
Rheology of Sunflower, Citrus and Apple Low Methoxyl Pectin
Uray Ulfah Nabilah
a
, Azis Boing Sitanggang
b
and Eko Hari Purnomo
c
Department of Food Science and Technology, IPB University, Bogor 16680, Indonesia
Keywords: Rheology, Low Methoxyl Pectin, Flow Behaviour, Consistency Index.
Abstract: Pectin is a hydrocolloid which widely used to form a specific desired texture and viscosity of food products.
Therefore, it is important to observe the rheological characteristics of pectin. There are three sources of
commercial pectin commonly used in the food industry i.e. sunflower, citrus, and apple. These three pectins
were characterized rheologically in this study using MCR 92 Rheometer (Anton Paar, GmbH, Germany). The
sunflower pectin had the highest viscosity and consistency index number (k) compared to citrus and apple
pectin. The sunflower pectin exhibited shear-thinning behaviour at, especially at concentrations 2.5% and
3.0% (i.e., with flow behaviour index number (n) was less than 1.0). The higher the pectin concentration, the
lower n value obtained with the increased k value. The information found in this study is considered useful
for consumers, industry, and researchers especially when pectin is used to modify the rheological properties
of food products.
1 INTRODUCTION
Pectin is a natural food additive which can be used as
a gelling agent, emulsifier, stabiliser, and thickening
agent (Yapo, 2011). The potential of pectin is closely
related to its chemical characteristics. Commonly, the
pectin structure consists of main chain (1→4)–β-D-
GalA (galacturonic acid) which is partly esterified by
methyl alcohol or acetic acid at the carboxylate acid
side (Round et al., 2010). Pectin has varied molecular
weights (MWs), ranging from 60 to 130,000 g mol
−1
.
The molecular weight of pectin depends on the
number of glucose-side and esterification level of
methyl (Khan et al., 2012). Commercial pectin, in
general, has at least 65% galacturonic acid content
(May, 1990). There are two types of commercial
pectin that commonly used: High Methoxyl Pectin
(HMP) and Low Methoxyl Pectin (LMP). Degree of
esterification (DE) number for HMP is more than 50
%, while LMP is less than 50% (De Oliveira et al.,
2015; Sundar Raj et al., 2012). LMP produced from
orange and apple is a derivative of its HMP (Fertonani
et al., 2009; Morales-Contreras et al., 2020).
Meanwhile, LMP produced from sunflower seed is
naturally formed (Iglesias & Lozano, 2004;
a
https://orcid.org/0000-0002-8741-7460
b
https://orcid.org/0000-0002-
1378-5367
c
https://orcid.org/0000-0002-4146-2549
Corresponding author
Miyamoto & Chang, 1992). Different kinds of pectin
have different properties due to variation of structure
in methyl ester chain located along the main chain.
Naturally, DE number of each pectin is different for
different resources (plant species), plant maturity, and
cell wall properties (Round et al., 2010). Varied
pectin properties might also be due to differences in
extraction methods and post-extraction treatments
(Constenla & Lozano, 2003).
Water-soluble pectin has potential as a thickening
or stabiliser agent for water-based food products
(Razak et al., 2018). The thickening process is the
transition condition from free flow behaviour
molecules (dilute) to binding molecules in a network
(Saha & Bhattacharya, 2010). Each pectin has
different concentrations to reach certain rheological
consistency, which is related to the chemical structure
of each pectin (Alba et al., 2015; Axelos et al., 1989;
Dimopoulou et al., 2019; Morales-Contreras et al.,
2020). The mechanism of pectin as a thickening agent
is closely related to viscosity. Hydrocolloid
molecules flow freely at a dilute solution and show no
thickening properties. Meanwhile, at viscous
solution, molecules interact with each other, and the
flow is limited (Saha & Bhattacharya, 2010).
178
Nabilah, U., Sitanggang, A. and Purnomo, E.
Rheology of Sunflower, Citrus and Apple Low Methoxyl Pectin.
DOI: 10.5220/0010568600003108
In Proceedings of the 6th Food Ingredient Asia Conference (6th FiAC 2020) - Food Science, Nutrition and Health, pages 178-184
ISBN: 978-989-758-540-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Table 1: Properties of pectin.
Sunflower (M) Citrus (C) Apple (A)
Degree of esterification (%DE) 31.2 29.34 27.6
De
g
ree of amidation
(
%DA
)
0 19.2 22.6
Galacturonic acid (%GA) 85.45 88.7 65
The thickening process of pectin is also related to
the ability of pectin to form a gel, emulsify and
stabilise (Ngouémazong et al., 2015). Gel forming
capacity in LMP is not dependent on the glucose
content of the product, and not pH-sensitive, the same
as HMP. Based on the previous study by Yang et al.
(2018), LMP could form a gel ranging from acidic
(pH 3.5) to saline condition (pH 9.5), by the addition
of divalent cation such as calcium. One of the main
purposes of pectin used in the food product is to form
a specific food texture, which makes rheological
study important. Prior to product formulation, it is
crucial to know the rheological characteristics of
pectin. Raw material selection is a crucial factor to
make the desired product. The important rheological
parameters, especially in the liquid system, are flow
behaviour index (n) and consistency index (k). It is
generally known that the greater the concentration
and viscosity of pectin, the higher the shear thinning
properties. However, the effect of concentration is
unique for each pectin. Therefore, the purpose of this
study was to investigate the effect concentration and
source of LMP (i.e. sunflower, citrus, and apple) on
the rheological properties of pectin solution. This
study can be used as a source of information to choose
suitable pectin source for certain food product
formulation.
2 MATERIALS AND METHODS
2.1 Materials
The three sources of pectin used in this study were
sunflower, citrus and apple with the specifications as
indicated in Table 1. The pectins were purchased
from Hunan Zhengdi Biological Resources
Development Co., Ltd., China. The solvent used was
deionised water.
2.2 Preparation of Pectin Solution
Pectin from each source was dissolved in 100 mL
deionised waters, stirred overnight at room
temperature. For each, there were eight
concentrations prepared (i.e., 0.1, 0.25, 0.5, 1.0, 1.5,
2.0, 2.5, 3.0 % (w/v)).
2.3 Viscosity Measurements
The flow properties of prepared pectin samples were
measured by using MCR 92 Rheometer (Anton Paar,
GmbH, Germany); with geometric cone and plate
(diameter = 50 mm and angle = 2 °). A sample of 5.0
mL was applied on the surface of the plate with a 0.1
mm gap between plates. The instrument was operated
at shear rates of 0.01 - 1000 s
-1
and temperature of 25
°C (Kontogiorgos et al., 2012). The data points were
set as 51 points, logarithmic ramp duration, 10 s
initial time and 1 s final time. The data set value was
at a shear rate variable, logarithmic ramp profile, 0.01
s initial time and 1000 s final time. Flow curve
analysis was done using RheoCompass
TM
software,
and the measurement of each sample was repeated
twice.
The information obtained were shear rate, shear
stress, and viscosity of the samples used to determine
flow behaviour. Experimental data were fitted using
the power-law model (Equation 1), where τ is shear
stress (Pa), ( 𝛾 ) is the shear rate (s
-1
), n is the
dimensionless flow behaviour index value and k is the
consistency index (Pa.s
n
). Slope of flow behaviour
curve plotted as log τ vs log 𝛾 shows the flow
behaviour index (n). The results show Newtonian
typical flow when n = 1, shear-thinning if 0 < n < 1
and shear thickening fluid if n > 1 (Rao, 2007; Steffe,
1996).
𝜏𝐾 𝛾
(1)
2.4 Statistical Analysis
The experimental design of this study was factorial
completely randomised design (CRD), and all tests
were repeated in duplo (N = 2). Statistical research
was carried out using two-way variance analysis
(ANOVA) to evaluate significant differences
between mean values, and continued by Duncan test.
3 RESULT AND DISCUSSION
3.1 Flow Curve Behaviour
The rheological properties indicate the ability of
pectin to fulfil a specific role in modifying the food
Rheology of Sunflower, Citrus and Apple Low Methoxyl Pectin
179
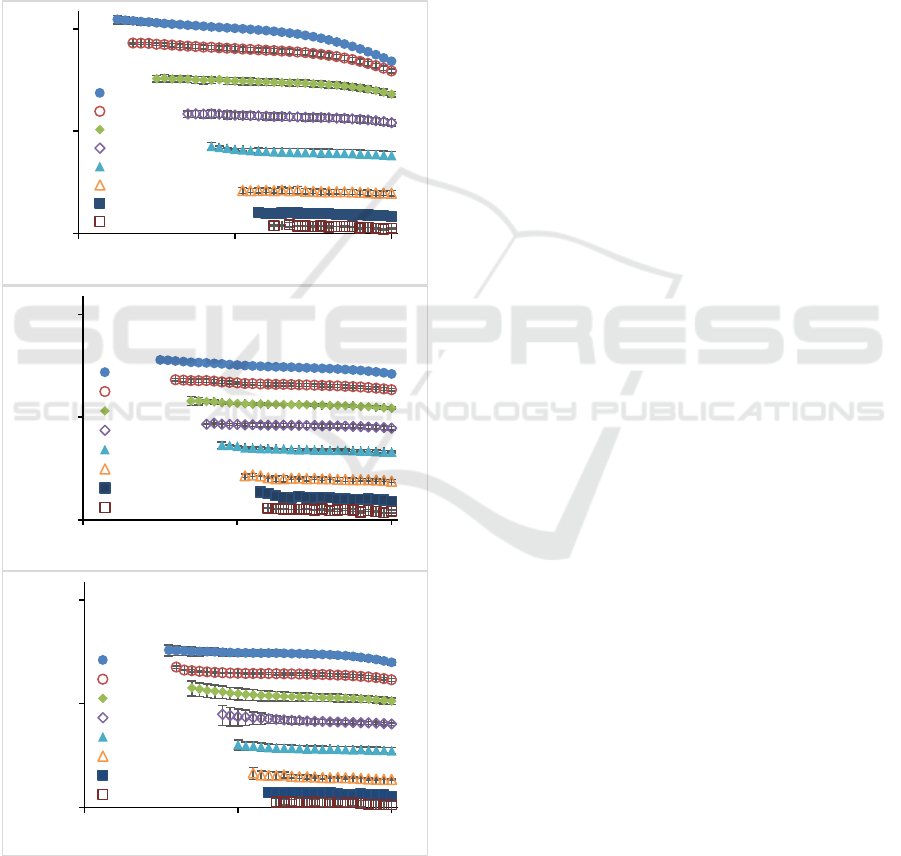
texture. One of the important parameters in
rheological studies of pectin as a thickening agent is
viscosity (Saha & Bhattacharya, 2010). The data of
pectin viscosity as function of shear rate measured at
different concentrations are shown in Figure 1. It was
found that increased concentration leads to the
increasing flow resistance in sunflower (M), citrus
(C) and apple (A) pectin, as indicated by higher value
of viscosity. This condition could be attributed to the
interaction between dispersed solids which caused
limited movement of water (Saha & Bhattacharya,
2010; Vinogradov & Titkova, 1968).
Figure 1: Viscosity of pection solution as function of shear
rate for (a) sunflower, (b) citrus and (c) apple.
In general, at low shear rates, there is a plateau or
Newtonian flow of the pectin. When the shear rate
increases, or after passing the critical shear rate, the
viscosity starts to decrease or displays shear thinning
behaviour. At high concentrations, the Newtonian
flow region is shorter than at low concentrations. The
lower the concentration, the longer the Newtonian
flow region and could extend to the highest shear rate.
At low concentrations, the dominant flow pattern is
Newtonian and there is no indication of viscosity
decrease under high shear rate conditions. At low
concentrations, pectin has Newtonian properties,
shown by the constant viscosity as the shear rate
increases. This condition can be linked to the fact that
of coil disentanglement due to shear forces is lower
than the rate of coil re-entanglement in all shear rate
range. Therefore, the shear force has no significant
effect on the decrease in viscosity. This condition is
similar to the study conducted by Li et al. (2013),
Chan et al. (2017) and Kalegowda et al. (2017),
where at low shear rates, pectin shows a Newtonian
flow pattern and become shear thinning at higher
shear rates. The ability of the coil to reconfigure a
network can be based on the ability of pectin to form
hydrogen bonds. LMP at low concentrations have
more probability to form hydrogen bonds and
consequently increasing re-entanglement rate of the
coil and thus, viscosity decrease was not observed.
3.2 Pectin Rheological Parameters
Quantitatively, Newtonian and non-Newtonian flow
patterns can be described using the power-law model
(Equation 1), extracted from the relationship between
shear rate and shear stress (Figure 2). Power law
model is widely used in viscosity modelling in food
processing during handling, heating, or cooling (Rao,
2007; Steffe, 1996). Coefficient of determination
obtained from the relationship between shear rate and
shear stress are shown in Table 2. The results showed
that the power-law model describes the flow
behaviour of all three pectin types. This is indicated
by the coefficient of determination which is close to
one. The results provided confidence in extracting the
fitting parameters (n and K) from the model for
further analysis.
0,001
0,01
0,1
0,1 10 1000
Viscosity (Pa.s)
Shear rate (1/s)
3%
2.50%
2%
1.50%
1%
0.50%
0.25%
0.10%
a
0,001
0,01
0,1
0,1 10 1000
Viscosity (Pa.s)
Shear rate (1/s)
3%
2.50%
2%
1.50%
1%
0.50%
0.25%
0.10%
b
0,001
0,01
0,1
0,1 10 1000
Viscosity (Pa.s)
Shear rate (1/s)
3%
2.50%
2%
1.50%
1%
0.50%
0.25%
0.10%
c
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
180
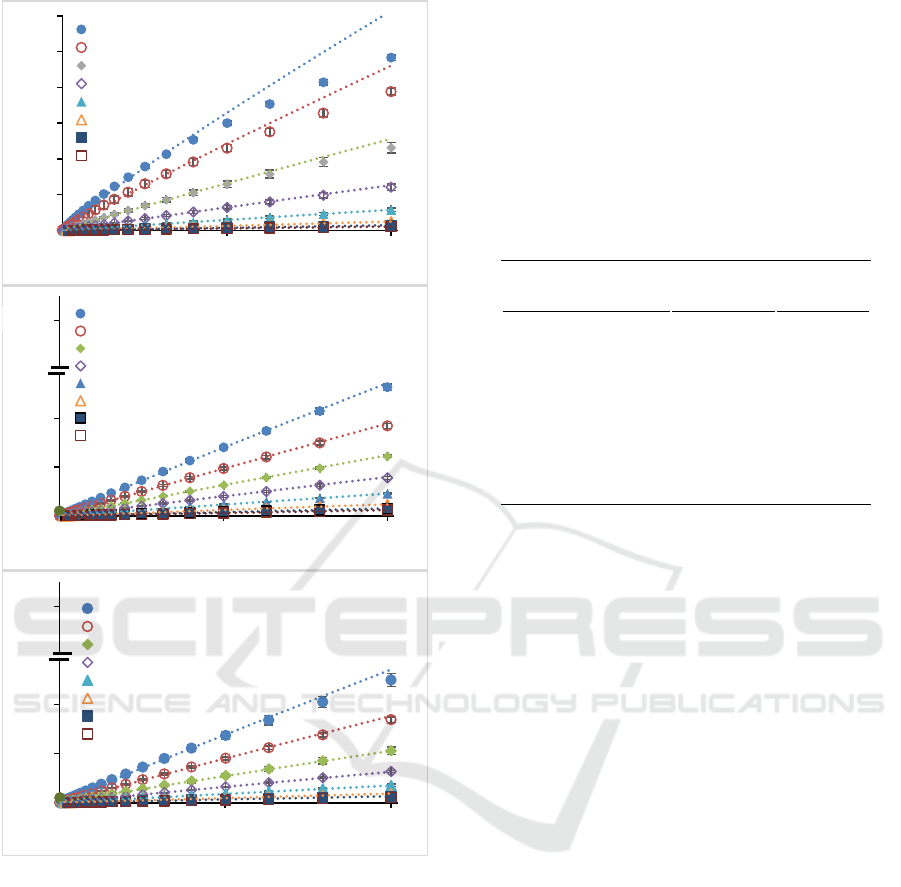
Figure 2: Flow curves of pectin solutions ((a) sunflower, (b)
citrus, and (c) apple)) measured at different concentrations.
Dotted lines are Power Law Model used to describe the
experimental data.
Figure 3 shows the effect of concentration of the
flow behaviour index (Figure 3a) and consistency
index (Figure 3b) of pectin solution. Flow behaviour
indexes (n) at various LMP concentrations decreased
as pectin concentration increased. A behaviour of
typical Newtonian-to-slightly shear thinning was
observed, as shown by the value of n <1. Based on
two-way ANOVA, it was known that the
concentration of pectin and their interactions had a
significant effect on n value. Pectin solutions showed
more shear thinning behaviour at higher
concentration. For example, at concentrations of
2.5% and 3.0 % for sunflower pectin, showed the
smallest flow behaviour index value which was
significantly different from other concentrations.
Whereas at low concentrations, the results showed
that n values close to one (less shear thinning). Pectin
solution practically has Newtonian characteristics
when the concentration was below 1% w/v.
Table 2: Coefficient of determination (R
2
) of linear
regressing between shear rate and shear stress for
sunflower, citrus and apple pectin.
Conc.
(%)
Coe
ff
icient o
f
determination
(
R
2
)
Sunflowe
r
Citrus A
pp
le
3 0.9985 1 0.9998
2.5 0.9993 1 0.9999
2 0.9997 1 0.9999
1.5 0.9999 1 0.9999
1 0.9999 0.9999 0.9999
0.5 1 0.9999 0.9999
0.25 0.9999 0.9996 0.9998
0.1 0.9999 0.9999 0.9999
Other study by Kontogiorgos et al. (2012) showed
similar observation. Okra pectin showed Newtonian
properties at a concentration below 1% w/v, and when
the concentration increased, it exhibited shear-
thinning properties. Whereas in the study of okra fruit
pectin DE 58% by Methacanon et al. (2013) showed
Newtonian flow behaviour at concentrations below
0.4% w/v. Based on the type of pectin used, sun
flower pectin (M) had the lowest n value and was
significantly different from the other two pectin
sources. At 3% concentration, n value of sunflower
pectin showed the lowest value (0.9021) or displayed
more shear-thinning behaviour than the other two
pectin sources (p < 0.05). The overall n values for all
pectin were close to one: M (0.9021- 0.9838), C
(0.9608 - 0.9855), and A (0.9599- 0.9817). The shear-
thinning behaviour of pectin solutions can be linked
to the inter-polymer chain. At high shear rate, the
formation rate of polymer entanglements is smaller
than the breaking rate. This causes the number of
cross-link bonds between polymers to decrease.
Moreover, the breakdown of previously formed
polymer-polymer hydrogen bonds reduces the final
polymer dimension. Thereby, the water or solvent can
easily flow out of the entangled polymer coils
(Thirawong et al., 2008). On the other side, the
aggregation that occurs between pectin polymers is
due to the formation of polymer-polymer hydrogen
bonds. This eventually leads to the formation of a
stable network that can trap water, leading to the
increase of viscosity (Karimi et al., 2016).
0
10
20
30
40
50
60
0 500 1000
Shear stress (Pa)
Shear rate (1/s)
3%
2.50%
2%
1.50%
1%
0.50%
0.25%
0.10%
a
0
10
20
30
40
0 500 1000
Shear stress ( Pa)
Shear rate (1/s)
3%
2.50%
2%
1.50%
1%
0.50%
0.25%
0.10%
60
b
0
10
20
30
40
0 500 1000
Shear streess ( Pa)
Shear rate (1/s)
3%
2.50%
2%
1.50%
1%
0.50%
0.25%
0.10%
60
c
Rheology of Sunflower, Citrus and Apple Low Methoxyl Pectin
181
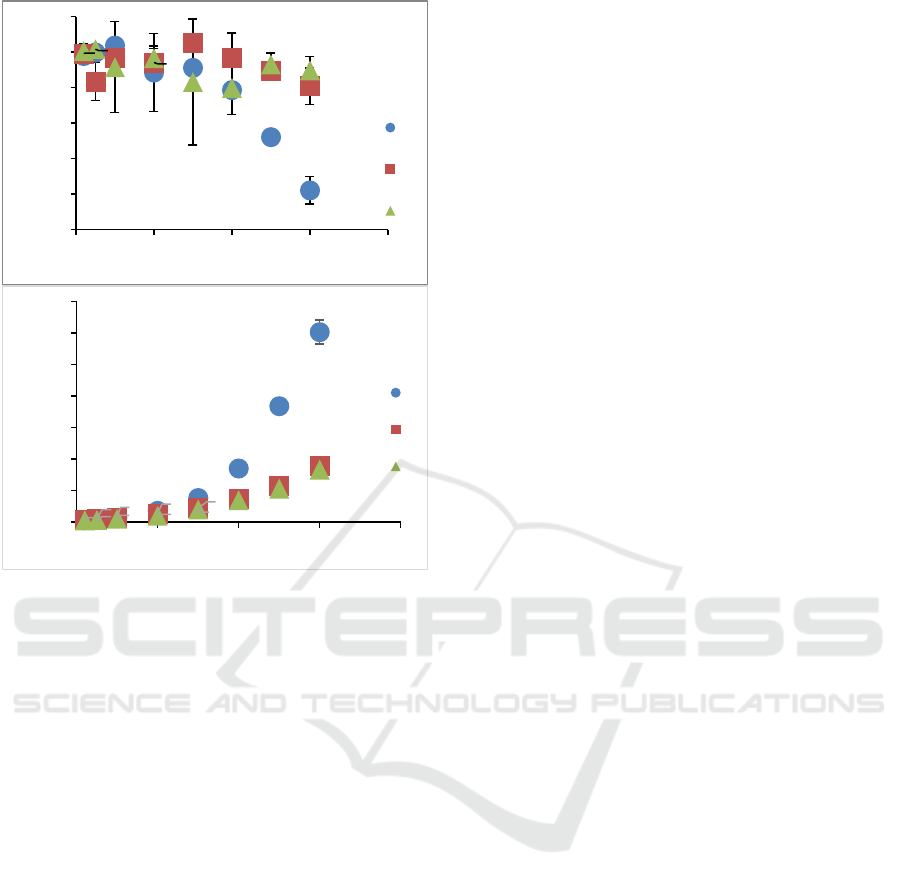
Figure 3: Flow behaviour index (a) and consistency index
(b) of pectin solution.
Two-way ANOVA showed that source of pectin,
concentration, and polymer interaction significantly
affected the consistency index (k) (Figure 3b). In
general, a higher concentration of pectin solution
yields higher k value, because at higher concentration,
the polymer chains overlap, so it increases
competition for free water access, leading to stronger
binding of the polymer chains. The overall k values
for all sources of pectin as follows: M (0.0013 -
0.1206), C (0.0014 - 0.0357) and A (0.0012- 0.0334).
Sunflower (M) pectin had the highest k value and was
significantly different from the other two pectin
sources, especially at 3% concentration (k = 0.1206
Pa.s
n
) (p < 0.05). This result was corresponding to the
viscosity profile of sunflower pectin as shown in
Figure 1.
Pectin M, C and A had different degrees of
esterification (DE), degrees of amidation (DA) and
galacturonic acid concentrations (GA) (see Table 1).
Differences in rheological properties of each source
of pectin can be influenced by chemical factors, such
as content of anhydrous galacturonic acid (AGA),
degree of methylation (DM) or DE, molecular size,
distribution of carboxyl groups, and charge of pectin
molecules (Constenla & Lozano, 2003; Cullen, 2012;
Singh & Heldman, 2009; Zhong & Daubert, 2013). In
some cases, the viscosity can be affected by
physicochemical properties and temperature of the
material (Cullen, 2012; Singh & Heldman, 2009;
Zhong & Daubert, 2013). Based on the chemical
properties of the pectin used (Table 1), the pectin with
the highest to lowest DE was M, C and A pectin.
Pectin A had higher DA than pectin C, while pectin
M had no DA. Based on the results, it can be shown
that there was a tendency for higher DE and low DA
to have a higher fluid consistency. Based on existing
study, higher DE showed higher intrinsic viscosity
(Morris et al., 2000; Pippen et al., 1953; Yoo et al.,
2006), but this condition occurred at the same pectin
source. Based on these results, it can be seen that the
chemical structure of pectin sourced from sunflowers
has the potential to increase viscosity higher than the
other two.
The information on dynamic viscosity can
provide information regarding material flow
characteristics, especially to mitigate product
handling during processing at food industry. In
general, sunflower pectin had a higher viscosity as
compared to citrus and apple pectin. Thus, it can be
considered for product formulation which requires
moderate-to-high viscosity level. In addition to this,
one must also consider the power input or energy
needed during mixing process of such highly viscous
solution.
4 CONCLUSIONS
Source and concentration of pectin were shown to
have influence on the rheological properties of pectin-
containing solution. In general, pectin solution
showed Newtonian behaviour at very low shear rate
distinctly exhibited shear thinning behaviour at
higher shear rate. Shear thinning behaviour of pectin
solution was more pronounced at higher
concentrations. Sunflower pectin had the lowest flow
behaviour index and displayed vivid shear-thinning
behaviour compared to other sources. Herein,
sunflower pectin might be useful as an alternative
thickening agent for formulating viscous food
products.
ACKNOWLEDGEMENTS
The authors are grateful to PT. Equiva Ligand
Indonesia for providing access to MCR 92 Rheometer
to perform this study. This study was funded by
Master of Education towards Doctoral Scholarship
a
b
c
c
c
c
c
c
c
c
c
a
c
c
c
c
c
c
c
c
c
c
c
c
0,88
0,9
0,92
0,94
0,96
0,98
1
01234
n
c % (b/v)
M
C
A
a
a
b
c
ef
hi
i
i
i
c
d
fg
fghi
i
i
i
i
c
de
fgh
ghi
i
i
i
i
0
0,02
0,04
0,06
0,08
0,1
0,12
0,14
01234
k (Pa.s
n
)
c % (b/v)
M
C
A
b
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
182

Program for Excellent Undergraduate (PMDSU)
from The Ministry of Research, Technology, and
Higher Education of The Republic of Indonesia.
REFERENCES
Alba, K., Laws, A. P., & Kontogiorgos, V. (2015). Isolation
and characterization of acetylated LM-pectins extracted
from okra pods. Food Hydrocoll, 43, 726–735.
https://doi.org/10.1016/j.foodhyd.2014.08.003
Axelos, M., Thibault, J., & Lefebvre, J. (1989). Structure of
citrus pectins and viscometric study of their solution
properties. Int. J. Biol. Macromol., 11, 186–191.
Chan, S. Y., Choo, W. S., Young, D. J., & Loh, X. J. (2017).
Pectin as a rheology modifier: Origin, structure,
commercial production and rheology. Carbohydr.
Polym., 161, 118–139.
https://doi.org/10.1016/j.carbpol.2016.12.033
Constenla, D., & Lozano, J. E. (2003). Kinetic model of
pectin demethylation. Lat. Am. Appl. Res., 33, 91–96.
Cullen, P. J. (2012). Fluid rheology in novel thermal and
non-thermal processes. In Novel Thermal And Non-
Thermal Technologies For Fluid Foods (pp. 35–61).
Academic Press. https://doi.org/10.1016/B978-0-12-
381470-8.00003-7
De Oliveira, C. F., Giordani, D., Gurak, P. D., Cladera-
Olivera, F., & Marczak, L. D. F. (2015). Extraction of
pectin from passion fruit peel using moderate electric
field and conventional heating extraction methods.
Innov Food Sci Emerg Technol, 29, 201–208.
https://doi.org/10.1016/j.ifset.2015.02.005
Dimopoulou, M., Alba, K., & Kontogiorgos, V. (2019).
Pectin recovery and characterization from lemon juice
waste streams. J Sci FoodAgric.
https://doi.org/10.1002/jsfa.9891
Fertonani, H. C. R., Scabio, A., Carneiro, E. B. B.,
Schemim, M. H. C., Nogueira, A., & Wosiacki, G.
(2009). Extraction model of low methoxyl pectin from
apple pomace effects of acid concentration and time on
the process and the product. Braz. Arch. Biol. Technol,
52(1), 177–185.
Iglesias, M. T., & Lozano, J. E. (2004). Extraction and
characterization of sunflower pectin. J Food Eng, 62,
215–223. https://doi.org/10.1016/S0260-
8774(03)00234-6
Kalegowda, P., Singh Chauhan, A., & Mysore NanjarajUrs,
S. (2017). Opuntia dillenii (Ker-gawl) haw fruit peel
pectin: Physicochemical, rheological, and functional
behavior. J. Food Process. Preserv., 41(5), 1–8.
https://doi.org/10.1111/jfpp.13165
Karimi, N., Sani, A. M., & Pourahmad, R. (2016). Influence
of carboxy methyl cellulose (CMC) and pectin on
rheological, physical stability and sensory properties of
milk and concentrated jujuba mixture. J. Food Meas.
Charact., 10(2), 396–404.
https://doi.org/10.1007/s11694-016-9318-z
Khan, M., Nakkeeran, E., & Umesh-Kumar, S. (2012).
Potential Application of Pectinase in Developing
Functional Foods. Annu Rev Food Sci Technol
, 4(1),
21–34. https://doi.org/10.1146/annurev-food-030212-
182525
Kontogiorgos, V., Margelou, I., Georgiadis, N., &
Ritzoulis, C. (2012). Rheological characterization of
okra pectins. Food Hydrocoll, 29(2), 356–362.
https://doi.org/10.1016/j.foodhyd.2012.04.003
Li, X., Al-Assaf, S., Fang, Y., & Phillips, G. O. (2013).
Characterisation of commercial LM-pectin in aqueous
solution. Carbohydr. Polym., 92(2), 1133–1142.
https://doi.org/10.1016/j.carbpol.2012.09.100
May, C. D. (1990). Industrial pectins: sources, production
and applications. Carbohydr. Polym., 12(1), 79–99.
https://doi.org/10.1016/0144-8617(90)90105-2
Methacanon, P., Krongsin, J., & Gamonpilas, C. (2014).
Pomelo (Citrus maxima) pectin: Effects of extraction
parameters and its properties. Food Hydrocoll, 35, 383–
391. https://doi.org/10.1016/j.foodhyd.2013.06.018
Miyamoto, A., & Chang, K. C. (1992). Extraction and
physicochemical characterization from sunflower head
residues of pectin. J. Food Sci., 57(6), 1439–1443.
Morales-Contreras, B. E., Wicker, L., Rosas-Flores, W.,
Contreras-Esquivel, J. C., Gallegos-Infante, J. A.,
Reyes-Jaquez, D., & Morales-Castro, J. (2020). Apple
pomace from variety “Blanca de Asturias” as
sustainable source of pectin: Composition, rheological,
and thermal properties. LWT, 117. https://doi.org/
10.1016/j.lwt.2019.108641
Morris, G. A., Foster, T. J., & Harding, S. E. (2000). The
effect of the degree of esterification on the
hydrodynamic properties of citrus pectin. Food
Hydrocoll, 14(3), 227–235. https://doi.org/10.1016/
S0268-005X(00)00007-2
Ngouémazong, E. D., Christiaens, S., Shpigelman, A., Van
Loey, A., & Hendrickx, M. (2015). The emulsifying
and emulsion-stabilizing properties of pectin: A
Review. Compr. Rev. Food Sci. Food Saf., 14(6), 705–
718. https://doi.org/10.1111/1541-4337.12160
Pippen, E. L., Schultz, T. H., & Owens, H. S. (1953). Effect
of degree of esterification on viscosity and gelation
behavior of pectin. J. Colloid Sci., 8(1), 97–104.
https://doi.org/10.1016/0095-8522(53)90010-5
Rao, M. A. (2007). Rheology of Fluid and Semisolid Foods:
Principles and Applications (2nd ed.). Springer.
Razak, R. A., Karim, R., Sulaiman, R., & Hussain, N.
(2018). Effects of different types and concentration of
hydrocolloids on mango filling. Int Food Res J, 25
(3),
1109–1119.
Round, A. N., Rigby, N. M., MacDougall, A. J., & Morris,
V. J. (2010). A new view of pectin structure revealed
by acid hydrolysis and atomic force microscopy.
Carbohydr. Res., 345(4), 487–497. https://doi.org/
10.1016/j.carres.2009.12.019
Saha, D., & Bhattacharya, S. (2010). Hydrocolloids as
thickening and gelling agents in food: a critical review.
Int. J. Food Sci. Technol., 47(6), 587–597.
https://doi.org/10.1007/s13197-010-0162-6
Singh, R., & Heldman, D. (2009). Introduction to Food
Engineering (4th ed.). Academic Press.
Rheology of Sunflower, Citrus and Apple Low Methoxyl Pectin
183

Steffe, J. (1996). Rheological Method in Food Procces
Engineering. Freeman Press.
Sundar Raj, A., Rubila, S., Jayabalan, R., & Ranganathan,
T. (2012). A Review on pectin: Chemistry due to
general properties of pectin and its pharmaceutical uses.
Sci. Rep., 1(12), 10–12. https://doi.org/10.4172/
scientificreports.5
Thirawong, N., Kennedy, R. A., & Sriamornsak, P. (2008).
Viscometric study of pectin – mucin interaction and its
mucoadhesive bond strength. Carbohydr. Polym., 71,
170–179.
https://doi.org/10.1016/j.carbpol.2007.05.026
Vinogradov, G. V., & Titkova, L. V. (1968). Critical
concentrations of polymers in solutions according to
measurements of the viscosity and specific surface area
of aerogels resulting after sublimation of the solvent.
Rheologica Acta, 7(4), 297–306. https://doi.org/
10.1007/BF01984842
Yang, X., Nisar, T., Liang, D., Hou, Y., Sun, L., & Guo, Y.
(2018). Low methoxyl pectin gelation under alkaline
conditions and its rheological properties: Using NaOH
as a pH regulator. Food Hydrocoll, 79, 560–571.
https://doi.org/10.1016/j.foodhyd.2017.12.006
Yapo, B. M. (2011). Pectic substances: From simple pectic
polysaccharides to complex pectins - A new
hypothetical model. Carbohydr. Polym., 86(2), 373–
385. https://doi.org/10.1016/j.carbpol.2011.05.065
Yoo, S., Fishman, M. L., Hotchkiss, A. T., & Gyu, H.
(2006). Viscometric behavior of high-methoxy and
low-methoxy pectin solutions. Food Hydrocoll, 20, 62–
67. https://doi.org/10.1016/j.foodhyd.2005.03.003
Zhong, Q., & Daubert, C. R. (2013). Food Rheology. In
Handbook of Farm, Dairy and Food Machinery
Engineering: Second Edition (Vol. 1, pp. 403–426).
Academic Press. https://doi.org/10.1016/B978-0-12-
385881-8.00015-X
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
184
