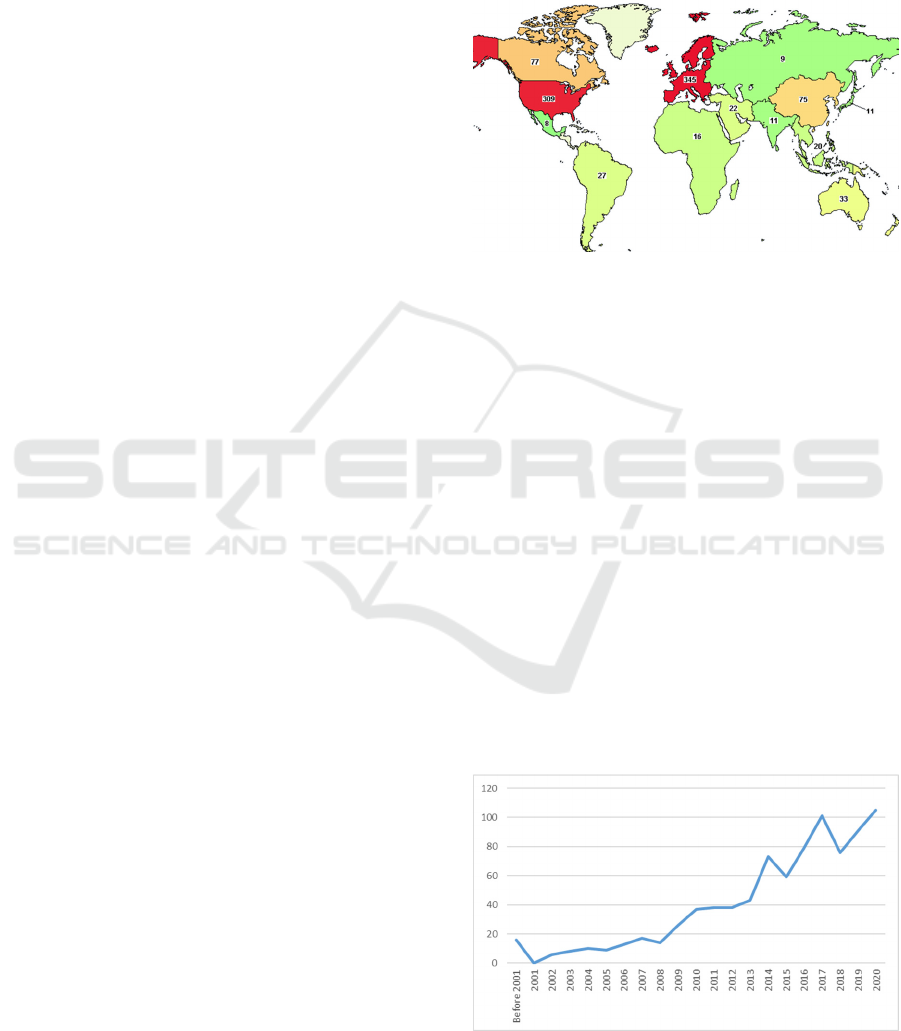
Figure 1: Repartition of the studies concerning high risk
medical devices and specific designs (source: map tool of
https://www.clinicaltrials.gov/).
We observed 7155 studies on high risk medical
devices. With methodology key-words, we detected
61156 studies. Crossing high risk + methodology
key-words, we obtained 859 studies.
On this total of 859 trials (on date November 25
th
,
2020) matching both, 341 were completed, 14
withdraw, 1 suspended, 54 not yet recruiting, 142
with results, 130 accepting healthy volunteers, 6 with
usability key-word.
A quick overview of the map provided by clinical
trials website presents that these kind of studies are
ainly performed in Europe (345), North America
(309+77), and to a lesser extent in India (75) (figure
1).
We can also observe an evolution in terms of
number of concerned studies (figure 2), with 16
referenced before 2001, and a regular increase (105 in
2020).
Figure 2: Evolution of trials on medical devices involving
specific methodologies, over time.
ClinMed 2021 - Special Session on Dealing with the Change in European Regulations for Medical Devices
286

Table 1 present also the distribution of the different
methodologies in the obtained results.
Table 1: Repartition of methodologies in the identified
studies: at least one key word concerning methodology –
some could have more than one, that is why we get here a
total of 933.
We performed the same extraction on Medline
(https://pubmed.ncbi.nlm.nih.gov/), with article type
filter “clinical trial”:
- we detected 10380 publications on high risk
medical devices,
- with methodology key-words, we obtained 88560,
- crossing high risk + methodology key-words, we
observed 864 articles (still on November 25
th
,
2020).
4 NEXT STEPS
4.1 Selection of the Studies of Interest
The selection phase will consist in defining criteria
allowing to retain relevant studies.
We will rank the trials by type of methodology.
The brief titles, official titles, then details of the
studies will be successivelly read by two experts; a
consensus will be reached on the retained trials to be
explored and retained in our discussion.
Criteria of selection will focus on the fact that the
study addresses well an high risk medical device (the
definition is not exactly shared), and that an
“innovative” / interesting design was provided; the
first identified methodologies will be discussed in
order to determine the originalty and relevancy for
medical devices field.
4.2 Analysis
In term of analysis, we will considerate the possible
problems, challenges, key points or strong points
enhanced by the investigators: we will pay attention
to the duration of the study compared to the planned
duration: was the study performed until the end? If
yes, quicker or slower than planned? If stopped, what
was the reason ? Devices was in question ? Was there
any discussion about the relevancy of the choosen
methodology ? ...
We will also extract the possible usability
information that could be detected with specific
methodologies, usability that is well know to be
important for considerations on technological
innovations.
For the results published, we will explore these
information thanks to https://pubmed.ncbi.nlm.
nih.gov/ website and based on our first articles
selection, to see if the extractions between the
registrered studies and the publications of the results
match, and in which way they are complementary or
different. We could have there an interesting view of
the ability of the available data to provide enough
information compared to our hypothesis. Are the
documentation provided in Clinical trials or
publications results systematically sufficient for
confirming our questions.
4.3 Extension to Other Databases
We plan to test the same strategy on adapted
algorithms for Medline website, Cochrane library
(https://www.cochranelibrary.com/ ), or databases for
clinical trials performed in other part of the world
(example in Asia: http://www.chictr.org.cn/abouten.
aspx, https://www.umin.ac.jp/ctr/ ).
5 PERSPECTIVES AND
PRACTICAL CASES
Mixing quantitative and qualitative analysis (looking
onto details inside the studies), we will then
considerate the state and number of employed
methodologies, depending of the device evaluated as
well as its stage of development.
Based on our experience, we extracted few
illustrations allowing to provide an idea of possible
typical cases and the way evaluations could be
provided to adequatly answer to requirements from
between authorities (for market assess, for studies
Type of methodology Number of concerned studies
Cross-Over 259
Trials within Cohorts 162
Flexible 132
Randomization Adjustment 124
Sequential 86
Adaptive 62
Trial without Informed Consent 46
Cluster 28
Treatment Switching 13
Tracker Study 7
Bayesian 6
Sample Size Adjustment 3
Dose Finding 2
Tracker Trial 2
Stepwise Multiple Arm 1
Exloration of High Risk Medical Devices Methodologies for Optimized Evaluations
287

approvals), industrials, scientists and cliniciens, and
for sure patients.
5.1 A Device without Assess to Market
Approval
In our practice, we had the case on which the strategy
of market acess was different depending of the
country/area of the world on which the manufacturer
would like to apply, which underlined different
procedures, but also lectures by authorities
concerning the way a device need to bring proofs.
5.2 A Software without Assess to
Market
The european regulation address specific sections for
software, that could have strong impact in the
diagnosis or care of the patients.
We can meet the case on which industrials
selected the strategy to play on some edges (or
adaptations?) in the lecture of the regulations, that
permit them to diffuse an “inoffensive observational”
version of their device onto hospitals and clinics on
very early phases; in that way, the aim consists in
aggregation of data, without any intervention on
patients. The data accumaleted need for sure to comply
with General Data Protection Regulation (GDPR
https://gdpr-info.eu/), but could help the manufacturer
to adapt its future device (especially in machine
learning/artificial intelligence considerations), and to
feed his future FDA or CE mark with in fact data
provided by the real life.
Another example relies on a software for which
we were involved since its very early stage for
development; the manufacturer had no experience
and was not structured for medical devices field. In
order to well understand the context, as well as giving
time to adapt the device and securize the things before
going on patients, we structured a two sequences
study, the first one being dedicated to observe the
current practices (without the software) and to define
the scenarii of use, the second one introducing the
software in simulation experimentation.
The regulatory positionning could then go on a
software first dedicated to training of caregivers,
before going on a high risk / class III medical device
- if enough proofs accumulated in these simulated
envirmnements.
5.3 A Device with a CE Mark
On medical devices already on the market since years,
we had to provide specific medico-economic
evaluations, taking into account these efficiency
considerations, in terms of duration of
hospitalization, back to work time, quality of life…
And in fact impacting the way the care and the
evaluations need to be arranged, with sometimes a
strong gap between what imagine the industrial at the
very beginning, and the proposed organization.
5.4 Use of Real Data
More and more and with the available big amount of
data, it is possible to imagine some “virtual”
controlled group, with possible pairing between a real
patient prospectively enrolled in a study, and his pair
selected onto database. This need strong thoughts on
criteria of inclusion (pairing), as well as available data
linked to the criteria of judgements. And then even if
it’s seems quite interesting (reducing the number of
patients involved in a research, gaining time, reducing
cost of a study…), it is not possible in many cases and
must not make forget to respect the chronological
steps of testing.
5.5 Implementation of a Device along
the Trial
For a very diruptive innovation involving not only
technology, but also organizations around patient and
its environment, we can pay attention to a tracker trial
that in fact allowed different phases introducing
implemented version of a prototype, each phases
turning profit from the previous ones, and feeding the
next ones (in term of adaptation of the device, but also
for the evaluations).
6 CONCLUSION
Considering our results, we plan to extend analysis
and to in end build recommendations, illustrating and
guiding the skateholders on the pathway to the
selection of a relevant methodology, depending of the
device, its level of risk but also its destination, its use,
or its stage of development. We will also think about
a “bottom up” approach by starting from the
methodology/design point of view.
We built a working group constituted of
methodologist, medical doctors, specialists in
medical devices evaluations, usability experts, in
order to set up this identification and study of cases.
Our results will be confronted to different actors of
the field, disseminated and adapted along the
feedbacks we’ll received, and finally to provide better
ClinMed 2021 - Special Session on Dealing with the Change in European Regulations for Medical Devices
288

evaluations, better medical devices, for better
healthcare for patients.
REFERENCES
Barker, D, et al., 2016. Stepped wedge cluster randomised
trials: a review of the statistical methodology used and
available. BMC Med Res Methodol 6 ;16:6..
Brunotte, G., Beuscart, R., Pariset, A. and Pazart, L. Place
of High-risk Medical Devices in European
recommendations with a Focus on End-users. In
Proceedings of the 13th International Joint Conference
on Biomedical Engineering Systems and Technologies
(BIOSTEC 2020) - Volume 1: BIODEVICES, pages
350-360. ISBN: 978-989-758-398-8.
Campbell, G, 2011. Bayesian statistics in medical devices:
innovation sparked by the FDA. J Biopharm Stat.
21:871-87.
Campbell, G, et al., 2016. Statistical innovations in the
medical device world sparked by the FDA. J Biopharm
Stat. 26 (1):3-16.
Guetterman, TC, et al., 2015. Reflections on the Adaptive
Designs Accelerating Promising Trials Into Treatments
(ADAPT-IT) Process-Findings from a Qualitative
Study. Clin Res Regul Aff. 32(4):121-130.
Hamilton, C,et al., 2012. Sequential design for clinical trials
evaluating a prosthetic heart valve. Ann Thorac Surg,
93(4): 1162-1166.
Jiang, F, et al., 2013. A Bayesian decision-theoretic
sequential response-adaptive randomization design.
Stat Med, 32 (12):1975-94.
Kim SY, Flory J, Relton C. Ethics and practice of Trials
within Cohorts: An emerging pragmatic trial design.
Clin Trials. 2018 Feb; 15(1):9-16.
Lai, TL, et al., 2019. Adaptive enrichment designs for
confirmatory trials. Stat Med 38 (4) :613-624.
Lihoreau, T., Chatelain, B., Rolin, G., Vidal, C., Butterlin,
N., Jacquet, E., Elouneg, Chambert, J., Bertrand, X.,
Meyer, C. and Louvrier, A. End-user Need based
Creation of a Medical Device: An Experience of Co-
design to Struggle Pathological Scars. In Proceedings
of the 13th International Joint Conference on
Biomedical Engineering Systems and Technologies
(BIOSTEC 2020) - Volume 1: BIODEVICES, pages
317-322. ISBN: 978-989-758-398-8.
Lilford, R, et al., 2000. Trials and fast changing
technologies: the case for tracker studies. BMJ, 320
(7226): 43–46.
Magirr, D, et al., 2016. Sample Size Reassessment and
Hypothesis Testing in Adaptive Survival Trials. PLoS
One. 11(2):e0146465.
Meurer, WJ, et al., 2017. Multiple assignment randomized
Trials: An opportunity for improved design of stroke
reperfusion trials. J Stroke Cerebrovasc Dis. 26 (4):717-
724.
Pennello, G, et al., 2008. Experience with reviewing
Bayesian medical device trials. J Biopharm Stat 18 :81-
115.
Simon, N, et al., 2013. Adaptive enrichment designs for
clinical trials. Biostatistics 14(4):613-25. Tamura, RN,
et al., 2016. A small n sequential multiple assignment
randomized trial design for use in rare disease research.
Contemp Clin Trials. ; 46:48-51.
Vidal, C., Beuscart, R. and Chevallier, T. Contribution of
Methodologies Adapted to Clinical Trials Focusing on
High Risk Medical Devices. In Proceedings of the 13th
International Joint Conference on Biomedical
Engineering Systems and Technologies (BIOSTEC
2020) - Volume 1: BIODEVICES, pages 337-343.
ISBN: 978-989-758-398-8.
Wei, B, et al., 2018. A Bayesian analysis of small n
sequential multiple assignment randomized trials
(snSMARTs). Stat Med 20 ; 37 (26) :3723-3732.
Weijer C, Goldstein CE, Taljaard M. TwiC or treat? Are
trials within cohorts ethically defensible?. Clin Trials.
2018;15(1):21-24. doi:10.1177/1740774517746622.
Zelen, M, et al., 1990. Randomized consent designs for
clinical trials: an update. Stat Med 9 (6): 645-56.
Exloration of High Risk Medical Devices Methodologies for Optimized Evaluations
289
