
Effect of Addition of Sea Water on Changes in Turbidity and Metal
Content in Industrial Wastewater Treatment into Drinking Water
using Electrocoagulation Process
Sutanto
1
and Rahmat
1
1
Department of Electrical Engineering, State Polytechnic of Jakarta, Depok, Indonesia
Keywords: Wastewater, sea water, electrocoagulation, turbidity, drinking water
Abstract: The treatment of industrial wastewater was studied by electrocoagulation process into drinking water. The
wastewater 4.5 liters was drained off into the three cells of electrocoagulation process tank. The process was
conducted at 12 Volt in voltage and observed of change of the turbidity and metal content in interval time
of 10 minutes. Subsequently, the same procedure was carried out by adding sea water with varying volumes
of 5,10 and 15 mL Based on the research results, the best processes are obtained 10 mL of sea water, 12 Volt
of voltage and 100 minutes for processing time, where turbidity can be removed from 44.08 NTU to 4.85
NTU or equivalent 88.99 %, copper content from 3.55 to 0.93 mg/L or equivalent 80.97 % and iron content
from 1.25 to 0.27 mg/L or equivalent 70,95 %.
1 INTRODUCTION
The wastewater is generally divided into two types,
industrial and domestic wastewater. The content of
pollutants in wastewater are organic and inorganic
materials. These two types of waste water can be
recycled into drinking water or clean water.
According to the regulation of the Minister of Health
of the Republic of Indonesia No. 492 / Menkes / Per /
IV / 2010, the maximum content of each parameter in
drinking water is as follows: are 5 NTU for turbidity,
0.3 mg/L for iron (Fe), 2 mg/L for copper (Cu), 0.01
mg/L for arsenic (As), 0.05 mg/L for chrome (Cr),
0,02 mg/L for aluminum (Al) and 0.003 mg/L for
cadmium (Cd).
One of the methods that can be used to treat
wastewater into clean or drinking water is the
electrocoagulation process. The
equipment needed to
carry out the electrocoagulation process are as
follows: direct current voltage source (DC), anode,
cathode, process tank made from insulating material,
voltage and current meter (Zaied et al,,2020) .
Aluminum or iron is a material that can be used as
anode or cathode (Salem,2020). When the
electrocagulation process is carried out, the anode
will release metal ions to form a coagulant which
absorbs all pollutants in the water. When aluminum is
used as an anode, the coagulant formed is the
compound Al(OH)
3
. However, if the anode used is
iron, the coagulant compound formed is Fe(OH)
3
.
Wastewater from domestic or restaurants contains
a lot of organic pollutants, so the electrical
conductivity is very weak. If the electrical
conductivity is very weak, the coagulant formed is
very small. So that the time needed to remove
pollutants is longer (Adegoke et al,2020).
Electrical conductivity can be increased by adding
seawater into wastewater. The sea water has the
ability to kill microorganisms and can also produce a
strong electrolyte solution (Pishgar et al,2020).
Sea water added into wastewater will accelerate
the removal of bacteria and pollutants in the
wastewater, while the electrocoagulation process is
being operated (Sefatjoo et al,2020).
The equation of the reaction in the
electrocoagulation process using aluminum as anode
is as follows ( Rigueto et al, 2020):
anode (oxidation process ):
2Al 2Al
+3
+ 6e
-
(1)
cathode (reduction process) :
6H
2
O+6e
-
6OH
-
+3H
2
(2)
in overall :
2Al+6H
2
O2Al(OH)
3
+3H
2
(3)
Sutanto, . and Rahmat, .
Effect of Addition of Sea Water on Changes in Turbidity and Metal Content in Industrial Wastewater Treatment into Drinking Water using Electrocoagulation Process.
DOI: 10.5220/0010536000003153
In Proceedings of the 9th Annual Southeast Asian International Seminar (ASAIS 2020), pages 85-92
ISBN: 978-989-758-518-0
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
85

In equation 3 shows that the final result of the
chemical reaction from the electrocoagulation
process is the coagulant compound Al(OH)
3
.
Coagulant Al(OH)
3
is a compound that has the
characteristic of easily absorbing pollutants in the
water.
The cadmium removal efficiency from the
electrocoagulation process carried out at a voltage of
20 volts is 90%. The initial content of Cadmium is 50
mg/L and a pH of 7 (Alameen,,2020).
In the electrocoagulation process of restaurant
wastewater within 30 minutes, the results show that
the efficiency of removal of dissolved oxygen (DO),
color, chemical oxygen demand (COD), phosphorus
pentoxide (P
2
O
5
), soluble reactive polyphosphate
(PO
4
3-
) and particulate phosphorus (PP) are 20.12%,
-32.88%, 12.58%, 17.15%, 19.33%, 16.85%
respectively (Temitope, 2020).
The results of the continuous process
electrocoagulation research on iron pollutant removal
showed that the removal efficiency of iron reached
99.9%. The process was carried out at a current
density of 7.3 mA/cm
2
, a processing time of 50
minutes and an initial concentration of iron 10 mg/L
(Abdulhadi et al,2021).
The electrocoagulation process which is added
with sodium chloride (NaCl) salt can accelerate the
corrosion of aluminum electrodes, but can increase
the electrical conductivity. So that the formation
process of the Al(OH)
3
coagulant compound is
increasing and can also accelerating the process of
removing pollutants in wastewater
Research decoloration in wastewater using
anode and cathode Al only able to remove the color
reaches 95.50%, whereas when using the anode and
the cathode Fe were able to remove the color can
reach 97.24% (Ebba, 2021).
The results of a study on the electrocoagulation
process in in brackish water and sea water showed
that process can reduce silica content (Zhang et al,
2019). The silica content in brackish water is easier to
remove than silica in seawater. The
electrocoagulation process of brackish water
containing 17 mg/L of silica dioxide can remove 60%
of silica dioxide. Meanwhile, the electrocoagulation
process of seawater containing 17 m/L of silica
dioxide was only able to remove 40% of silica
dioxide.
Salinity was removed electrochemically from
saline water through electrocoagulation process
(Raad et al, 2020). In saline water contain ions of Br
−
,
Cl
−
, TDS, and SO
4
2−
.
The study on the electrocoagulation process in
brine which contain of lithium ions showed that more
than 95% of lithium ions (1000 mg/L) could be
recovered with a relatively low energy consumption
of 0.064 kWh/g Li, under operating conditions of
76.9 mA/cm
2
of current density, 6.45 of pH, and
reaction time of 150 minutes (Zhang et al, 2020). That
the lithium recovery from brine by electrocoagulation
was mainly attributed to the chemical precipitation of
aluminum hydroxide coagulants.
Research on aluminum removal from biomass
has been carried out using 7.1 mA/cm
2
current
density, asymmetrical aluminum electrodes and
10 minutes electrolysis time (Hawari et al, 2020). The
aluminum content in the harvested biomass which
decreased by 52% compared to the conventional
symmetrical electrocoagulation electrodes.
Research on cadmium removal in wastewater
containing high concentrations of inorganic salts
using electrocoagulation process. The results showed
that cadmium can be removed up to 99.5% (Xu et al,
2019).
Research on the removal of ammonia and nitrite
in seawater was carried out by electrocoagulation.
The results showed that ammonia can be removed up
to 95% (Song et al, 2020).
The study has been carried out on the effect of
adding sodium salt on electrocoagulation wastewater
treatment. The results of study shown that sodium salt
is very influential on the corrosion of aluminum
surface and found the aluminum metal in the
sediment (Wellner et al, 2018)
The combination of electrocoagulation and
osmosis processes can remove more than 90%
phosphate, more than 80% carbonate and more than
40% dissolved organic pollutants (Azerrad et al,
2019).
Electrocagulation process to completely
eliminate 41 mg/L lead content in wastewater
requires the following process conditions: electric
current density of 0.3 A, pH 6, processing time of 13
minutes and energy of 0.77 watt-hours per gram of
lead removal (Khan et al,2020)
Another alternative to kill or eliminate bacteria
or other microorganisms contained in wastewater can
be done by adding seawater into the wastewater
which is being processed electrocoagulation. Because
the content in seawater consists of 55% chloride salt,
31% sodium salt, 8% sulfate salt, 4% magnesium salt,
1% calcium salt, 1% potassium salt, and the
remaining less than 1% is bicarbonate, bromide, boric
acid, strontium, and fluoride salts. The composition
of the chloride salt content which reaches 55% is very
likely that sea water can be used as a disinfectant or
sterilizer for wastewater, because it will form a
compound hypochlorite (OCl
-
) when combined with
ASAIS 2020 - Annual Southeast Asian International Seminar
86
the electrocoagulation process. The hypochlorite
compound (OCl
-
) will act as a killing agent or oxidize
microorganisms (Wellner et al,2018).
The electrocoagulation process that is carried
out under strong acid conditions is the best choice,
because chlorine (Cl
2
) is the strongest oxidizing agent
compared to HClO. Therefore, a higher pH value
could theoretically increase the electrochemical
oxidation of pollutants where HClO and ClO
-
are not
affected by gas desorption and can act as oxidizing
agents (Anglada,2009). The following equation for
the reaction is the evolutionary reaction of chlorine in
the indirect oxidation process which is
influenced by
pH:
Cl → 1/2 Cl
2
+ e− (4)
Cl
2
+ H
2
O → HOCl + H
+
+ Cl
−
(5)
HOCl → ClO
−
+ H
+
(6)
Increasing the concentration of Cl
-
can increase
removal or decrease the content of pollutants, but
there is also an increase in the formation of
chlorinated organic compounds which are quite toxic.
High concentrations of electrolytes can increase the
conductivity of the solution and reduce the demand
for electrical voltage from the electrocoagulation cell.
Thus it can be explained that the electrochemical
oxidation process will be more economical or cheaper
if the treated wastewater has a high level of salinity.
An important phenomenon that occurs in the
electrocoagulation process is the formation of an
oxidizing agent such as HOCl, OCl
−
, ClO
2
and Cl
2
.
The oxidizing agent that is formed is to kill
microorganisms The reaction mechanism can be
explained as follows (Ghernaout et al,2020) :
2Cl
−
→ Cl2 + 2e− (7)
Cl
2
+ 2OH− → H
2
O + OCl
−
+ Cl
−
(8)
Cl
2
+ 4H
2
O → 2ClO
2
+ 8e
−
(9)
2 METHOD
The research method that has been carried out
consists of preparation of materials, preparation of
equipment and implementation of research
2.1 Materials
Materials needed include seawater, industrial
wastewater and aluminum HTC 16-35.
2.2 Equipments
The equipments needed include: electrocoagulation
cells, waste water tanks, settling tanks, clean water
storage tanks, electric power sources, electric voltage
measuring devices, flow meters, turbidimeter and
AAS (Atomic Absorption Spectrophotometer).
2.3 Implementation
2.3.1 Wastewater Quality Measurement
Several parameters are measured to determine the
quality of wastewater, including: turbidity is
measured by a turbidimeter, metal content is
measured by AAS and the level of acidity is measured
by a pH meter.
2.3.2 Constructing Research Equipments
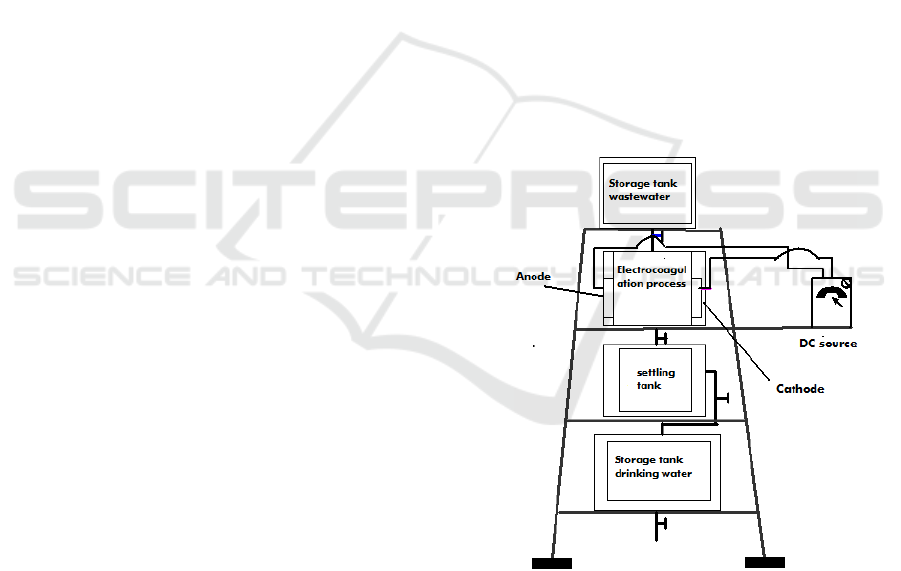
The equipments used for research can be seen in
Figure 1, consists of a voltage source,
electrocoagulation process cells, waste water tanks,
settling tanks, clean water tanks, electric current and
voltage meters.
Figure 1: The series of research tools
2.3.3 Research Implementation
The wastewater 4.5 liters was drained off into the
three cells of electrocoagulation process tank. The
process was conducted at 12 Volt in voltage and
observed of change of the turbidity and metal content
in interval time of 10 minutes. Subsequently, the
same procedure was carried out by adding sea water
with varying volumes of 5, 10 and 15 mL. The
Effect of Addition of Sea Water on Changes in Turbidity and Metal Content in Industrial Wastewater Treatment into Drinking Water using
Electrocoagulation Process
87
turbidity is measured using the turbidimeter and
metals content is measured using Atomic Absorption
Spectrophotometer (AAS).
3 RESULT AND ANALYSIS
Laboratory test results of electronics industry
wastewater parameters shown in table 1. According
to table 1, it appears that the initial content of the
metals in wastewater is 3.55 mg/L for copper, 0.78
mg/L for aluminum and 1.25 mg/L for iron.
According to the regulation of the minister of health
No.492/Menkes/Per/IV/2010, shown that all content
of metals in wastewater has not met the requirements
for drinking water standards.
Table 1: Results of measurements of electronics industry
wastewater
Parameters Measurement results
Copper (Cu) 3.55 mg/L
Aluminum (Al) 0.78 mg/L
Chrome (Cr) not detected
Iron (Fe) 1.25 mg/L
pH 7.54
Turbidity 44.08 NTU
The results of research by impact of addition of sea
water in wastewater treatment in electrocoagulation
process presented in 3 views, namely: impact of
addition of sea water to changes turbidity, impact of
addition of sea water to changes copper content and
impact of addition of sea water to changes iron
content. Each research result, then can be seen in the
sub-section discussion from 3.1 until 3.4.
3.1 Impact of Addition of Sea Water to
Changes in Water Turbidity
The laboratory test results of water turbidity, shown
in table 2. From table 2, can be seen that the addition
of sea water in the electrocoagulation process is able
to remove the turbidity in wastewater. The
electrocoagulation process which is carried out
without the addition of sea water, the turbidity level
can be reduced from 44.08 NTU to 18.20 NTU or
equal to 58.71% in 110 minutes for process time. If
the process is carried out for up to 120 minutes, it will
not be able to produce drinking water standard,
because the turbidity of the water is 12.21 NTU or
more than 5 NTU. Furthermore, the
electrocoagulation process which was carried out for
120 minutes with the addition 5 mL of sea water had
not yet obtained the water drinking standard. Because
turbidity of water is 6.27 NTU. Meanwhile, the
electrocoagulation process, which was carried out for
110 minutes with the addition of 10 mL of sea water,
was able to produce water that has a drinking water
standard with turbidity of 3.25 NTU or less than of 5
NTU. In this case the turbidity of water can be
reduced from 44.08 to 3.25 or equal to 92.63 %.
When the electrocoagulation process is carried
out using aluminum as an anode, an Al(OH)
3
coagulant will be formed. The Al(OH)
3
coagulant is
an adsorbant that can absorb pollutants in water, so
that the turbidity will be reduced in wastewater.
Table 2: Results of measurements of turbidity
Time addition of sea water
(minute) 0 mL 5mL 10 mL 15 mL
0 44.08 44.08 44.08 44.08
10 43.98 42.24 41.55 40.85
20 43.18 41.14 39.25 38.15
30 42.68 39.24 37.85 34.56
40 41.21 36.27 34.25 32.76
50 39.51 34.87 32.54 30.12
60 37.48 31.29 28.25 26.76
70 34.21 28.27 24.85 22.23
80 30.71 22.54 19.25 16.76
90 27.29 18.37 12.65 12.34
100 22.14 14.43 4.85 3.76
110 18.20 10.27 3.25 1.23
120 12.21 6.27 1.25 0.16
3.2 Impact of Addition of Sea Water to
Changes in Copper Content
The laboratory test results of copper content, shown
in table 3. From table 3, can be seen that the addition
of sea water in the electrocoagulation process is able
to remove the copper content in wastewater. The
copper content can be reduced from 3.55 mg/L to
2.02 mg/L or equal to 43.10 % at 80 minutes for
process time without adding sea water. If the
electrocoagulation process is carried out for up to 120
minutes, it will produce the water that is as drinking
water standard. Because the copper content in the
water was 0.98 mg/L or less than 2 mg/L. In this case
it is clearly shown that the copper content can be
reduced from 3.55 mg/L to 0.98 mg/L or equal to
72.39%. Furthermore, the electrocoagulation process
which was run for up to 70 minutes with the addition
of 5 mL of sea water has been able to produce
drinking water. Because copper content in the water
was 1.85 mg/L or less than 2 mg/L or copper content
can be reduced from 3.55 to 1.85 mg/L that was
ASAIS 2020 - Annual Southeast Asian International Seminar
88

equivalent of 47.44 %. Meanwhile, the
electrocoagulation process which was carried out for
60 minutes with the addition of 10 mL of sea water,
was able to produce water that has a drinking water
standard with copper content was 1.91 mg/L or less
than of 2 mg/L. In this case the copper content in the
water has been removed from 3.55 to 1.91 mg/L or
equal to 45.74 %. Furthermore, the electrocoagulation
process which was run for up to 50 minutes with the
addition of 15 mL of sea water has been able to
produce drinking water. Because copper content in
the water was 1.98 mg/L (less than 2 mg/L) or copper
content can be reduced from 3.55 to 1.98 mg/L that
was equivalent of 43.75 %.
Table 3: Results of measurements of copper content
Time addition of sea water
(minute) 0 mL 5mL 10 mL 15 mL
0 3.55 3.55 3.55 3.55
10 3.50 3.48 3.28 3.00
20 3.42 3.32 3.02 2.67
30 3.21 3.08 2.82 2.45
40 3.01 2.83 2. 45 2.18
50 2.86 2.54 2.12 1.98
60 2.56 2.21 1.91 1.57
70 2.21 1.85 1.74 1.23
80 2.02 1.56 1.54 1.01
90 1.83 1.32 1.12 0.91
100 1.47 1.03 0.93 0.73
110 1.21 0.89 0.67 0.54
120 0.98 0.67 0.47 0.32
3.3 Impact of Addition of Sea Water to
Changes in Iron Content
The laboratory test results of iron content, shown in
table 4. From table 4, can be seen that the addition of
sea water in the electrocoagulation process is able to
remove the iron content in wastewater.
If the process is conducted without the addition
of sea water, then to get water that is drinking water
standard it takes a minimum of 120 minutes. In this
case iron content can be removed from 1.25 to 0.21
mg/L or equivalent to 83.20 %. The maximum
recommended iron content in drinking water is 0.3
mg/L (according to drinking water requirements).
If the process is conducted with the addition 5
mL of sea water, then to get water that is drinking
water standard it takes a minimum of 100 minutes for
process time. In this case iron content can be reduced
from 1.25 to 0.26 mg/L (less than 0.3 mg/L) or
equivalent to 78.51%. It appears that the time
required for the process is 10 minutes faster than the
process without adding sea water.
If the electrocoagulation process is carried out
with the addition 10 mL of sea water, then to get water
that is drinking water standard it takes a minimum of
100 minutes. In this case iron content can be reduced
from 1.25 to 0.26 mg/L (less than 0.3 mg/L) or
equivalent to 79.20 %. It appears that the time
required for the process is 20 minutes faster than the
process without adding sea water or 10 minutes faster
than the process with adding 5 mL sea water.
If the electrocoagulation process is carried out
with the addition 15 mL of sea water, then to get water
that is drinking water standard it takes a minimum of
80 minutes. In this case iron content can be reduced
from 1.25 to 0.22 mg/L (less than 0.3 mg/L) or
equivalent to 82,40 %. It appears that the time
required for the process is 40 minutes faster than the
process without adding sea water or 20 minutes faster
than the process with adding 10 mL of sea water.
The addition of sea water must be limited so that
the quality of the water produced is maintained
according to drinking water standard. However,
turbidity and metal content are maintained so that the
quality of the water produced is in accordance with
drinking water standards. The volume of sea water
recommended is 10 mL and the time for the
processing is 100 minutes and 12 Volts in voltage.
The water turbidity in this condition is 4.85 NTU, the
copper content is 0.93 mg/L and the iron content is
0.27 mg/L.
The process of removing iron content in water
can be accelerated by adding seawater. Because the
added sea water can increase the electrical
conductivity, so that the electric current that flowed is
greater than the initial current. The increasing electric
current can accelerate the formation rate of the
Al(OH)
3
coagulant. The coagulant of Al(OH)
3
which
functions as an absorbent compound and precipitates
iron pollutants in the water.
Effect of Addition of Sea Water on Changes in Turbidity and Metal Content in Industrial Wastewater Treatment into Drinking Water using
Electrocoagulation Process
89
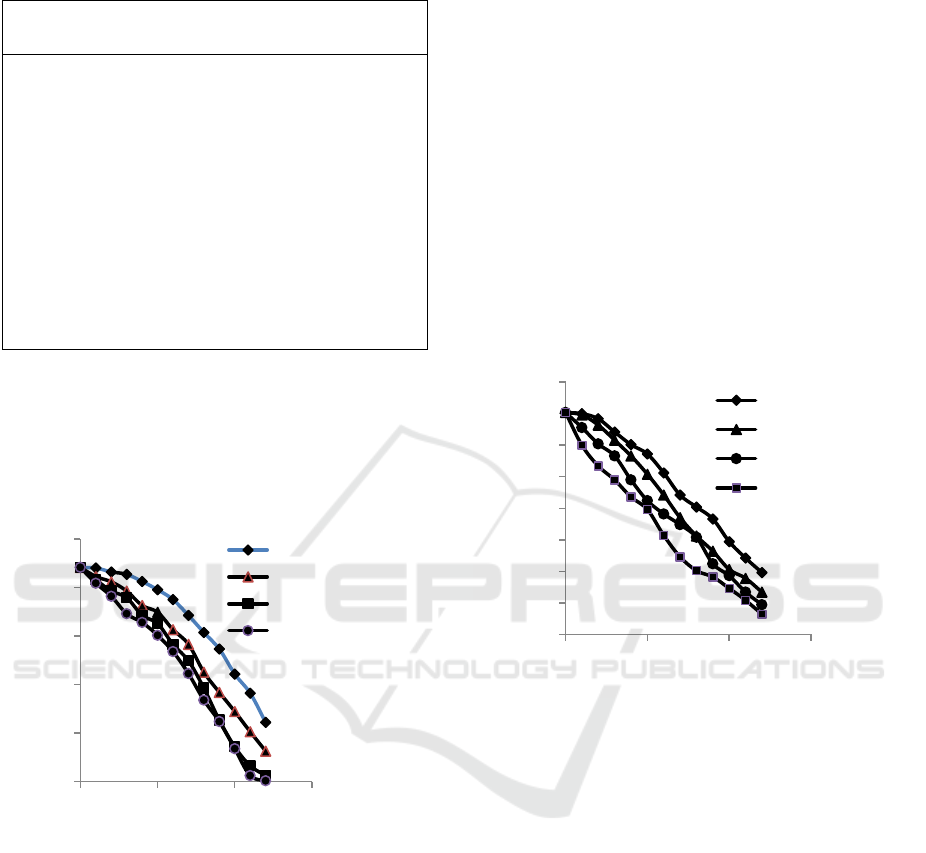
Table 4: Results of measurements of iron content
Time addition of sea water
(minute) 0 mL 5mL 10 mL 15 mL
0 1.25 1.25 1.25 1.25
10 1.20 1.18 1.12 1.10
20 1.19 1.09 1.01 0.99
30 1.12 0.93 0.90 0.87
40 1.00 0.89 0.76 0.71
50 0.91 0.72 0.67 0.58
60 0.82 0.63 0.56 0.47
70 0.71 0.52 0.43 0.31
80 0.62 0.43 0.38 0.22
90 0.55 0. 37 0.31 0.19
100 0.41 0.32 0.27 0.12
110 0.31 0.26 0.18 0.09
120 0.21 0.18 0.07 0.01
3.4 Curves of Changes of Turbidity
and Metal Content
Figure 2 is made from the data in table 2 which shows
the relationship of the effect of changes in sea water
addition to water turbidity.
Figure 2: The relationship between changes of sea water
additions to water turbidity
According to figure 2, it can be seen that the
performance of the electrocoagulation process to
remove turbidity increases when the sea water is
added into the wastewater. If more and more sea
water is added to the electrocoagulation process, the
process of reducing water turbidity will be
accelerated. The addition of sea water into
wastewater will increase the electrical conductivity.
The increasing electrical conductivity will accelerate
the formation of the Al(OH)
3
coagulant. The Al
(OH)
3
compound is a material that absorbs pollutants
in water and precipitates it to the bottom of the
process tank, so that the water is getting clearer.
Figure 3 is made from the data in table 3 which
shows the relationship of the effect of changes in sea
water addition to remove copper content in water.
According to figure 3, it can be seen that the
performance of the electrocoagulation process to
remove copper content increases when the sea water
is added into the wastewater. If more and more sea
water is added to the electrocoagulation process, the
process of reducing copper content in water will be
accelerated. The addition of sea water to wastewater
will increase the electrical conductivity. The
increasing electrical conductivity will accelerate the
formation of the Al(OH)
3
coagulant. The Al(OH)
3
compound is a material that absorbs copper content
in water and precipitates it to the bottom of the
process tank, so that the copper content in the water
is getting lower.
Figure 3: The relationship between changes of sea water
additions to copper content
Figure 4 is made from the data in table 4 which
shows the relationship of the effect of changes in sea
water addition to remove iron content in the water.
According to figure 4, it can be seen that the
performance of the electrocoagulation process to
remove iron content increases when the sea water is
added into the wastewater. If more and more sea
water is added to the electrocoagulation process, the
process of reducing iron content in water will be
accelerated. The addition of sea water into
wastewater will increase the electrical conductivity.
The increasing electrical conductivity will accelerate
the formation of the Al(OH)
3
coagulant. The Al
(OH)
3
compound is a material that absorbs iron
content in water and precipitates it to the bottom of
the process tank, so that the iron content in the water
is getting lower.
0
10
20
30
40
50
050100150
Turbidity,NTU
Time, minute
seawater0mL
seawater5mL
seawater10mL
seawater15mL
0
0,5
1
1,5
2
2,5
3
3,5
4
050100150
Copper content, mg/L
Time, minute
sea water 0 mL
sea water 5 mL
sea water 10 mL
sea water 15 mL
ASAIS 2020 - Annual Southeast Asian International Seminar
90
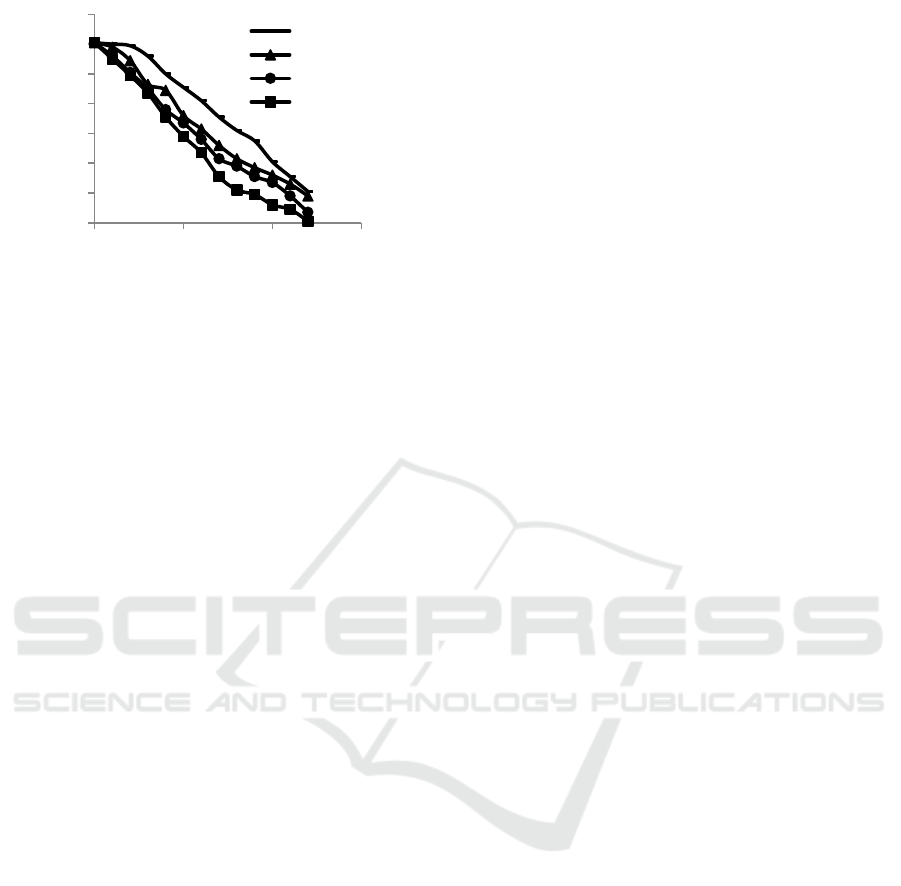
Figure 4: The relationship between changes of sea water
additions to iron content
4 CONCLUSION
Sea water added to wastewater which is being
processed electrocoagulation can remove turbidity,
copper and iron content. The recommended variable
values to operate the electrocoagulation process are
12 V for voltage, 10 mL for seawater requirements
and 100 minutes for processing time. Efficiency
removal of turbidity is 88.99 %, copper content is
80.97% and iron content is 70.95 % repectively.
ACKNOWLEDGEMENTS
The Authors would like to thank the head of research
and community unit, Jakarta State Polytechnic for
financial support, so that the authors are able to
complete this research. Thank a lot also to Afiliation-
Laboratory FMIPA University of Indonesia UI for
giving laboratory equipment support.
REFERENCES
Abdulhadi, B., Kot, P., Hashim, K., Shaw, A., Muradov, M.
and Khaddar, R.A., 2021. Continuous-flow
Electrocoagulation (EC) Process for Iron Removal
from Water: Experimental, Statistical and Economic
Study, Science of The Total Environment.
Adegoke, A.T, Abayomi and Temitayo, E.,2020. A
Preliminary Study on The Treatment of Restaurant
Wastewater Using Electrocoagulation Technique,
Journal of Degraded and Mining Lands Management.
Alameen, M. and Majeed, N., 2020. Removal of Cadmium
from Industrial Wastewater using Electrocoagulation
Process, Chemical, Petroleum and Environmental
Engineering.
Anglada, A., Urtiaga, A. and Ortiz, I., 2009. Contributions
of Electrochemical Oxidation to Waste-water
Treatment: Fundamentals and Review of Applications,
Society of Chemical Industry.
Azerrad, S. P., Isaacs, M. and Dosoretz, C. G., 2019.
Integrated Treatment of Reverse Osmosis Brines
Coupling Electrocoagulation with Advanced Oxidation
Processes, Chemical Engineering Journal.
Ebba, M., 2021. Application of Electrocoagulation for the
Removal of Color from Institutional Wastewater:
Analysis with Response Surface Methodology, Journal
of Environmental Treatment Techniques.
Ghernaout, D. and Elboughdiri, N., 2020.
Electrocoagulation Process in the Context of
Disinfection Mechanism, Scientific Research.
Hawari, A.H., Alkhatib, A.M., Das, P., Thaher, M. and
Benamor, A.,2020. Effect of the Induced
Dielectrophoretic Force on Harvesting of Marine
Microalgae (Tetraselmis sp.) in Electrocoagulation,
Journal of Environmental Management.
Khan, S.U., Mahtab, M.S.and Izharul Haq Farooqi, I.H.,
2020. Enhanced Lead (II) Removal with Low Energy
Consumption in an Electrocoagulation Column
Employing Concentric Electrodes: Process
Optimisation by RSM Using CCD, International
Journal of Environmental Analytical Chemistry.
Pishgar, Z., Samimi, A., Kalhori, D.M. and
Shokrollahzadeh, S.,2020. Comparative Study on The
Harvesting of Marine Chlorella Vulgaris Microalgae
from a Dilute Slurry Using Autoflocculation-
Sedimentation and Electrocoagulation-Flotation
Methods, International Journal of Environmental
Research.
Raad, A. A. A., Hanafiah, M. M, Naje, A. S., Ajeel, M.A.,
2020. Optimized parameters of the electrocoagulation
process using a novel reactor with rotating anode for
saline treatment, Environmental Pollution.
Rigueto,C.V.T., Nazari,M.T., Souza,C.F.D, Cadore,J.S.,
Brião, V.B. and Jeferson Steffanello Piccin, J.S, 2020.
Alternative Techniques for Caffeine Removal from
Wastewater: an Overview of Opportunities and
Challenges, Journal of Water Process Engineering
Salem, M. A., 2020. Removal of Cadmium from Industrial
Wastewater Using Electrocoagulation Process, Journal
of Engineering.
Sefatjoo, P.,Moghaddam,M.R.A.and Mehrabadi,A.R.,
2020. Evaluating Electrocoagulation Pretreatment Prior
to Reverse Osmosis System for Simultaneous Scaling
and Colloidal Fouling Mitigation: Application of RSM
in Performance and Cost Optimization, Journal of
Water Process Engineering
Song, J., Yin, Y., Li, Y.,Gao, Y. and Liu,Y.,2020. in-Situ
Membrane Fouling control by Electrooxidation and
Microbial Community in Membrane electro-bioreactor
Treating Aquaculture Seawater, Bioresource
Technology.
Wellner, D. B., Couperthwaite, S. J. and Millar, G.J.,2018.
Influence of Operating Parameters During
Electrocoagulation of Sodium Chloride and Sodium
0
0,2
0,4
0,6
0,8
1
1,2
1,4
050100150
Ironcontent,mg/L
Time,minute
seawater0mL
seawater5mL
seawater10mL
seawater15mL
Effect of Addition of Sea Water on Changes in Turbidity and Metal Content in Industrial Wastewater Treatment into Drinking Water using
Electrocoagulation Process
91

Bicarbonate Solutions Using Aluminium Electrodes,
Journal of Water Process Engineering.
Xu, L., Wu, D., Liu, W., Xu, X., Cao, G., 2019.
Comparative Performance of Green Rusts Generated in
Fe
0
–Electrocoagulation for Cd
2+
Removal from High
Salinity Wastewater: Mechanisms and Optimization,
Journal of Environmental Management.
Zaied, B.K, Rashid, M., Nasrullah,,M..Zularisam,
A.W.,Pant,D.and Singh, L., 2020. A Comprehensive
Review on Contaminants Removal from
Pharmaceutical Wastewater by Electrocoagulation
Process, Science of The Total Environment.
Zhang, X.,Lu, M., Idrus, M.A.M., Crombie,C.,Veeriah and
Jegatheesan,V., 2019. Performance of Precipitation and
Electrocoagulation as Pretreatment of Silica Removal
in Brackish Water and Seawater, Process Safety and
Environmental Protection.
Zhang,Y., Xu, R., Sun, W. , Wang, L. and Tang, H., 2020.
Li Extraction from Model Brine via Electrocoagulation:
Processing, kinetics, and Mechanism, Separation and
Purification Technology.
.
.
ASAIS 2020 - Annual Southeast Asian International Seminar
92
