
Methods for Preclinical Validation of Software as a Medical Device
Alice Ravizza
1
, Federico Sternini
2a
, Alice Giannini
3
and Filippo Molinari
4b
1
USE-ME-D srl, I3P Politecnico di Torino, Torino, Italy
2
Politecnico di Torino, Torino, Italy
3
Stefanelli & Stefanelli Law Firm, Bologna, Italy
4
Biolab, Department of Electronics and Telecommunications, Politecnico di Torino, Torino, Italy
filippo.molinari@polito.it
Keywords: Software as a Medical Device, Preclinical Validation.
Abstract: Software as a medical device is subject to dedicated regulatory requirements before it can be used on human
beings. The certification process in Europe requires that sufficient data on clinical benefits are available before
the device is CE marked. This position paper describes our proposal of a risk-based approach to technical and
preclinical validation of software as medical devices. This approach ensures that all technical solutions for
safety are implemented in the software and that all information for safe use is consistent before the software
can be made available to patients. This approach is compliant to the main international standards ISO 13485
on quality systems and ISO 14971 on risk management and therefore ensures regulatory compliance as well
as patient protection. This integrated approach allows the designers of the software to integrate regulatory and
safety testing in the technical testing of the candidate release version of the device. This approach ensures
patient safety and regulatory compliance at the same time as technical functionality.
1 INTRODUCTION
Currently, among all software solutions related to
health, only few can be considered as medical
devices, at least from a regulatory point of view. For
a software to be considered as a medical device it
shall be specifically intended to perform a medical
action: vital parameters monitoring for diagnosis;
provision of information for subsequent diagnosis or
therapy; therapy of a disease; alleviation of a
disfunction; control of conception. Specifically, the
medical device definition from World Health
Organization (Executive Board, 2019) states that it
shall be “used in the prevention, diagnosis or
treatment of illness or disease, or for detecting,
measuring, restoring, correcting or modifying the
structure or function of the body for some health
purpose”; this definition is also in the European
Regulation 2017/745 (European Parliament &
European Council, 2017).
This definition rules out all software used for
wellness, self monitoring and self enhancement such
a
https://orcid.org/0000-0002-5510-2296
b
https://orcid.org/0000-0003-1150-2244
as apps for the monitoring of body weight of healthy
subjects, wearable sensors for the monitoring of heart
beat during sport activities, software for guided
meditation. So, not all software about the human body
is actually a medical device: this guideline applies
only to those software that fall into the definition of
medical devices.
Medical software is specifically designed to
provide a measurable clinical benefit to an individual
patient. Being the “zero-risk” condition impossible,
regulations prescribes that medical software shall not
only provide a measurable and evidence based
clinical benefit, but also that this expected benefit
shall overweight any risk posed by the software to the
patient, the software users and the general
environment. We present this position paper about the
preclinical validation of software as a medical device,
the first step in a long process that leads from ideation
to certification of a medical software.
648
Ravizza, A., Sternini, F., Giannini, A. and Molinari, F.
Methods for Preclinical Validation of Software as a Medical Device.
DOI: 10.5220/0009155406480655
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 5: HEALTHINF, pages 648-655
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 EUROPEAN REGULATORY
LANDSCAPE
In Europe, the core regulatory requirements on
medical devices can be summarised as “the risk-
benefit profile of the device is favourable to the
patient” and “all risks have been adequately
minimised”. Specifically, new requirements on
medical software are laid out in the Medical device
Regulations 2017/745 (European Parliament &
European Council, 2017) for medical devices and
2017/746 (European Parliament & European Council,
2017) for in vitro medical devices.
Those regulations set very detailed requirements
that ensure that device, before being provided to
patients, has shown an adequate level of safety and
benefit. Moreover, the software life cycle plan shall
ensure an adequate level of quality over time. In this
position paper, we focus particularly on safety aspects
that should be ensured before the device is rendered
available to patients.
We have given special attention to the use of
international standards, and have given preference to
European harmonized standards: this approach
ensures that the methods described in this paper are
adequate not only for design of medical software but
also match the regulatory requirements and allow for
a faster certification path in Europe and in countries
non-members of the European Union that
implemented or harmonized European Regulations
and Directives (De Maria et al., 2018). Additionally,
the use of European harmonized standards allows
presumption of compliance to the European
Regulations.
In terms of medical device design, the core
requirements are set in the standard EN ISO 13485
(International Organization for Standardization,
2016). Software developers shall list the design inputs
to describe the user needs, the expected functionality
and performance and to define the main risks that
should be overcome to ensure patient safety. The risk
identification and reduction is managed by the
application, during the input phase and also during
development and testing, of the standard EN ISO
14971 (European Committee for Standardization, &
International Organization for Standardization,
2012). These general standards shall be applied
together with the specific standard on software life
cycle management, IEC 62304 (International
Electrotechnical Commission & International
Organization for Standardization, 2015) that sets
requirements not only for the input list, but also on
methods and minimum contents of the software
testing. A core requirement is that all the risk
minimisation measures shall be included in the
software testing plan.
Another core standard that should be taken into
account is IEC 62366 (International Electrotechnical
Commission, 2015) that sets methods to design and
test the device for usability.
At the present time, to our knowledge, the state of
the art in approaching to regulatory requirements for
the European market is a “vertical approach” method.
In fact, the industry standard in the management of
the software life cycle is typically to approach the
compliance to each international standard as a
separate task, that is carried out by a different
development or testing team. The Quality Assurance
testing is performed according to a test plan that is
based on software functionality and is not based on
the assessment of the potential impact of software
malfunctioning on patient health. Additionally,
usability is taken care of by a dedicated, specialised
team that is not involved in the setup of the functional
testing. Lastly, privacy requirements are forcibly
added in the software functionalities by another,
separate team. A comprehensive test plan, with full
coverage of all regulatory requirements, is rarely
available. This is reflected in a shortage of
information for Competent Authorities when they
assess the compliance to European regulations.
Additionally, this lack of comprehensive planning
impacts those developers that have to take care of the
software changes over time, as they design small sets
of testing especially for the change under evaluation,
while losing control of the main medical features of
the device.
3 SOFTWARE LIFE CYCLE
MANAGEMENT
3.1 Input Requirements
According to ISO 13485 (International Organization
for Standardization, 2016) the first design step should
be a clear list of requirements for the developers. The
standard cites amongst others "functional,
performance, safety, regulatory requirements" and
clearly requires to include usability requirements and
the definition of the main risks.
Every medical software is different, but we
propose to define at the early stages of the
development at least the following core inputs, that
are presented in an order that reflects their impact on
the development:
Methods for Preclinical Validation of Software as a Medical Device
649

Clear expected clinical benefit: what target
patient population the software is meant for,
what pathology or condition it is addressed to
and what are the expected benefits on those
patients and those health conditions. The
clearer this definition is, the more focused the
design and testing activities
Expected users: the use by professionals,
laypersons, special needs persons or different
age groups shapes all aspects of design and
leads the risk analysis
Means to measure the clinical benefits: once
the clinical benefits are clearly expressed, they
shall be measured in a reliable and repeatable
way. This means defining which data are
collected, the frequency and quality of this
collection, and how the data are analysed to
detect variations that measure the actual
improvements on the patient status
Data storage: data should be safely stored
whatever is the support of the storage. Consider
a catastrophe recovery system and a backup
database. In case of a centralised database,
specify proper countermeasures to minimise
errors in data synchronisation.
Associated software and hardware: Changes in
the Software Of Unknown Provenance (SOUP)
should be controlled with appropriate policies
that plan consequent changes. We propose to
classify SOUPs in three risk levels: “red”
SOUPs are SOUPs whose malfunctions can
expose patients to risk, “yellow” SOUPs are
SOUPs that affects the use of the device,
“green” SOUPs are all other SOUPs. After the
SOUP classification, we propose a reaction
policy to SOUP changes.
We have a special policy regarding software as a
medical device as medical apps on smart devices. The
Operating system is under all points of view a “red”
SOUP, because it sustains the Software architecture.
A malfunction may interfere with the software proper
use or availability and can lower the patient state of
health, if the patient management is primarily based
on the app itself. iOS and Android have their own
versioning and the medical software shall follow the
versioning; this means that the developers shall
support the latest available version to iOS and
Android. It should be noted that Android and iOS
provide notifications in advance for major changes.
At each change, the development team shall
evaluate the change and define:
All the required changes
Timeline for changes
Verification testing
Table 1: Reaction policy to SOUP changes.
RED YELLOW GREEN
The
SOUP
complies
to a
LEGAL
REQUI
REMENT
Update is top
urgent and
requires to
repeat the
technical
validation
Update is
top urgent
and
requires
repeat the
usability
testing
Update is
top urgent
but re-
validation
may not
be
required
The
SOUP
change
affects the
user
experience
Update is top
urgent and
requires to
repeat the
technical
validation, the
assessment of
the risk
minimization
measures, and
requires to
repeat the
usability
validation
Update is
top urgent
and
requires to
repeat the
usability
testing
Update
can be
planned
and re
validation
may not
be
required
For each software update, the design team shall
assess the impact. Impact can be classified as a major
change (update that modifies the risk profile of the
device), minor change (update that changes the user
experience without modifying risk or usability profile
of the device,) and bugfix (any other update). While
major changes require a new clinical and safety
validation, minor requires only new safety validation.
Also, the quality of the data transfer between modules
shall be proved, including the accuracy and reliability
of the measurements originating from sensors
(Ravizza, De Maria, et al., 2019).
To propose this approach, our method includes
the application of brain storming techniques and
FMEA techniques to risk analysis, as the very first
step of planning of the validation testing. In fact, the
risk control measures that can be implemented at
design stage (safe-by-design risk control) shall be not
only implemented but also verified and therefore shall
be part of the test protocol.
3.2 Identification of Main Risks and of
Risk Control Measures
The standard ISO 14971 (European Committee for
Standardization & International Organization for
Standardization, 2012) on risk management foresees
that all possible risks posed to the patient safety and
also to data security are identified; the standard
HEALTHINF 2020 - 13th International Conference on Health Informatics
650
suggests structured methods such as Failure Mode
and Effects Analysis (FMEA). Subsequently,
developers are requested by the standard to
implement all those control measures that are
technically available, in order to lower and minimise
the risks. The preferred order to the risk minimisation
measures, as stated in the European Regulation, is to
give priority to a safe-by- design approach. If no safe-
by-design solutions are available, then developers
should include adequate protections and alarms;
additionally, all the required information for safe use
shall be provided.
Again, every medical software is different, but we
have identified some core issues that may be
applicable to a vast majority of medical device
software; for each we also have identified a possible
risk minimization measure. Hereby we list the risks
in an order that reflects the potential impact on patient
safety.
Table 2: Examples of core issues and their risk control
measures.
Risk Risk control measures
Risk of delayed
treatment
Streamlined use flow chart
Risk of alarm
overload
Alarms should be differentiated
in priority levels according to
the associated risk.
Risk of improper
treatment
Alarms should be triggered for
any use that is inconsistent with
previous data or clinical
guidelines
Risk of patient
incomplete or
wrong profiling
Guided data insertion procedure,
patient ID always visible,
minimal screen cluttering
Risk of improper
professional use
Clear definition of profile
privileges and procedure for
password management
Risk of data loss Backup, activity log of all user
interactions
Risk of data breach Basic cybersecurity measures
Risk of patient
privacy break
Data pseudonymization, strict
control on profiles with data
access privilege
3.3 Specific Activities for Privacy
Requirements
In Europe, the privacy rights of citizens are defined
and protected by the Regulation 2016/679, so called
GDPR (European Parliament & European Council,
2016).
The main rights include:
Article 15 GDPR – Right of access: function to
export data upon request. Data should be
exported in a common format (according to art.
20 GDPR – Right to data portability);
Article 16 GDPR – Right to rectification:
function to update data upon request with a
record of the update;
Article 17 GDPR – Right to erasure: design
function that perform record deletions of all
data points connected to one subject quickly
and efficiently (deleting the record shouldn’t
affect the integrity of the whole database) with
a report of deletion
Article 18 GDPR – Right to restriction of
processing.
Art. 22 GDPR- Automated individual decision-
making, including profiling: functions which
allow to turn on or off certain processing
parameters
As medical devices collect a great entity of
personal data, it is necessary to deal with the privacy
aspects of this data collecting and processing. In
literature, there are applicable position papers that
provide an in depth analysis of how to apply concepts
of the GDPR to Artificial Intelligence for specific
medical purposes (Pesapane, Volonté, Codari, &
Sardanelli, 2018)
. Minimum measures required to be
GDPR compliant might include:
Conduction of a Data Protection Impact
Assessment before the medical software is in
use;
Design the software to collect and store only
data which is necessary for the core activity of
the medical software;
Regularly pseudonymize data for back-end
processing.
Restrict access and privileges of developers to
sensitive data if not strictly necessary;
Design software functions which allow for data
subjects to correctly exercise their rights.
Conduct periodical tests of effectiveness of
data security measures of the software;
Pseudonymization represents, from a privacy
standpoint, both a type of data processing and a
Methods for Preclinical Validation of Software as a Medical Device
651
measure to ensure data security. As such, it lowers the
risks connected to the processing of particular sets of
data such as those related to health.
Moreover, according to the GDPR,
pseudonymized data are to be considered as personal
data. On the other hand, anonymization consists of a
processing which results into an irreversible de-
identification of the data subjects. Data subjects need
to be informed, in a transparent way, that their clinical
data might be anonymized and used for further
research. Thus, the anonymization of patients’ data
represents a secondary use which does not require the
consent of the patient. The GDPR is not applicable to
fully anonymized data but, differently from other
privacy standards, it does not include a set of
variables that when removed from a dataset render the
data anonymous, making this specific type of
processing riskier.
3.4 Output of the Design: Software
Functionalities
Designers should be able to define, for each end user,
a set of privileges and a list of functionalities that the
software provides.
Such functionalities are those software actions
that, once used on the patient or for the patient, will
provide the clinical performance and therefore ensure
the patient clinical benefit.
For example, a medical device software for
medical adherence may have different functionalities:
plan a therapy, alert the patient when the therapy
should be taken, measure the amount of drug that was
taken, monitor the adherence in terms of timing and
dosing, coach the patient to improve adherence,
provide suggestions to manage symptoms or
collateral effects and so on. In this case, not all
functionalities will be available to the patient directly:
for example, only a user with a “physician” profile
may be able to access to the therapy planning
function.
On the other hand, a software for cognitive or
behavioural therapy will have completely different
functionalities such as provision of images, text, or
sounds at specific times of the day or in reaction to
patient inputs.
It should be noted that software modules can be
discriminated according to the functionality for the
user, where each of these features is related to a
module. Some of these modules have a medical
purpose, others do not.
Some features without a medical purpose are
widely present in medical software, for example:
collect and retain the patient's administrative details
or archive the patient's medical history
Some modules may have ancillary functions, for
example: audit trail, access and security,
cryptography, archiving and backup of personal data.
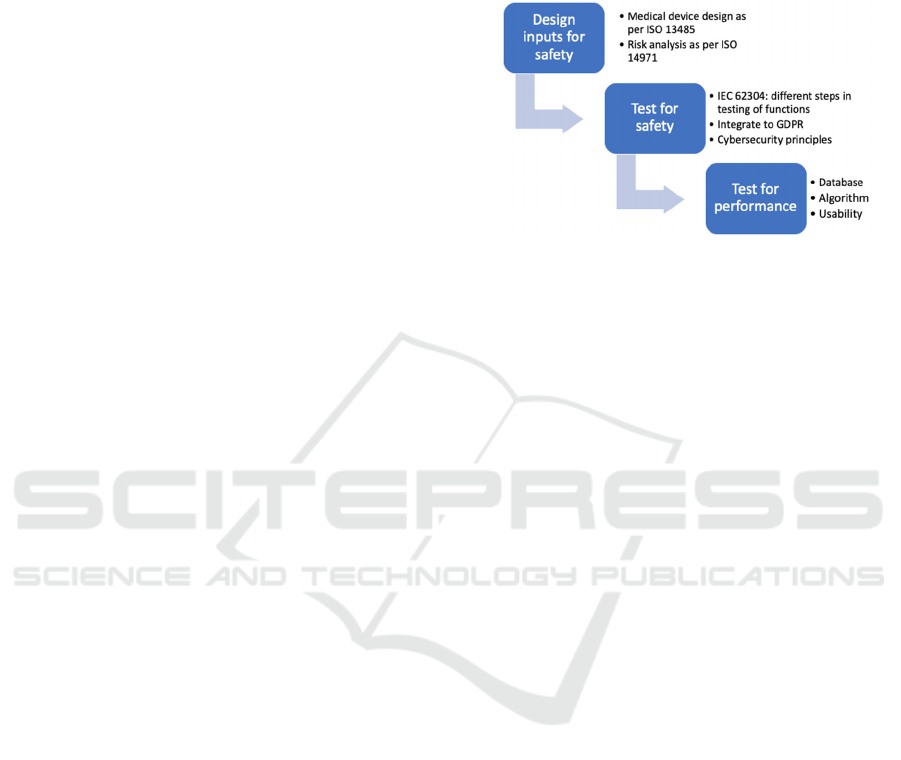
Figure 1: The risk-based approach.
4 SOFTWARE TECHNICAL
VERIFICATION
4.1 Technical Verification of
Functionalities and Risk Control
Measures
After the development of the Minimum Viable
Product of the Medical Device, its safety and clinical
benefit shall be tested. The testing should follow a
fixed plan built on the base of the input requirements
drafted at the beginning of the design stage. This plan
shall not only declare the required input, but also
define, for each input, the testing method and the
expected result. The tests are passed if the result is
consistent with the expected pre-declared deliverable
and if it does not introduce any additional risk to the
patient. While some requirements may need actual
functional tests on the device, others can be satisfied
with documentation and procedures. Technical
requirements that need functional tests may include
the correct alarm activation, which requires to prove
that it is activated when needed, and that does not
activate when it is not needed. On the other hand,
other requirements are not strictly related to the
software development, such as the password policy
and the personnel training.
For risk control measures that are information for
safe use, the verification is in two steps. First verify
the instructions for safe use is in place, then verify
that it is clear (during the usability test).
The outcome of the test defines the needs for
additional development. If all tests are passed, the
product is ready to be tested for usability and clinical
HEALTHINF 2020 - 13th International Conference on Health Informatics
652

benefit. Otherwise, if some tests failed, but they do
not affect risk for the patient or do not modify the
usability profile of the device, the manufacturer can
still test the device for usability but should refrain to
use it for clinical benefit until all the technical
functions have been confirmed.
4.1.1 Usability Verification
Usability verification aims to confirming the safety of
the user interface and also of the provided
information, that together enable effective use and
protect against potentially harmful use errors. The
reference standard is IEC 62366 (International
Electrotechnical Commission, 2015) that proposes
various methods for usability verification. We
proposed in the past the choice of the most adequate
methods according to the device kind and the step in
the device design process (Ravizza, Lantada,
Sánchez, Sternini, & Bignardi, 2019).
For validation activities, we propose to define a
structured approach to user testing in real or
simulated use conditions, with real users. The
preparation of such user tests requires that the
usability experts define, together with the designers,
a list of expected use scenarios, that shall be chosen
in order to simulate the most common use and also
any hazardous situations in the expected use
experience. We usually prefer to describe the use
experience by a flow chart of each use scenario and
then to provide detailed description by a task list
analysis. For detailed risk management, this task list
may be used as the entrance information to apply
Failure Mode and Effect Analysis techniques. We
also propose to involve real users in the usability
evaluation activities, by applications of the use
scenarios in a real or, most probably, simulated
clinical setting and by observation of user errors and
any safety-critical errors.
Analysis of user errors and of critical errors as
observed leads to the approval of the user interface or
to the identification of non-acceptable use risks, that
would lead to device interface re-design (Ravizza,
Lantada, et al., 2019; Zhang, Johnson, Patel, Paige, &
Kubose, 2003).
4.2 Aim of the Verification Activities:
Compliance with the Medical
Device Regulation and to GDPR
In Medical Device Regulation (MDR), Annex I
section 17 declares that medical device software shall
“be designed to ensure repeatability, reliability and
performance in line with their intended use”.
For compliance with the essential requirement 17
of the MDR, the manufacturer is required to apply
management principles to the entire software life
cycle: these principles are listed in the EN 62304
(International Electrotechnical Commission &
International Organization for Standardization,
2015).
Another core Essential Requirement, is the point
5 of Annex I, that requires that risk minimisation
measures include use error minimisation principles
and usability principles. The manufacturer may apply
the methods described in the standard IEC 62366;
while this standard is not harmonized, it is considered
as state-of-the- art and is cited as a means of
compliance in both ISO 13485:2016 and IEC 62304
in its current version (indirectly cited, by citation of
IEC 60601-1-6) and in its proposed draft (directly
cited) (International Electrotechnical Commission &
International Organization for Standardization, 2015;
International Organization for Standardization,
2016).
The new cybersecurity requirements of
Regulation (EU) 2019/881 (European Parliament &
European Council, 2019) referred to below also apply
to software:
Consider the risks related to the possible
negative interaction between the software and
its IT environment (Annex I, Chapter II,
Section 14.2 (d)).
Develop the software taking into account
information security (Section 17.2).
Establish minimum requirements relating to
hardware, IT network features and IT security
measures, including protection against
unauthorized access (Section 17.4)
Include these instructions in the instructions for
use (Annex I, Chapter III, Section 23.4 (ab)).
Furthermore, the principles of art. 24 of the
GDPR for Software as Medical Device. Since
software treats data: that is to say that they must be
designed according to the principles of privacy by
design and by default pursuant to art. 24 of the GDPR.
Very briefly, the software must comply with the
principles set out in art. 5 (in particular the principles
of minimization, accuracy, security, integrity and
confidentiality of the data). It must also allow the user
(as Data Controller or Data Processor) to respect the
general principle of accountability.
4.3 Proposed Test Methods
For regulatory purposes, device validation shall give
proof that the device is adequate for its intended
Methods for Preclinical Validation of Software as a Medical Device
653

purpose and to confirm its estimated risk benefit
profile. No major modifications are expected after the
device validation, in terms of design characteristics
for risk minimisation. On the other hand,
improvement suggestions can be collected, for the
subsequent future iterations.
4.3.1 For Technical Verification
The device design process has defined the input
requirements, including expected results of real use.
Therefore, to complete the technical validation, a
simulation of the real-world condition is proposed.
The simulation should include the use of dummies
that simulate typical patients/users; these dummies
that can be designed to reflect what is expected by real
user interactions; we propose, to simulate the real
users, to define a known dataset of real user activities
and then apply the principle of the mode, as opposed
to the principle of the average. The proposed strategy
simulates the real users with the most frequent
activity, ensuring that the simulation is representative
of the majority of the users. We propose to not use the
average principle because it could create simulations
that are statistically representative of the users but
that are not consistent with any user activity. For
example, if the interaction is defined as the time
reaction to a stimulus, the mode provides real users
time reactions, while average may be biased. In the
case of high-risk applications, manufacturers should
complete additional simulations with additional
dummies, built to represents high-risk user/patients or
worst-case scenarios.
4.3.2 For Usability
The international standard on usability requires that
designers validate those parts of the interface that are
meaningful for clinical use, including all the critical
ones. For example, all the software interactions with
the patients should be included, while some on-
boarding activities of the professional users and the
administrative or logistic modules may be subject to
very light usability assessment.
Since all the critical use scenarios should be
tested, we propose that a complete task analysis is
available and checked for coherence to the user
manual or instruction leaflet. This should be available
for all the software intended users.
The usability validation should be performed with
real users and in a very well simulated or real use
environment and should include a significant number
of participants. International guidelines (Center for
Devices and Radiological Health, 2016) suggest a
minimum of 15 participants per user profile. We
suggest, in this phase, to involve as applicable the
patient associations, such as for example EUPATI,
that may be able to assist not only in the recruitment
of the participants but also in raising awareness on the
importance of their participation in the whole life
cycle of the development of new technologies
(Haerry et al., 2018).
During user tests, we also suggest to evaluate, if
applicable to the device, the length of time needed for
each task (by time-and-motion studies) and the
workload of the user; additionally, the readability and
understandability of the privacy disclosure
documents and the privacy contents can be added as
usability endpoints. In order to collect valuable
information from the end users, destined to root cause
analysis of any encountered user error, we suggest to
include a de-briefing interview at the end of the user
testing. We typically apply heuristic principles while
drafting the interview questions (Zhang et al., 2003).
As for all other validation activities, risk control
measures in the user interface such as passwords,
pop-up and notifications, should be formally
reviewed for final implementation and effectiveness
and the risk-benefit profile confirmed.
5 CONCLUSIONS
An integrated risk-based approach for software
validation allows to plan and execute the validation
activities in a complete and efficient manner. The test
plan should include requirements from the main
medical international standards and should give proof
that all the risk minimisation measures have been
effectively implemented. Such testing, for technical
features, can be based on simulation, thanks to the
creation of adequate simulated clinical use
conditions. Such conditions should be created by
application of the principle of the mode, to define a
significant simulated patient and clinical conditions.
At the completion of such activities, real users and
patients should be involved to test the usability
aspects of the interface. The outcome of the testing is
the proof that all technical features of the device show
an adequate performance and that all risks have been
minimised. The device is therefore compliant to
regulatory requirements and can be subject to clinical
trials.
Future work for this position paper will include
the application of this method to the preclinical
testing of different devices and the evaluation of a
similar risk-based approach for clinical testing.
HEALTHINF 2020 - 13th International Conference on Health Informatics
654

ACKNOWLEDGEMENTS
The authors would like to acknowledge the European
Patients’ Academy (EUPATI), a pan-European
project implemented as a public-private partnership
by a collaborative multi-stakeholder consortium from
the pharmaceutical industry, academia, not-for-profit,
and patient organisations. The Academy was started,
developed and implemented as a flagship project of
the Innovative Medicines Initiative
(http://www.imi.europa.eu/), and continues to be led
by the European Patients’ Forum.
REFERENCES
Center for Devices and Radiological Health. (2016).
Applying Human Factors and Usability Engineering to
Medical Devices. Retrieved November 21, 2019, from
U.S. Food and Drug Administration website:
http://www.fda.gov/regulatory-information/search-
fda-guidance-documents/applying-human-factors-and-
usability-engineering-medical-devices
De Maria, C., Di Pietro, L., Díaz Lantada, A., Madete, J.,
Makobore, P. N., Mridha, M., … Ahluwalia, A. (2018).
Safe innovation: On medical device legislation in
Europe and Africa. Health Policy and Technology,
7(2), 156–165.
https://doi.org/10.1016/j.hlpt.2018.01.012
European Committee for Standardization, & International
Organization for Standardization. (2012). EN ISO
14971:2012, Medical devices: Application of risk
management to medical devices. London: BSI.
European Parliament, & European Council. Regulation
(EU) 2016/679 of the European Parliament and of the
Council of 27 April 2016 on the protection of natural
persons with regard to the processing of personal data
and on the free movement of such data, and repealing
Directive 95/46/EC (General Data Protection
Regulation) (Text with EEA relevance). , Pub. L. No.
32016R0679, 119 OJ L (2016).
European Parliament, & European Council. Regulation
(EU) 2017/745 of the European Parliament and of the
Council of 5 April 2017 on medical devices, amending
Directive 2001/83/EC, Regulation (EC) No 178/2002
and Regulation (EC) No 1223/2009 and repealing
Council Directives 90/385/EEC and 93/42/EEC (Text
with EEA relevance. ). , Pub. L. No. 32017R0745, 117
OJ L (2017).
European Parliament, & European Council. Regulation
(EU) 2017/746 of the European Parliament and of the
Council of 5 April 2017 on in vitro diagnostic medical
devices and repealing Directive 98/79/EC and
Commission Decision 2010/227/EU (Text with EEA
relevance. ). , Pub. L. No. 32017R0746, 117 OJ L
(2017).
European Parliament, & European Council. Regulation
(EU) 2019/881 of the European Parliament and of the
Council of 17 April 2019 on ENISA (the European
Union Agency for Cybersecurity) and on information
and communications technology cybersecurity
certification and repealing Regulation (EU) No
526/2013 (Cybersecurity Act) (Text with EEA
relevance). , Pub. L. No. 32019R0881, 151 OJ L
(2019).
Executive Board, 145. (2019). Standardization of medical
devices nomenclature: International classification,
coding and nomenclature of medical devices: report by
the Director-General. Retrieved from
https://apps.who.int/iris/handle/10665/328220
Haerry, D., Landgraf, C., Warner, K., Hunter, A.,
Klingmann, I., May, M., & See, W. (2018). EUPATI
and Patients in Medicines Research and Development:
Guidance for Patient Involvement in Regulatory
Processes. Frontiers in Medicine, 5.
https://doi.org/10.3389/fmed.2018.00230
International Electrotechnical Commission. (2015). IEC
62366-1:2015, Medical devices—Part 1: Application of
usability engineering to medical devices. Retrieved
from
http://www.iso.org/cms/render/live/en/sites/isoorg/con
tents/data/standard/06/31/63179.html
International Electrotechnical Commission, & International
Organization for Standardization. (2015). IEC
62304:2006+AMD1:2015 CSV, Medical device
software: Software life cycle processes (1st ed.).
Geneva: IEC.
International Organization for Standardization. (2016).
ISO 13485:2016, Medical devices—Quality
management systems—Requirements for regulatory
purposes (3rd ed.). Geneva: ISO.
Pesapane, F., Volonté, C., Codari, M., & Sardanelli, F.
(2018). Artificial intelligence as a medical device in
radiology: Ethical and regulatory issues in Europe and
the United States. Insights into Imaging, 9(5), 745–753.
https://doi.org/10.1007/s13244-018-0645-y
Ravizza, A., De Maria, C., Di Pietro, L., Sternini, F.,
Audenino, A. L., & Bignardi, C. (2019).
Comprehensive Review on Current and Future
Regulatory Requirements on Wearable Sensors in
Preclinical and Clinical Testing. Frontiers in
Bioengineering and Biotechnology, 7.
https://doi.org/10.3389/fbioe.2019.00313
Ravizza, A., Lantada, A. D., Sánchez, L. I. B., Sternini, F.,
& Bignardi, C. (2019). Techniques for Usability Risk
Assessment during Medical Device Design.
Proceedings of the 12th International Joint Conference
on Biomedical Engineering Systems and Technologies,
207–214. https://doi.org/10.5220/0007483102070214
Zhang, J., Johnson, T. R., Patel, V. L., Paige, D. L., &
Kubose, T. (2003). Using usability heuristics to
evaluate patient safety of medical devices. Journal of
Biomedical Informatics, 36(1), 23–30.
https://doi.org/10.1016/S1532-0464(03)00060-1
Methods for Preclinical Validation of Software as a Medical Device
655
