
Usability Evaluation of Medical Devices during Clinical Studies: First
Results of a Scoping Review
Laura Douze
1a
, Jessica Schiro
1b
and Sylvia Pelayo
1,2 c
1
Univ. Lille, Inserm, CHU Lille, ULR 2694 - METRICS (Évaluation des Technologies de Santé et des Pratiques Médicales),
F-59000 Lille, France
2
Inserm, Tech4Health, F-59000 Toulouse, France
Keywords: Usability, Medical Device, Clinical Study.
Abstract: This scoping review is interested in mapping the clinical studies protocols of medical devices on usability
evaluation. The research question is as follows: How is usability of medical devices evaluated in clinical
studies? The paper presents some first results from a sample of 47 protocols within a set of 188 potentially
eligible protocols. Results highlight that a non-negligible part of usability evaluations are carried out
combined with clinical studies. Very often, usability outcomes are part of the secondary outcomes of the
clinical study. The most claimed usability-related outcomes are ease of use, handling and satisfaction.
Usability is mainly addressed through questionnaires which provide actually perceived usability (not usability
per se). Some protocols appear to be quite comprehensive in terms of usability evaluation methods.
1 INTRODUCTION
Medical devices diagnose, prevent, monitor, treat,
alleviate, or compensate for disease or injury (World
Health Organization, 2018). Their importance is
rising due to several factors, including advances in
technology, increases in lifestyle-associated disease
(Menotti, Puddu, Maiani, & Catasta, 2015;
Weisburger, 2002), and an aging population. Medical
devices developed with usability principles and
methods not only make devices easier to learn, more
efficient to use, more satisfying, and better able to fit
into peoples’ lives, but they also reduce the likelihood
of injury to patients, caregivers, and health-care
providers (Wiklund & Weinger, 2011).
The EU’s Medical Device Regulations (The
European Parliament and the Council of the European
Union, 2007, 2017) regulate the market access of new
medical technology. Since 2010, these regulations
have included the obligation to adopt a usability
engineering process. The main objective is to
optimize medical device usability as it relates to
safety, but also to task accuracy, completeness and
efficiency, and user satisfaction.
a
https://orcid.org/0000-0003-1759-0273
b
https://orcid.org/0000-0002-6710-8310
c
https://orcid.org/0000-0003-2830-2548
The usability engineering process is supposed to
be applied as early as possible during the
development process of a medical device. It includes
iterative usability evaluations of medical devices (i.e.
formative evaluations) and a final validation (i.e.
sommative evaluation). This final validation must
prove that the residual risk as it relates to usability is
acceptable. The EU regulation also mentions the
importance of the usability post-deployment
monitoring to follow-up the usage of the medical
device.
One of the challenges of this usability process is
to anticipate as well as possible the risks of use errors
before the deployment in real life of the medical
device. This supposes to conduct usability
evaluations as close as possible to the reality of
clinical settings. There is a need to “bring context into
the design and evaluation of usable and safe health
information technologies” (Kushniruk et al, 2013).
With this in mind, clinical studies are a good
opportunity to test usability and gather information
about the usage of a device. To our knowledge, no
studies have focused on usability studies conducted in
combination with clinical studies. This is precisely
Douze, L., Schiro, J. and Pelayo, S.
Usability Evaluation of Medical Devices during Clinical Studies: First Results of a Scoping Review.
DOI: 10.5220/0010385602930299
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 1: BIODEVICES, pages 293-299
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
293

the aim of this scoping review. The objective is to
identify the outcomes of clinical studies related to
usability evaluation and the methods used to collect
corresponding data. This paper presents the method
and first results of the study.
2 MATERIALS AND METHODS
We used the scoping review as the method for this
study. Our aim is to map the clinical studies protocols
of medical devices on usability evaluation. The
research question is as follows: How is usability of
medical devices evaluated in clinical studies?
2.1 Information Sources
The US National Library of Medicine database,
ClinicalTrials.gov, was searched. It is a well-known
database of privately and publicly funded clinical
studies conducted around the world.
2.2 Search Strategy
The search strategy was developed by two authors (JS
and LD). The general search terms were usability,
human factor, usage, use errors, satisfaction,
acceptability, acceptance, utility. Searches were
conducted between September 2020 and October
2020.
The following search string was used: (usability
OR satisfaction OR usage OR use errors OR
acceptability OR utility OR acceptance OR human
factors OR adherence OR adoption).
Any protocol about medical devices using
empirical methods of usability evaluation published
between January 2015 and October 2020 with the .pdf
protocol attached was considered. This means that the
following additional criteria were used as filters: only
Study Protocols as Study documents in the
ClinicalTrial.gov database, 01/01/2015 as Study start
date, and Medical device as Intervention/treatment.
2.3 Inclusion and Exclusion Criteria
The eligibility criteria were developed by two authors
(JS and LD). The usability definition provided by the
ISO 9241-11 was considered: “Extent to which a
product can be used by specified users to achieve
specified goals with effectiveness, efficiency and
satisfaction in a specified context of use.” All
protocols that included the collection of device usage
data to link device effectiveness and efficiency to its
intrinsic qualities were included in the analysis.
Objective data (e.g. use of a device, handling, ease of
use, safety of the procedure, adverse events,
successes) as well as subjective data (e.g. satisfaction,
perceived usability, barriers to adherence) were
considered for the analysis. The terms usage,
compliance or adherence if motivations were
collected, in terms of barriers for example, were
included in the analysis.
All in all, a protocol was included for analysis if
the following criteria were met:
The protocol included evaluation of a medical
device or a combination product.
The protocol concerned usability evaluation as
described in the outcomes of the protocol (e.g.
satisfaction, perceived usability, ease of use,
difficulties to use, handling, safety of the
procedure, utility).
A protocol was excluded of the analysis if the
following criteria were met:
The protocol evaluated a product that was not a
medical device or a combination product (e.g.
a drug, a behaviour, a procedure).
The protocol didn’t evaluated usability (e.g.
evaluate rather comfort, time spent for
procedure, clinical performance).
The protocol focused only on the satisfaction of
a patient and/or his/her family while they were
not the end users (e.g. medical device used by
healthcare professionals, while the patients and
their families were the beneficiaries).
The protocol concerned adherence to the
medical device without gathering information
about the motivations/reasons for the
adherence (or non-adherence) or acceptability.
The protocol was not a clinical study (e.g.
authors claimed in the protocol that it was not
a clinical study, but a “classical” evaluation).
2.4 Search Results and Selection
Two authors (JS and LD) searched the
ClinicalTrials.gov database which yielded 883
protocols for possible inclusion in the scoping review
(Figure 1). In a first step, two of the authors (JS and
LD) independently screened 50 protocols on titles and
outcome measures.
Then, they pooled the results and discussed non-
agreements until consensus was reached. This first
step allowed a refinement of the eligibility criteria. In
a second step, the same two authors (JS and LD)
independently screened 300 other protocols on titles
ClinMed 2021 - Special Session on Dealing with the Change in European Regulations for Medical Devices
294
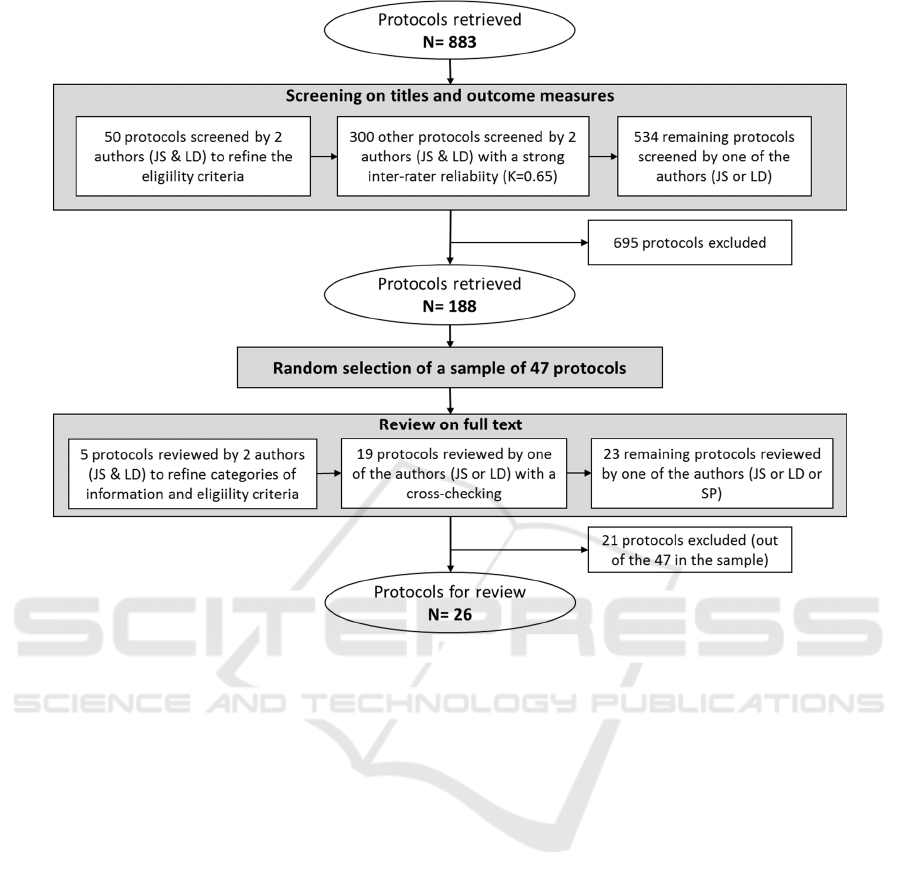
Figure 1: Flowchart of protocol selection.
and outcome measures. Then, they pooled the results
to assess the inter-rater reliability; the Cohen’s kappa
was 0.65 which indicated a strong agreement
(Krippendorff, 2013). The remaining protocols (534
protocols) were therefore screened by one of two
authors (JS and LD).
The screening of the protocols based on the titles
and the outcome measures led to 188 protocols to be
reviewed on their full text. A random selection of 47
protocols among the 188 was made for analysis.
Among these 47 protocols, 21 were excluded since
the studies did not met the eligibility criteria. Twenty-
six protocols were finally included for the next step.
2.5 Data Extraction and Categorisation
As for the selection of the protocols, the different
categories of information extracted from the
protocols were the result of an iterative and
collaborative work. In a first step, 5 protocols were
independently reviewed by two of the authors (JS and
LD) and then pooled in order to validate the
categories of information and their definition and to
refine the eligibility criteria. Then, in a second step,
19 protocols were reviewed by one of the authors (JS
or LD with respectively 9 and 10 protocols) to extract
the information. All the 19 protocols were cross-
checked to verify the extraction and completion of the
information. The remaining 23 protocols were
reviewed by one of the authors (JS or LD or SP).
General and specific information about the 26
protocols was extracted from the .pdf document
protocol and some metadata provided by the
ClinicalTrials.gov database in the study design. Table
1 lists all the extracted information along with their
definition.
Data extracted from each protocol was recorded
on an Excel computer worksheet in order to
categorise and compare characteristics. This study
was a scoping review with a focus on mapping the
clinical studies protocols of medical devices on
usability evaluation. As the objective was not to
collect the best available evidence, critical appraisal
of the selected articles was not performed.
Usability Evaluation of Medical Devices during Clinical Studies: First Results of a Scoping Review
295
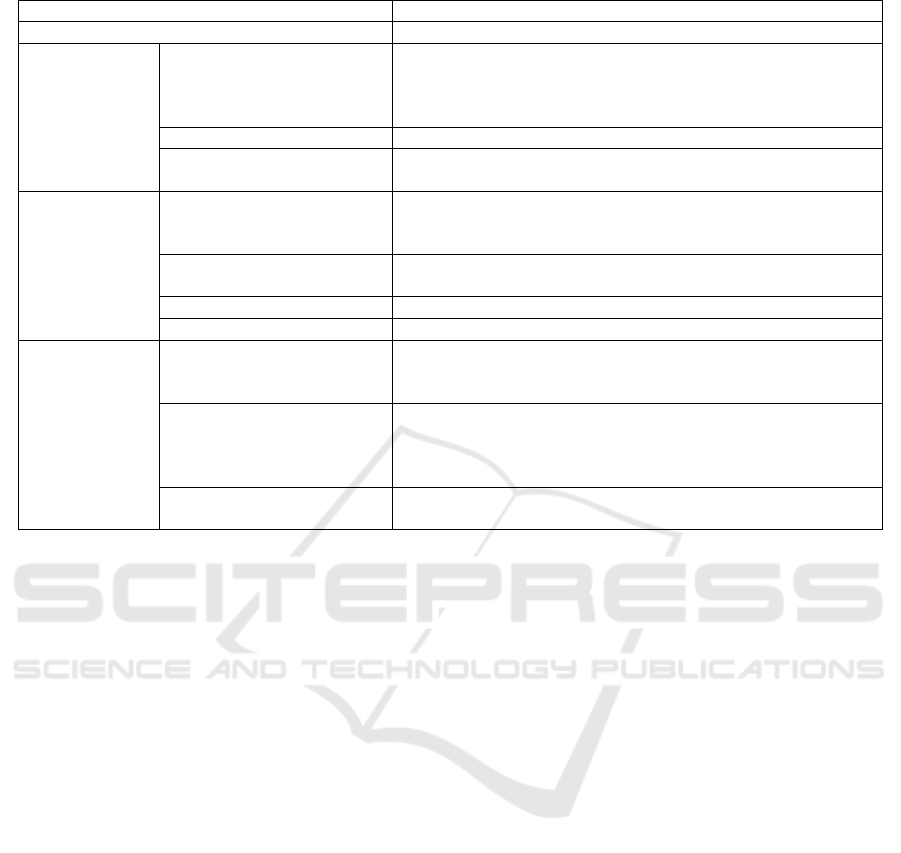
Table 1: Information categories extracted during the analysis and their definition.
NCT ClinicalTrials.gov Identifier.
Title Title of the protocol.
Medical device
specification
Condition or disease
Disease, disorder, syndrome, illness, or injury that is being
studied. On ClinicalTrials.gov, conditions may also include
other health-related issues, such as lifespan, quality of life, and
health risks.
Device Medical device that is the focus of the clinical study.
End user considered in the
clinical study
Person that will use the medical device during the clinical study,
i.e. Patient and/or Healthcare professional or Other
Study design
Study type
Nature of the clinical study, includes interventional studies (also
called clinical trials), observational studies (including patient
registries), and expanded access.
Intervention model
Intervention model of the study includes one group assignment,
parallel assignment, crossover assignment or cohort.
Number of participants Number of participants that is planned to be recruited.
Post Market Surveillance Is the clinical study a post market surveillance study?
Usability
evaluation*
Category of primary
outcome measure related to
usability
Category of the planned outcome measure that is the most
important for evaluating the effect of the medical device, if
related to usability evaluation.
Category of secondary
outcome measure related to
usability
Category of the planned outcome measure that is not as
important as the primary outcome measure for evaluating the
effect of the medical device but that is still of interest, if related
to usability evaluation.
Type of usability-related
methods
Methods specified in the protocols to collect usability-related
outcomes.
* The values of these categories were established by the authors based on the information provided in the protocols.
3 RESULTS
3.1 Medical Device Specifications
Several types of medical devices are concerned by the
clinical studies including usability evaluations (Table
2), e.g. digital health devices (e.g. app to monitor
glucose, virtual reality systems, image guidance
system), biomaterials, orthoses, contact lenses,
therapeutic boot or shoe. The medical devices are
intended for both patients and healthcare
professionals.
3.2 Study Design
From our sample, the clinical studies incorporating
usability assessment that are reported in
ClinicalTrials.gov are essentially intervention studies
(Table 3), only one observational study has been
identified, a cohort study. These clinical studies are
either follow-up studies of the use of a medical device
with one group of participants, crossover studies or
comparative studies (2 groups). Only one post market
surveillance study has been identified. Sample sizes
are highly variable.
3.3 Usability Evaluation
Among the 26 analysed protocols, 5 protocols have
primary outcomes related to usability evaluation.
Figure 2 presents the different categories of outcomes
considered in each of the 26 protocols. Seventeen
protocols include one outcome related to usability
while 9 protocols include at least two usability-related
outcomes. The three most claimed outcomes (at least
8 protocols from our sample) correspond to the ease
of use of the medical device, its handling and the
satisfaction it provides when using it. Some studies
are interested in use errors or barriers to medical
device adherence (at least 3 protocols from our
sample). Other outcomes are also sometimes used,
such as willingness to use, acceptability, user-
friendliness, clarity of information or usefulness as
related to usability.
Figure 3 shows the different methods on which the
protocols are based on. The classical methods of the
usability field are used, e.g. shadowing,
questionnaires, interviews, user testing. The
questionnaire is the most widely used technique,
followed by the interview. Most of the protocols
(16/26) rely on one technique to collect usability-
ClinMed 2021 - Special Session on Dealing with the Change in European Regulations for Medical Devices
296

Table 2: Specifications of the medical device concerned by the clinical studies included in the analysis.
Condition or disease Device (NCT)
End user considered in
the clinical study
Diabetes
Sealed therapeutic shoe (NCT04085926) Patient
Offloading boot (NCT02783066) Patient
Diabetes app to assess diabetes control
(NCT03252964)
Patient
Continuous glucose monitor combined with an
activity tracker (NCT03165110)
Patient
Myopia, Astigmatism, visual
acuity
Contact lens (NCT03086447; NCT03024970;
NCT03006458; NCT03139578;
NCT03098745; NCT03707821;
NCT03679741)
Patient
Accidental falls Ankle Foot Orthoses (NCT02819011) Patient
Hearing Loss Successor hearing aid (NCT03086018) Patient
Amblyopia
Virtual reality based digital therapeutic that
applies therapeutic modifications in real-time to
cinematic content to rebalance visual input and
treat amblyopia (NCT03608150; )
Patient
Fecal Incontinence Anal tape (NCT02989545) Patient
Stroke
Smart Glove (home based virtual reality
biofeedback system) (NCT03559829)
Patient
Asthma
Propeller Health device + asthma navigator
(NCT03065205)
Healthcare professional
& Patient
Medication Adherence
Device for Dispensing Pain Medications in
Hospice Patients (NCT03940534)
Healthcare professional
& Patient
Feeding tube dysphagia
Enhanced enteral feeding device
(NCT03007511)
Healthcare professional
Hypothermia Neonatal Non-Electric Infant Warmer (NCT03031431) Healthcare professional
Spinal Diseases New image-guidance software (NCT03015142) Healthcare professional
Wounds and Injuries,
Lacerations, Surgical Incision
Polyurethane-based skin adhesive
(NCT03688880)
Healthcare professional
Coronary Artery Bypass
Grafting
Pliable and absorbable bone hemostats
(NCT03085017)
Healthcare professional
Aortic Valve Stenosis
Portico TF and ALT Delivery System
(NCT03056573)
Healthcare professional
Table 3: Study designs of the clinical studies included in the analysis.
Interventional model
Single group
assignment
Parallel
assignment
Crossover
assignment
Cohort
Interventional
study
PMS 1 / 1 /
No PMS 7 10 6 /
Observational
study
PMS / / / /
No PMS / / / 1
Number of participants:
Mean (SD)
Min-Max
95, 33 (122,04)
15-400
116, 4 (91, 34)
10-267
49, 42 (36, 41)
20-120
240 screw
placements (15
to 25 patients)
related data, mostly on questionnaires. Almost 40%
of protocols (10/26) combine several techniques,
quite often interviews and questionnaires, but also
shadowing and interview. The category Other refers
to techniques used once in one of the protocols; the
log analysis was used once, as the technique of the
diary study (the end user document in a journal some
Usability Evaluation of Medical Devices during Clinical Studies: First Results of a Scoping Review
297

Figure 2: Categories of outcome concerned by each of the
protocols.
elements related to the use of the device), or the
analysis of adverse events to identify use errors.
When crossing outcomes with methods (Table 4),
not surprisingly questionnaires are used to address
ease of use or satisfaction of the participants with the
medical device, but more surprisingly also to
collectinformation on the handling of the device
which is supposed to be more objective data.
Interestingly the shadowing technique is exclusively
used with healthcare professionals (Table 5).
Figure 3: Methods concerned by each of the protocols.
Table 5: Methods depending on the participants in clinical
studies included in the analysis.
Healthcare
professional
Patient Other
Shadowing 6 0 0
User testing 0 1 0
Interview 3 8 1
Questionnaire 7 26 0
Other 2 2 0
Table 4: Categories of outcomes and methods of clinical studies included in the analysis.
Shadowing
User
testing
Interview Questionnaire Other
Ease of use 5 0 5 7 2
Handling 1 1 3 11 2
Satisfaction 0 0 0 8 0
Use error 3 0 3 2 1
Barrier to adherence 0 0 3 3 1
Willingness to use 0 0 0 1 0
Usefulness/Utility 0 0 0 2 0
Acceptability 0 0 0 1 0
User friendly/User Experience 0 0 0 2 0
Clarity of information 0 0 0 1 0
ABCDEFGHI J
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
AEase of use
BHandling
CSatisfaction
DUse error
E Barriers to adherence
F Willingness to use
G Acceptability
H Userfriendliness/UX
I Clarity of information
J Usefulness/Utility
ABCDE
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
A Shadowing
B User testing
C I nterview
D Questionnaire
EOther
ClinMed 2021 - Special Session on Dealing with the Change in European Regulations for Medical Devices
298

4 DISCUSSION
The first results of this scoping review highlight that
a non-negligible part of usability evaluations is
carried out combined with clinical studies (or planned
to be carried out as only protocols have been
analysed). Very often, usability outcomes are part of
the secondary outcomes of the clinical study. The
most claimed usability-related outcomes are ease of
use, handling and satisfaction. Usability is mainly
addressed through questionnaires which provide
actually perceived usability of the medical device
instead of usability per se. While several protocols
appear to be quite comprehensive in terms of usability
evaluation methods, the vast majority of protocols
refer to notions close to that of usability, but not to
usability.
But this paper presents only some first results of
the scoping review and are maybe not representative
of the results out of the total of 188 protocols.
Moreover, not all usability studies conducted with
medical devices are necessarily reported on a
database such as ClinicalTirals.gov. But these first
results show the validity of the methodology and
some interesting trends.
REFERENCES
Krippendorff K. Content analysis. An introduction to is
methodology. Third. USA: Sage; 2013.
Kushniruk, A., Nohr, C., Jensen, S., Borycki, E. M. From
usability testing to clinical simulations: Bringing
context into the design and evaluation of usable and
safe health information technologies, Yearb. Med.
Inform. 22 (01) (2013) 78–85, http://dx.doi.org/
10.1055/s-0038-1638836.
Menotti, A., Puddu, P. E., Maiani, G., & Catasta, G. (2015).
Lifestyle behaviour and lifetime incidence of heart
diseases. International Journal of Cardiology, 201,
293_299.
The European Parliament and the Council of the European
Union, Regulation (EU) 2017/745 of the European
Parliament and of the Council of 5 april 2017 on
medical devices, 2017, https://eur-lex.europa.eu/legal-
content/EN/TXT/PDF/?uri=CELEX:32017R0745&fro
m=ENThe European Parliament and the Council of the
European Union, Directive 2007/47/EC of the
European Parliament and the Council of the European
Union of 5 september 2007 on medical devices, 2007,
https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?
uri=OJ:L:2007:247:0021:0055:en:PDF
World Health Organization. (2018). Medical device -
Full definition. http://www.who.int/medical_devices/
full_deffinition/en/. Retrieved September 16, 2018.
Weisburger, J. H. (2002). Lifestyle, health and disease
prevention: the underlying mechanisms. European
Journal of Cancer Prevention, 11, 1_7.
Wiklund, M. E., & Weinger, M. B. (2011). General
principles. In M. B. Weinger, M. E. Wiklund, & D. J.
Gardner-Bonneau (Eds.), Handbook of human factors
in medical device design. New York: CRC Press.
Usability Evaluation of Medical Devices during Clinical Studies: First Results of a Scoping Review
299
