
Enhancing Anti-pathogenic Bacteria Activity of Lactobacillus
Plantarum AKK-30 Cultured on the Medium Containing
Fructose-Oligosaccharides
Nisa Grendpina
1
, Dyah Fitri
1
, Hardi Julendra
2
, Ahmad Sofyan
2
and Ema Damayanti
2
1
Faculty of Biology, Jenderal Soedirman University, Purwokerto, Central Java, Indonesia
2
Research Division of Natural Product Technology (BPTBA) - Indonesian Institute of Sciences (LIPI), Indonesia
Keywords: Antimicrobial Metabolites, Fructose-Oligosaccharide, L. plantarum, Probiotic.
Abstract: The purpose of this study was to evaluate the concentration of Fructose-Oligosaccharides (FOS) in correlation
with incubation time for growing - Lactobacillus plantarum AKK-30, and to assess metabolites of L.
plantarum AKK-30 as antimicrobial substances against to pathogenic bacteria. L. plantarum AKK-30 was
isolated from the small intestine of native chicken. L. plantarum AKK-30 was grown on MRSB medium
containing FOS (0%, 0.5%, 1%, and 1.5%), and incubated at different times (6, 12, 18, and 24 hours) at 37°C.
Antimicrobial activity of L. plantarum AKK-30 metabolites was tested on four species of pathogenic bacteria
consisted of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Salmonella pullorum.
The results showed that the concentration of 1% FOS and 24-hours incubation were most effective in
increasing L. plantarum AKK-30 growth (2.11 x 10
8
CFU/ml). Antimicrobial activity extract of L. plantarum
AKK-30 metabolites was able to inhibit the growth of E. coli, P. aeruginosa, S. aureus, and S. pullorum. The
highest inhibition of bacteria was observed on S. aureus which was 10.8 mm, followed by E. coli at 9.9 mm,
S. pullorum at 9.083 mm, and P. aeruginosa 8.783 mm.
1 INTRODUCTION
Lactobacillus plantarum AKK-30 is a lactic acid
bacteria (LAB) isolated from Indonesian native
chicken (Damayanti et al., 2014) and has been
identified microbiologically, biochemically, and
molecularly (Istiqomah et al., 2017). This species
reported that has an activity of enzyme cholesterol
reductase (Julendra et al., 2017; Palaniyandi et al.,
2019). L. plantarum AKK-30 has an inhibitory agent
for pathogenic bacteria and produces antimicrobials
(Julendra et al., 2018; Sophian et al., 2018) and could
be used as probiotics for poultry (Wei et al., 2018).
Previous studies have reported that potency
antibacterial activity of L. plantarum (Kabir et al.,
2009), (Yang et al., 2017), (Lin and Tzu-M, 2017).
The growth of probiotic bacteria can be increased by
the addition of oligosaccharides in their medium
(Pranckute et al., 2016). The use of inulin and mono-
oligosaccharides as prebiotics has been investigated
for increasing viability of L. plantarum AKK-30
(Julendra et al., 2018). However, Julendra et al.
(2018) reported that a combination of L. plantarum
AKK-30 and oligosaccharides were not significant
influences of antibacterial activity. Addition of
mannan oligosaccharides (MOS) at 0.5 - 2% could
increase L. plantarum AKK-30 growth with 0.5%
MOS. However, the possibility of improving L.
plantarum growth by combining FOS has not been
reported. FOS is an oligosaccharide composed of 2-
10-unit fructose monomers with bonds -(2-1)
glycoside and one glucose monomer with bonds -(2-
1) glycoside at the ends (Yuliana et al., 2014).
Addition of FOS in probiotics was for microbial
nutrition (Setiarto et al., 2017), it could improve
metabolism of probiotic bacteria and increase the
number of bacterial cell biomass, bacteriocin
increases (Ogunbanwo et al., 2003), inhibited the
growth of pathogenic bacteria (Pranckute et al.,
2016).
Addition of FOS prebiotic in media is expected to
stimulate the growth of L. plantarum AKK-30
because a FOS was more soluble than inulin (about
80% in water at room temperature) (Gibson et al.,
2017). Therefore, a current study was conducted to
evaluate the effect of FOS addition in growth medium
Grendpina, N., Fitri, D., Julendra, H., Sofyan, A. and Damayanti, E.
Enhancing Anti-pathogenic Bacteria Activity of Lactobacillus Plantarum AKK-30 Cultured on the Medium Containing Fructose-Oligosaccharides.
DOI: 10.5220/0009991800002964
In Proceedings of the 16th ASEAN Food Conference (16th AFC 2019) - Outlook and Opportunities of Food Technology and Culinary for Tourism Industry, pages 205-209
ISBN: 978-989-758-467-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
205
on enhancing the anti-pathogenic bacteria activity of
L. plantarum AKK-30.
2 MATERIALS AND METHODS
2.1 Materials and Research Design
The research was conducted at the Research Division
of Natural Product Technology (BPTBA)-
Indonesian Institute of Sciences (LIPI) at the
Microbiology Laboratory, from November to
December 2018 using L. plantarum AKK-30 isolates
belonging to the BPTBA-LIPI Microbiology
Laboratory and the commercially obtained of Fructo-
oligosaccharides (FOS). FOS was dissolved in
distilled water until homogeneous and then sterilized
using 0.22 µm Millipore then implanted in MRSA. 2
mL of MRSB and 1% of L. plantarum AKK-30 were
added into microtube and then were vortexed and
incubated 24 hours at 37ºC. A series of falcon tubes
filled with 10 mL of sterile MRSB were added with
FOS (0%, 0.5%, 1%, 1.5%) and 1% of L. plantarum
AKK-30 culture (Setiarto et al., 2017). Pathogenic
bacteria used were Escherichia coli FNCC 0194,
Staphylococcus aureus FNCC 6049, Pseudomonas
aeruginosa FNCC 0063, and Salmonella pullorum
ATCC 13036, the bacteria were grown in nutrient
agar (NA) [Merck].
The study used an experimental method with two
stages using a Factorial-Completely Randomized
Design (FCRD). The experiment was arranged using
two stages; the first stage was optimization of L.
plantarum AKK-30 growth added with FOS 0%,
0.5%, 1%, and 1.5% and the second stage using a
Completely Randomized Design (CRD) tested the
antimicrobial activity of metabolites from the best
growth results of L. plantarum AKK-30 in the first
stage of which each treatment consisting of 4
replications. The parameters measured were bacterial
growth and antibacterial activity.
2.1.1 Total Plate Count (TPC)
Colonies of L. plantarum AKK-30 were enumerated
by the TPC method as previously reported by Setiarto
et al. (2017). Briefly, 1 ml of L. plantarum AKK-30
culture (6, 12, 18 and 24 hours) was diluted with 9 ml
of sterile distilled water until 10
-7
. 1 ml of culture was
inoculated on MRSA by pour plate method, then
incubated at 37°C for 48-hours.
2.1.2 Antimicrobial Activity
Antimicrobial activity was assessed according to
Damayanti et al. (2014). Briefly, L. plantarum AKK-
30 culture was centrifuged at 12.500 rpm and 4°C for
15 minutes. The supernatant was neutralized using
0.5N NaOH and sterilized using 0.22 μL millipore.
Agar diffusion method (Bonev et al., 2008) was used
to test the inhibitory activity of pathogenic bacteria
by inoculating pathogenic bacteria on NA media
(Merck) and 50 μL supernatant dripped on sterile disk
paper. Then incubated for 24-hours at 37°C. Positive
results of antimicrobial activity were revealed by the
formation of clear zones around the disk paper.
2.2 Data Analysis
Quantitative data from bacterial growth and
antibacterial activity were analyzed by using analysis
of variance (ANOVA) and followed by Duncan’s
multiple range test to distinguish the effect of
different treatment mean using CoSTAT statistical
software (Cohort, 2008).
3 RESULT AND DISCUSSION
3.1 The Growth of Lactobacillus
plantarum AKK-30
The results of the growth L. plantarum AKK-30 with the
addition of different FOS in MRSA were presented in
Figure 1.
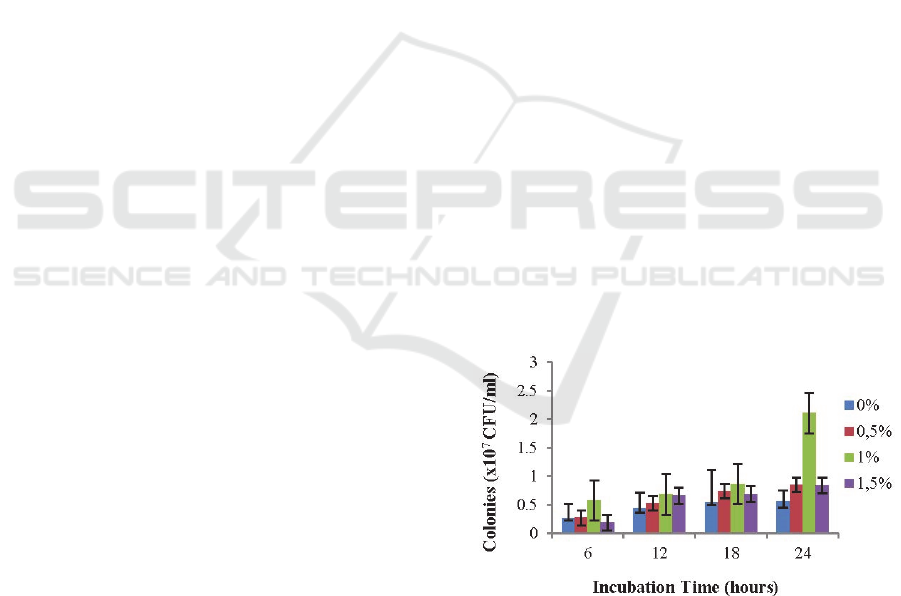
Figure 1: Growth of Lactobacillus plantarum AKK-30 at
different FOS and incubation times.
The results showed that the growth of L.
plantarum AKK-30 with FOS 1% better than other
treatments, it was seen starting from 6-hours of
incubation and the highest number of bacterial
colonies of L. plantarum AKK-30 (2.11 x 10
8
CFU/ml) occurred when 24-hours incubation. It was
16th AFC 2019 - ASEAN Food Conference
206
explained that the growth of L. plantarum AKK-30
was influenced by the addition of 1% FOS and
significantly different (P <0.05) with no addition of
FOS. The interaction between FOS and incubation
time effect on L. plantarum AKK-30 growth was
explained in Table 1.
Table 1: Interactions Between FOS Concentration and
Incubation Time (Log CFU/mL).
FOS
(%)
Time of Incubation (hours)
6 12 18 24
0
7.411 ±
0.052
a
7.639 ±
0.059
b
7.722 ±
0.129
bc
7.738 ±
0.117
bc
0.5
7.417 ±
0.140
a
7.718 ±
0.03
bc
7.886 ±
0.129
bc
7.929 ±
0.040
c
1.0
7.758 ±
0.03
bc
7.834 ±
0.009
bc
7.926 ±
0.129
c
8.261 ±
0.274
e
1.5
7.225 ±
0.249
a
7.815 ±
0.068
bc
8.039 ±
0.129
d
7.736 ±
0.223
bc
a,b,c,d
: Means in the same column and row differ significantly
(P<0.05).
The results showed that at the 24-hour incubation
of L. plantarum AKK-30 with the addition of FOS
were as follows FOS 0% (7.738 ± 0.117), FOS 0.5%
(7.929 ± 0.040), FOS 1% (8.226 ± 0.274) and FOS
1.5% (7.736 ± 0,223). At 24-hour incubation, growth
of L. plantarum AKK-30 adding 1% FOS was
significantly higher (P<0.05) (8.226 ± 0.274) than
other treatments.
3.2 Antibacterial Activity
The antibacterial in Lactobacillus is obtained from its
metabolite compounds (Setiarto et al., 2017) called
bacteriocin (Rawal et al., 2013).
Table 2: The Diameter of Inhibition Zones of L. plantarum
AKK-30 with FOS 1%.
Metabolic
Extract
The Diameter of Inhibition (mm)
S.
aureus
P.
aeru
g
ino
s
a
S.
p
ullorum
E. coli
L.
plantarum
AK
K
-30
10.8
b
8.783
a
9.083
ab
9.9
ab
a,b
; Means in the same column differ significantly (P<0.05).
In Table 2, L. plantarum AKK-30 with FOS 1%
demonstrated antibacterial ability as evidenced by the
inhibition zone in the growth of pathogenic bacteria,
Staphylococcus aureus FNCC 6049, Pseudomonas
aeruginosa FNCC 0063, Salmonella pullorum ATCC
13036 and Escherichia coli FNCC 0194. The
antibacterial activity of L. plantarum AKK-30 against
Staphylococcus aureus was significantly higher
(P<0.05) of 10.8 ± 3.59 compared to other pathogenic
bacteria. In Table 2, it can be said that the bacteriocin
in L. plantarum AKK-30 has inhibited Gram-positive
or Gram-negative bacteria. Bacteriocin has a broad
spectrum and could inhibit the growth of pathogenic
bacteria (Sifour et al., 2012; Arief et al., 2013;
Khikmah, 2015; Sulistiani, 2017).
The bacteriocin was an extracellular protein that
has antimicrobial activity (Sari et al., 2018).
Mechanism of inhibition of microbial growth by
bacteriocin is the cell wall damage and have causing
lysis (Pranckute
et al., 2016), the cell's metabolic
system was disrupted by inhibiting the activity of
intracellular enzymes (Pelczar and Chan, 1998) and
disruption of cytoplasmic membrane permeability
(Hasan and Wikandari, 2018).
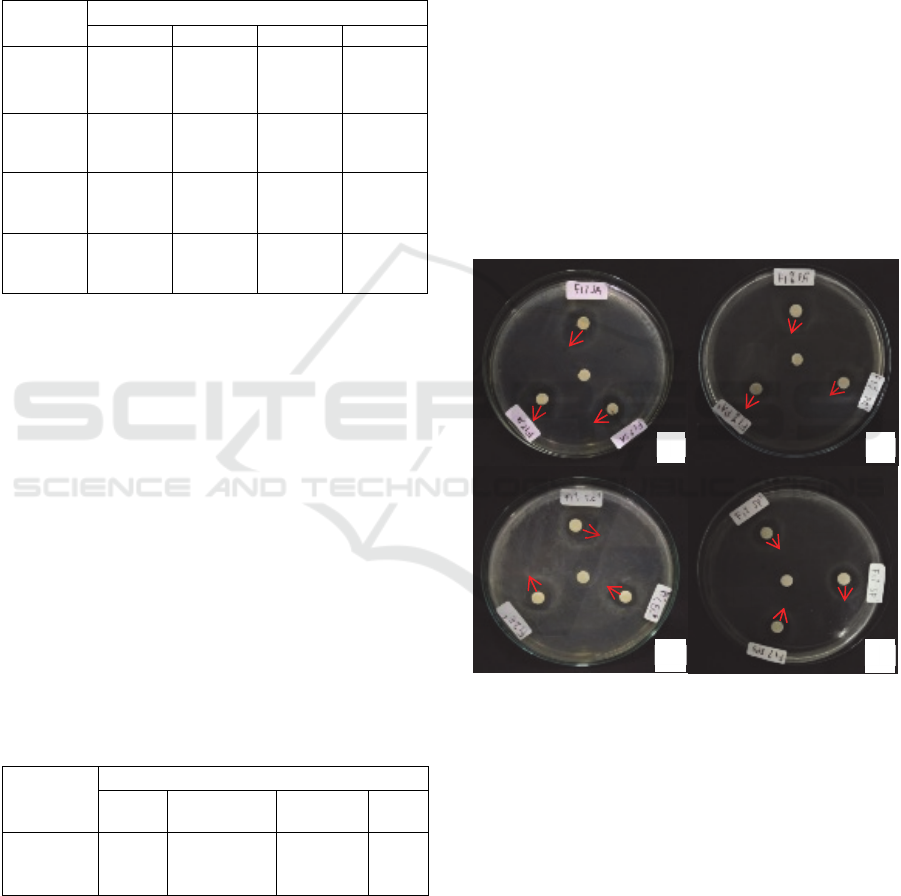
Figure 2: Inhibitory Activity Metabolic Extracts of L.
plantarum AKK-30 (mm) (a) Staphylococcus aureus (b)
Pseudomonas aeruginosa (c) Escherichia coli and (d)
Salmonella pullorum.
In Figure 2. it was shown that all pathogenic
bacterial growth was inhibited by L. plantarum AKK-
30 metabolite, the widest inhibitory zone was
Staphylococcus aureus and the lowest was
Pseudomonas aeruginosa. Inhibition zones were
influenced by bacteriocin concentrations (Julendra et
al., 2018), bacteriocin activity (Forte et al., 2016),
types of lactic acid bacteria (Kasi et al., 2017) and
different bacterial lipid layers (Jawetz et al., 2005),
(Radji, 2011). The active substance in bacteriocin
from L. plantarum was plantaricin (Gonzalez et al.,
a
b
dc
14
8.1
9.2
10.5
11.2
9.35
9.95
9.25
7.7
7.9
10.1
8.25
Enhancing Anti-pathogenic Bacteria Activity of Lactobacillus Plantarum AKK-30 Cultured on the Medium Containing
Fructose-Oligosaccharides
207

1996), antibacterial (Lim et al., 2007) that could lysis
cell membranes of pathogenic bacteria (Lu et al.,
2017).
The difference in width of the inhibition zone can
be caused by the bacterial lipid layer (Radji, 2011).
Gram-negative bacteria have thin peptidoglycan but
there are three polymers outside of peptidoglycan
namely lipoprotein, outer membrane, and
lipopolysaccharide. Permeable outer membranes are
resistant to low molecular weight substances and
hydrophilic solutes but are relatively quickly
penetrated by high molecular weight substances such
as bacteriocin (Jawetz et al., 2005). The mechanism
of bacteriocin is to damage the cell wall causing lysis
(Hasan and Wikandari, 2018), and inhibit cell wall
growth, change the permeability of cytoplasmic
membranes, denaturation of cell proteins, and
damage the metabolic system (Pelczar and Chan,
1998).
The action of plantaricin, in inhibition of
Staphylococcus aureus is by blocking the
permeability of the cytoplasmic membrane and
inducing the release of Adenosine Triphosphate
(ATP), chloroplast factor (CF), and glutamate
(Gonzalez et al., 1996). Plantaricin can disrupt cell
membranes of Gram-negative bacteria such as
Escherichia coli and Pseudomonas aeruginosa (Lu et
al., 2017), and causes the release of intracellular
components of enzymes and ions (Lim and Im.,
2007).
4 CONCLUSIONS
The highest total plate count of L. plantarum AKK-
30 was found at the medium containing 1% FOS with
24-hour incubation. The highest inhibition of L.
plantarum AKK-30 was observed against
Staphylococcus aureus (10.8 mm), followed by
Escherichia coli (9.9 mm), Salmonella pullorum
(9.083 mm), and Pseudomonas aeruginosa (8.783
mm).
ACKNOWLEDGMENTS
This research was financially supported by the Insinas
research program from the Indonesian Ministry of
Research, Technology and Higher Education. The
authors addressed to thank to drh. Ade Ema Suryani,
M.Sc., Mrs. Lusty Istiqomah, M. Biotech., Mrs.
Rumini., Mr. Nurhadi., Fitri Nurhayati., Wahyu Dwi
Saputra, S.Si., Awwaluz Zahroh Mahya A, S.Si., and
Isna Fitriana, S.Si for technical assistance and
supporting during the experiment.
REFERENCES
Arief, I., Jakaria, T., Suryati, Z., Wulandari. & Andreas, E.,
2013. Isolation and Characterization of Plantaricin
Produced by Lactobacillus plantarum Strains (IIA-
1A5, IIA-1B1, IIA2B2). Media Peternakan, 36(2),
pp.91-100.
Bonev, B., James H, & Judacael P., 2008. Principles of
Assessing Bacterial Susceptibility to Antibiotics Using
the Agar Diffusion Method. J. Antimicrob.
Chemotheraphy, (61), pp.1295-1301.
Cohort., 2008 CoSTAT Version 6.400 Cohort Software 798
(Moneterey).
Damayanti, E., Indriati, R., Sembiring, L., Julendra, H and
Sakti, A. A., 2014. Antifungal Activities of Lactic Acid
Bacteria Against Aspergillus flavus, A. parasiticus and
Penicillium citrinum as Mycotoxin Producing Fungi.
Proceedings of the 16th AAAP Animal Science
Congress, (II), pp.1742- 1745.
Damayanti, E., Julendra, H., Sofyan, A. & Hayati, S.N.,
2014. Bile Salt and Acid Tolerant of Lactic Acid
Bacteria Isolated from Proventriculus of Broiler
Chicken. Media Peternakan, 37(2), pp.80-86.
Forte, C., Acuti, G., Mamuali, E., Projetti, P. C., Pavone,
S., Marinucci, M. T., Moscati, L., Onofri, A.,
Lorenzetti, C and Franciosini. M. P., 2016. Effects of
two different probiotics on microflora, morphology,
and morphometry of gut in organic laying hens. Poultry
Science, 95, pp.2528-2535.
Gibson. G. R., Robert, H., Mary, E.S., Susan, L.P., Raylene,
A.R., Seppo, J.S., Karen, S., Catherine, S., Kelly, S.S.,
Patrice, D.C., Kristin, V and Gregor, R., 2017. The
International Scientific Association for Probiotics and
Prebiotics (ISAPP) Consensus Statement on The
Definition and Scope of Prebiotics. Gastroenterology &
Hepatology, (14), pp.491-502.
Gonzalez, B., Glaasker, E., Kunji, E.R.S., Driessen, A.J.M.,
Rez, J.E.S. and Konings, W.N., 1996. Bactericidal
Mode of Action of Plantaricin C. Applied and
Environmental Microbiology, 62(8), pp.2701–2709.
Hasan, A. & Wikandari, P.R., 2018. Penentuan Waktu
Produksi Optimum Bakteriosin Asal Lactobacillus
plantarum B1765 Berdasarkan Aktivitas
Penghambatannya Terhadap Staphylococcus aureus.
Journal of Chemistry, 8(1), pp.15-20.
Istiqomah, L., Damayanti, E., Julendra, H., Suryani, A. E.,
Sakti, A.A. and Anggraeni, A.S., 2017. Effect of
Methionine and Lactic Acid Bacteria as Aflatoxin
Binder on Broiler Performance. AIP Conference
Proceedings, (I), pp.2017- 2022.
Jawetz, E., Melnick, J.L. and Adelberg, E.A., 2005.
Mikrobiologi Kedokteran. Edisi XXII. Salemba
Medika: Jakarta.
Julendra, H., Suryani, A.E., Istiqomah, L., Damayanti, E.,
Anwar, M. and Fitriani, N., 2017. Isolation of Lactic
16th AFC 2019 - ASEAN Food Conference
208

Acid Bacteria with Cholesterol-Lowering Activity from
Digestive Tracts of Indonesian Native Chickens. Media
Peternakan, 40(1), pp.35-41.
Julendra, H., Sofyan, A., Abinawanto and Yasman., 2018.
Improving Antibacterial Activity and Viability of
Lactobacillus plantarum AKK-30 as Feed Additive by
Addition of Different Oligosaccharides. 2nd
International Conference on Natural Products and
Bioresource Sciences. IOP Conf. Series: Earth and
Environmental Science. (251) 012050:1-7. IOP
Publishing. doi:10.1088/1755-1315/251/1/012051.
Kabir, S.M.L., 2009. The Role of Probiotics in the Poultry
Industry. Int. J. Mol. Sci, (10), pp.3531-3546.
Kasi, M., Simsek, H., Ahlschlager, S., Ritterman, K.,
Hausauer, J., Hoff, J. and Khan, E., 2017. Impact of
Operations and Cleaning on Membrane Fouling at a
Wastewater Reclamation Facility. J. Environ. Manag,
(193), pp.326–333.
Khikmah, N., 2015. Uji Antibakteri Susu Fermentasi
Komersial pada Bakteri Patogen. Jurnal Penelitian
Saintek, 20 (1), pp.45-53
Lim, S.M. and Im, D.S., 2007. Bactericidal Effect of
Bacteriocin of Lactobacillus plantarum K11 Isolated
from Dongchimi on Escherichia coli O157. Journal
Food Hygiene and Safety, 22(3), pp.151-158.
Lin, T.H. and Tzu-M, P., 2017. Characterization of an
Antimicrobial Substance Produced by Lactobacillus
plantarum NTU 102, (xx), pp.1-9.
Lu, X., Lin, Y., Jie, Y., Sun, M., Zhang, B., Bai, F., Zhao,
H. and Li, J., 2017. Purification, Characterization, and
Action Mechanism of Plantaricin DL3, a novel
Bacteriocin Against Pseudomonas aeruginosa
Produced by Lactobacillus plantarum DL3 from
Chinese Suan‑Tsai. Eur Food Res Technol, 244(2)
pp.323-331.
Ogunbanwo, S.T., Sanni, A.I. and Onilude, A.A., 2003.
Influence of Cultural Conditions on the Production of
Bacteriocins by Lactobacillus brevis OG1. African
Journal of Biotechnology, 2(7), pp.179-184.
Palaniyandi, S.A., Karthiyaini Damodharan, K., Joo-Won
Suh. and Yang, S.H., 2019. Probiotic Characterization
of Cholesterol-Lowering Lactobacillus fermentum
MJM60397. Probiotics and Antimicrobial Proteins.
First Online: 20 August 2019.
https://doi.org/10.1007/s12602-019-09585-y
Pelczar, M.J. and Chan, E.C.S., 1998. Dasar-Dasar
Mikrobiologi Jilid II. UI Press: Jakarta.
Pranckute. R., Arnoldas, K., Nomeda, K. and Donaldas, J.,
2016. Combining Prebiotics with Probiotic Bacteria
Can Enhance Bacterial Growth and Secretion of
Bacteriocins. International Journal of Biological
Macromolecules, (89), pp.669–676.
Radji, M., 2011. Mikrobiologi. Buku Kedokteran. ECG:
Jakarta.
Rawal, K., Nirav, B., Gopal, R., Raol, B.V. and Patel, J.D.,
2013. Bacteriocin: Production and Optimization by
Lactobacillus Species. Journal of Microbiology and
Biotechnology Research
, 3(6), pp.64-76.
Sari, N.P., Sari, R. and Untari, E.K., 2018. Antibacterial
Activity Test of Bacteriocin from Lactobacillus brevis,
Lactobacillus casei and Lactobacillus plantarum
Against Gram Positive Pathogenic Bacteria. Journal of
Tropical Biodiversity and Biotechnology, (3), pp.85-91.
Setiarto, R.H., Widhyastuti, N. and Rikmawati, N.A., 2017.
Optimasi Konsentrasi Fruktooligosakarida untuk
Meningkatkan Pertumbuhan Bakteri Asam Laktat
Starter Yoghurt. Jurnal Veteriner, 18(3), pp.428-440.
Sifour, M., Tayeb, I., Haddar, H.O., Namous, H. and
Aissaoi., 2012. Production and Caracterizatition of
Bacteriocin of Lactobacillus plantarum F12 with
Inhibitory Activity Againts Listeria monocytogenes.
TOJSAT, 2(1), pp.55-61.
Sophian, A., Julendra, H., Sofyan, A., Karimy, M.F. and
Abinawanto., 2018. Adhesion Activity Assay of
Lactobacillus plantarum AKK30 Combined with
Oligosaccharides. 2nd International Conference on
Natural Products and Bioresource Sciences. IOP Conf.
Series: Earth and Environmental Science, (2), pp.1-5.
IOP Publishing. doi:10.1088/1755-1315/251/1/012050.
Sulistiani., 2017. Senyawa Antibakteri yang Diproduksi
oleh Lactobacillus plantarum dan Aplikasinya untuk
Pengawetan Bahan Ikan. Jurnal Biologi Indonesia,
13(2), pp.233-240.
Wei, M., Shaoyang, W., Pan, G., Xiaoyu, O., Shuxung, L.,
Yiqin, L., Boling, Z., Baoqing, Z., 2018. Comparison of
Physicochemical Indexes, Amino Acids, Phenolic
Compounds and Volatile Compounds in Bog Bilberry
Juice Fermented by Lactobacillus plantarum Under
Different pH Conditions. J Food Sci Technol, 55(6),
pp.2240–2250.
Yang, J., Qian, K., Wu, D., Zhang, W., Wu, Y. and Xu, Y.,
2017. Effects of Different Proportions of Two Bacillus
sp. on the Growth Performance, Small Intestinal
Morphology, Caecal Microbiota and Plasma
Biochemical Profile of Chinese Huainan Partridge
Shank Chickens. Journal of Integrative Agriculture,
16(6), pp.1383–1392.
Yuliana, R., Kusdiyanti, E. and Izzati, M., 2014. Potensi
Tepung Umbi Dahlia dan Ekstrak Inulin Dahlia sebagai
Sumber Karbon dalam Produksi Fruktooligosakarida
(FOS) oleh Khamir Kluyveromyces marxianus DUCC-
Y-003. Berkala Ilmiah Biologi, 16(1), pp.39-49.
Enhancing Anti-pathogenic Bacteria Activity of Lactobacillus Plantarum AKK-30 Cultured on the Medium Containing
Fructose-Oligosaccharides
209
