
Modification of Composite Structure on Cobalt Free Cathode
for Solid Oxide Fuel Cells
Iwan Susanto
1,2
, Dianta Mustofa Kamal
1
, Fitri Wijayanti
1
, Belyamin
1
, Tia Rahmiati
1
, Fuad Zainuri
1
,
Rahmat Subarkah
1
, Yen-Pei Fu
2
, Adi Subardi
3
, Sulaksana Permana
4
1
Department of Mechanical Engineering, Politeknik Negeri Jakarta, Depok 16424, Indonesia
2
Department of Materials Science and Engineering, National Dong Hwa University, Hualien 97401, Taiwan ROC
3
Faculty of Vocational, Institut Teknologi Nasional Yogyakarta, Yogyakarta 55281, Indonesia
4
Center of Mineral Processing and Corrosion Research, Department of Metallurgy and Materials Engineering,
Universitas Indonesia, Depok 16424, Indonesia
Keywords: Composite Cathode, IT-Solid Oxide Fuel Cells, Perovskite Structure, Cobalt-Free Cathode
Abstract: A novel cobalt-free cathode of composite Sm
0.5
Sr
0.15
Ba
0.35
Fe
3-δ
for IT-SOFCs is developed using the solid-
state reaction technique. Thermal gravimetric was carried out for monitoring the weight loss on the cathode
system. In contrast, the X-ray diffraction was employed for the structure phase constructed on the model.
Reduction of weight value during the calcination process was achieved to be 10.5%. Thus, a low temperature
of the reduction reaction was obtained less than 920 °С. The decomposition reaction related to oxygen vacancy
in the composite cathode-free cathode was begun at 410 °С. It takes the advantage in the device system for
IT-SOFCs in application. The established structure of the composite cathode system was in the perovskite
phase
1 INTRODUCTION
Intermediate temperature solid oxide fuel cells (IT-
SOFCs) is an exciting device with high energy
conversion efficiency (Zhang et al., 2017). In
traditional SOFCs, its device operated approximately
at 1000 °С, which limited to using the material (Shao
& Haile, 2004). Today, IT-SOFCs offer an excellent
device with a wide range of material uses due to a
lower temperature application (Mahato et al., 2015).
However, decreasing operating temperature led to an
oxygen reduction reaction that suffers the mobility of
its atom in the system (Kulkarni et al., 2016). The
perovskite structure for cathode material was
introduced to SOFCs due to its mixed ionic-electronic
conducting (Zhou et al., 2016). It facilitates the
oxygen reduction reaction on both the triple-phase
boundary and along with the cathode bulk (Julián et
al., 2020). So, developing the perovskite structure
could also be interesting to reduce the area-specific
resistance in the IT-SOFCs cathode system.
The perovskite structure containing cobalt have
been studied, namely SmBa
0.5
Sr
0.5
Co
2
O
5+δ
,
Sm
0.5
Sr
0.5
CoO
3−δ
(Subardi et al., 2017 and Li et al.
2012). Attending cobalt in the cathode system could
demonstrate the high electro-catalyst activity.
However, a higher thermal expansion coefficient
(TEC) these cathodes than electrolyte such as
Sm
0.2
Ce
0.8
O
1.9
(SDC) restricted it for long term
application (Li et al. 2012). It is caused by the damage
structure in the interface between cathode layer and
electrolyte. So, the reduction of TEC difference value
for both cathode and electrolyte was considered to
develop the IT-SOFCs system (Zhang et al., 2014 and
Zhang et al., 2013). Thus, many TEC value
differences can spoil the cathode/electrolyte layer,
accelerating cell degradation initiated from the
interface up to the surface of the layers (Susanto et al.,
2019). So that, the attending of composite cathode-
free with a novel oxide composition was in interesting
study to be investigated.
In the report, the composite cathode
Sm
0.5
Sr
0.15
Ba
0.35
Fe
3-δ
was produced by the solid-state
reaction method. The characterization will be
employed to observe the decomposition step and the
structure constructed on the model. The thermal
gravimetric related to the analysis of weight loss and
reduction reaction will be discussed in detail. While,
the perovskite structure of the cathode model was
Susanto, I., Kamal, D., Wijayanti, F., Belyamin, ., Rahmiati, T., Zainuri, F., Subarkah, R., Fu, Y., Subardi, A. and Permana, S.
Modification of Composite Structure on Cobalt Free Cathode for Solid Oxide Fuel Cells.
DOI: 10.5220/0010538000003153
In Proceedings of the 9th Annual Southeast Asian International Seminar (ASAIS 2020), pages 97-100
ISBN: 978-989-758-518-0
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
97
further identified comprehensively by XRD
characterization and it be analysed in detail as well.
2 EXPERIMENTAL METHOD
The cathode material composition consists of Sm
2
O
3
,
SrCO
3
, BaCO
3
, and Fe
2
O
3
powders (>99%) was
calculated by the stoichiometry method. The
synthetic of composites cathode was preparation via
the solid-state reaction (Susanto, et al., 2020). The
cathode powder was milled using alumina balls in
liquid alcohol for 12 hours (to obtain an even mixture)
and then dried at 65 °С for 24 hours. The cathode
material was subsequently filtered using 200 mesh
screening. The cathode material of 10 µg was heated
using the thermal gravimetric machine from room
temperature to 1200 °С, heating rate of 10 ℃/minute.
It cooled it(SBSF35) to room temperature in the air.
Further, the 5 gr cathode powders put on the Al
2
O
3
-
cup and calcined it up to 1000 °С with 3 °С/minute in
a heating rate and it cooled to room temperature as
well. The SBSF35 cathode structure was detected by
Rigaku D/MAX-2500V of XRD using a scanning of
3
o
/minute with a degree range of 20–80
o
. The total of
The sample crystal structure was analyzed by
applying the JADE 5 program to match the XRD
pattern obtained from the XRD database at the
International Center for Diffraction Data.
3 RESULT AND DISCUSSION
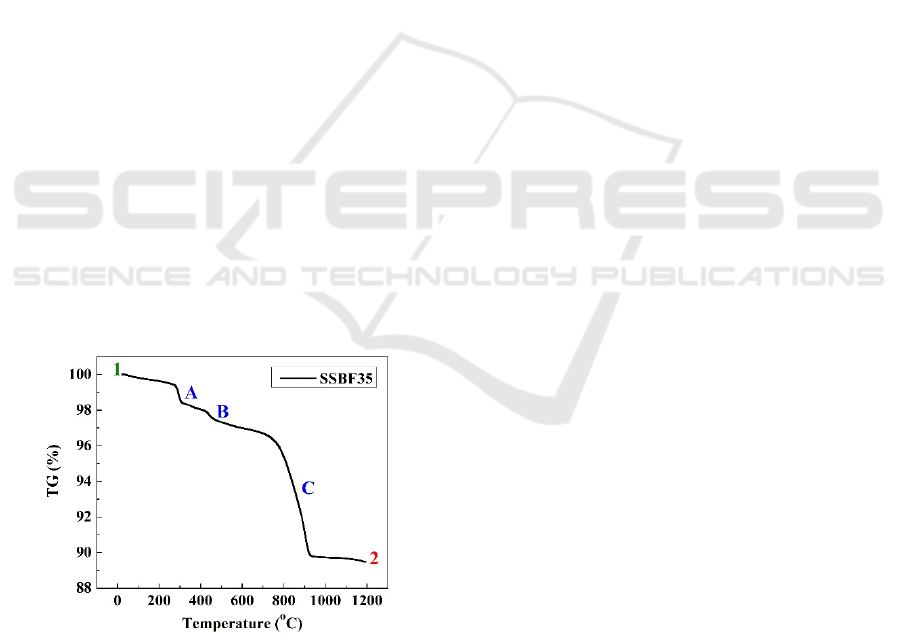
Figure 1. Thermal gravimetric of calcination process for
SSBF35 cathode powders
Fig. 1 shows curve thermal gravimetric (TG) of
SBSF35 oxide powders, which are calcined in the
range of 1200 °С from the room temperature. There
are three steep decreasing on the curve attended at
300 °С, 440 °С, and 760 °С for point A, B and C,
serially. At point A, the TG value reduction was
0.8%, and 0.4 % was in point B. In comparison, the
decreasing in its value was 7 % at point C. The total
TG % value was 10.5 % as long as 900 °С in range.
The thermal gravimetric was monitored the
stability of SBSF35 related to a reduction of the
weight on temperature. The calcination process of
composite powders was carried out to generate the
reaction of solid-state in the system. It decreasing the
TG curve from point 1 to 2 corresponded to the
reduction of weight loss of composite powders. For
the calcination process, the curve's reduction at the
first phase occurred at room temperature to 400 °С.
In point A, reduction of TG related to the release of
water content from the materials. The water content
could evaporate due to the system's heat energy in the
composite powders (Zhang and Zhao, 2020).
Furthermore, in the point B, The decreasing of TG
value suspected with purity of the materials. The
evaporating was demonstrated by the samarium
powder, which was performed by the lost weight in
the range temperature (Susanto, et al., 2020). It also
is proven by the endothermic process at the same
temperature due to the dehydration of materials.
Furthermore, in point C, the rate of subsequent weight
loss occurs that is caused by the decomposition of
strontium carbonate, forming the oxide materials of
SBSF35. The solid-state reaction was generated on
which held from this temperature up to 920 °С [31].
The endothermic could be constructed the formation
of the perovskite structure, confirmed by XRD in
Fig.3.
Moreover, the TG curve of SSBF35 after
calcination was displayed in Fig 2. The heating
process was given to 1000 °С. Three regions on the
curve related to lost weight on the materials during
calcination. The decreasing curve gradually in the
range of point one to point two, about 0.36 TG %, was
created from room temperature to 410 °С. It
demonstrated the lost weight influenced by the water
content that evaporated in the air. Furthermore, the
degradation of a curve from point 2 to 3 was
simultaneous to be 1% at 755 °С. Finally, the
reduction curve trend was only 0.2% which was
relatively stable up to 1000 °С. The total reduction in
the curve value was about 1.2 % which is smaller than
the lost weight during the calcination process. The
decreasing of weight corresponded with heat energy
encourage the oxygen atom to loose from the bonding
system. Decomposition process correlated with the
release of oxygen atoms in the structure system at
higher temperature, which was generating oxygen
vacancy. It could also facilitate the transport of
mobility oxygen in the cathode to be easier when
ASAIS 2020 - Annual Southeast Asian International Seminar
98
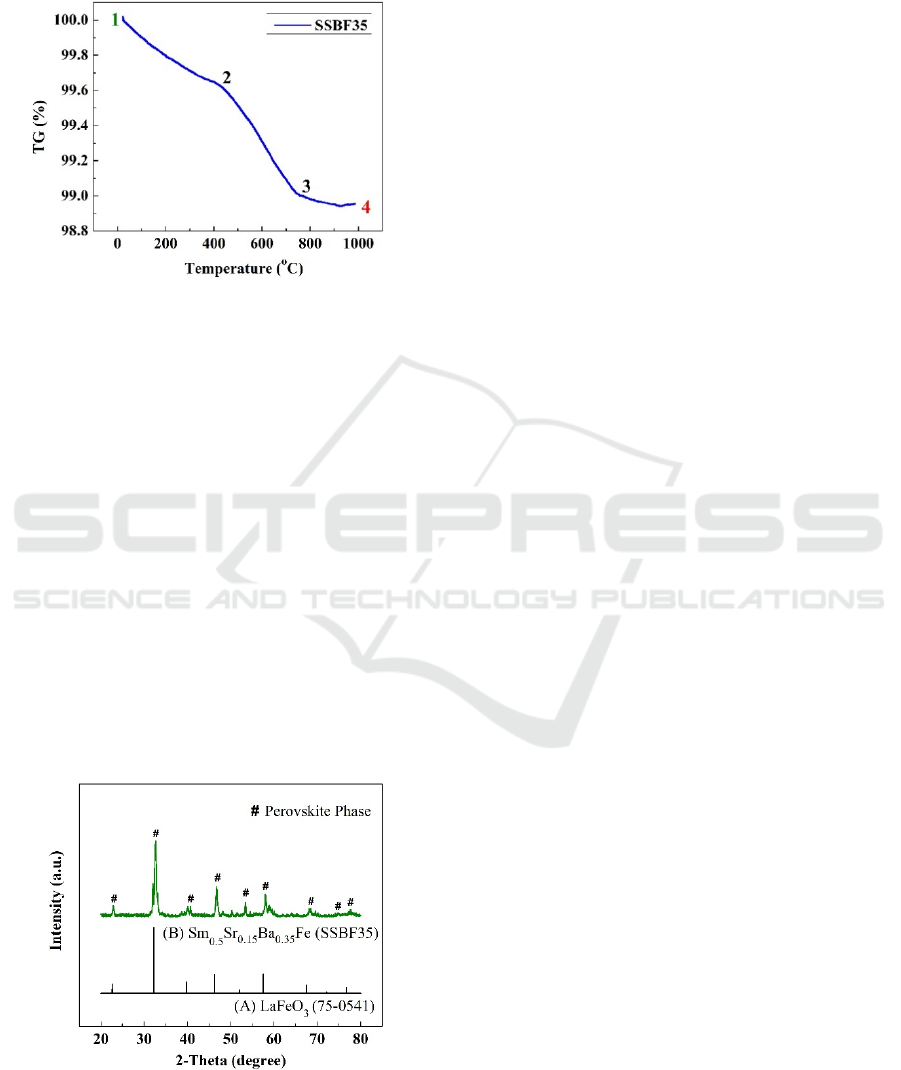
applied in the IT-SOFC system. So, based on the
thermal gravimetric characterization, the transport
mobility of oxygen ion make it possible to be started
at 410 °С.
Figure 2. Thermal gravimetric of SSBF35 cathode powders
after calcination
Fig 3 displays the XRD pattern of a composite
system of SSBF35 cathode (B) and the LaFeO3 (A)
as a reference. The observation was carried out from
20 to 80° of 2θ with a scan rate of 3°/minute. There
are nine peaks namely 22.88°, 32.64°, 40.46°, 46.68°,
52.32°, 57.68°, 67.42°, 72.48°, and 77.14° closed
with reference, respectively. The structure of the
cathode model system has a mixture phase of
rhombohedral with PDF number of 20-0130 and the
structure of cubic phase with PDF number of 14-0180
(Liu. et al., 2018). It indicates that the structure of the
cathode system was the perovskite phase. Based on
the XRD result, the composite cathode structure
related to design in the beginning for creating the
perovskite structure. Its structure could be fasilitated
the oxygent ions to be easier for movement in the
cathode element. At the device system, it takes the
avantages for IT-SOFCs system in application.
Figure 3. The XRD pattern of composite SSBF35 cathode
4 CONCLUSIONS
In this research, the composite cathode was
successfully modified using the solid-state reaction.
Thermal gravimetric confirmed that weight loss was
created during the heated process up to 1000 °С. The
decomposition reaction influenced the weight loss of
model SSBF35 in the range of 410 to 755 °С
drastically. The calcination temperature for
generating the perovskite structure was obtained less
than 920 °С, which can be used to references for the
calcination process. The perovskite phase related to
the structure modified was successfully constructed
on the composite cathode related to XRD result.
ACKNOWLEDGMENTS
The authors are grateful to the financial support
provided by Unit Penelitian dan Pengabdian
Masyarakat, Politeknik Negeri Jakarta (UP2M-PNJ)
under contract number:B.150/PL3.18/PN.00.03/2020
that made this work possible. The authors are also
grateful for the financial support of this research by
Ministry of Science and Technology of Taiwan under
contract number: MOST 106-2113-M-259-011.
REFERENCES
Julián A. Y, et al. (2020). The oxygen reduction reaction in
solid oxide fuel cells: from kinetic parameters
measurements to electrode design. J. Phys. Energy. doi:
10.1088/2515-7655/abb4ec
Kulkarni. A, et al. (2016). Enhancing Oxygen Reduction
Reactions in Solid Oxide Fuel Cells with Ultrathin
Nanofilm Electrode–Electrolyte Interfacial Layers. J.
Phys. Chem. doi: 10.1021/acs.jpcc.5b09345
Li. C. H, et al. (2012). Electrochemical characterization of
gradient Sm
0.5
Sr
0.5
CoO
3−δ
cathodes on Ce
0.8
Sm
0.2
O
1.9
electrolytes for solid oxide fuel cells, Ceramics
International, doi.org/10.1016/j.ceramint.2011.09.041
Liu. et al. (2018). Structure and electrochemical properties
of cobalt-free perovskite cathode materials for
intermediate-temperature solid oxide fuel cells,
Electrochimica Acta. doi:
org/10.1016/j.electacta.2018.05.086
Mahato. N, et al. (2015). Progress in material selection for
solid oxide fuel cell technology: A review, Progress in
Materials Science, 72 (2015) 141–337
Shao Z. P. & Haile S. M. (2004). A high-performance
cathode for the next generation of solid-oxide fuel cells,
Letter to Nature, Nature. doi: 10.1038/nature02863
Subardi. A, et al. (2017). Oxygen transportation, electrical
conductivity and electrochemical properties of layered
perovskite SmBa
0.5
Sr
0.5
Co
2
O
5+δ
, International Journal
Modification of Composite Structure on Cobalt Free Cathode for Solid Oxide Fuel Cells
99

of Hydrogen Energy.
doi:org/10.1016/j.ijhydene.2016.11.123
Susanto, I., Tsai, C., et al. (2019) ‘Morphology and surface
stability of GaN thin film grown on the short growth
time by Plasma Assisted Molecular Beam Epitaxy’, J.
Phs : Conference Seriese, 1364(012067). doi:
10.1088/1742-6596/1364/1/012067.
Susanto. I, et al. (2020) Development of cobalt-free oxide
(Sm
0.5
Sr
0.5
Fe
0.8
Cr
0.2
O
3-δ
) cathode for intermediate-
temperature solid oxide fuel cells (IT-SOFCs). Eastern-
European Journal of Enterprise Technologies. doi:
10.15587/1729-4061.2020.217282.
Zhang. Y, et al. (2017). Recent Progress on Advanced
Materials for Solid-Oxide Fuel Cells Operating Below
500 °C. Adv. Mater. doi: 10.1002/adma.201700132
Zhang. L, et al. (2013). Electrical conductivity, thermal
expansion and electrochemical performances of Ba-
doped SrCo
0.9
Nb
0.1
O
3−δ
cathodes for IT-SOFCs.
International Journal of Hydrogen Energy.
doi:org/10.1016/j.ijhydene.2013.04.107
Zhang. L, et al. (2014). Improved thermal expansion and
electrochemical performances of
Ba
0.6
Sr
0.4
Co
0.9
Nb
0.1
O
3−δ
–Gd
0.1
Ce
0.9
O
1.95
composite
cathodes for IT-SOFCs, International Journal of
Hydrogen Energy.
doi:org/10.1016/j.ijhydene.2014.03.055.
Zhang.C and Zhao. H, (2020). A novel cobalt-free cathode
material for proton-conducting solid oxide fuel cells,”
J. Mater. Chem. doi: 10.1039/c2jm32627b.
Zhou. Y, et al. (2016). Strongly correlated perovskite fuel
cells, Letter Nature. doi: 10.1038/nature17653
ASAIS 2020 - Annual Southeast Asian International Seminar
100
