
Characteristic of Garcinia Mangostana’s Pericarp Prepared by
Mechanical Milling
Dwi Wahyu Nugroho
1,3
, Dyah Ayu Daratika
1
, Elly Kristiyanti Agustin
2
, Muthia Kamila
1
,
Mohammad Aulia Rifada
1
, Lusiana Togatorop
4
, Wahyu Bambang Widayatno
5
, Syahrizal Maulana
6
,
Damai Ria Setyawati
7
, Etik Mardliyati
7
and Nurul Taufiqu Rochman
5
1
Center of Research and Development Product, Nano Center Indonesia, Puspiptek, South Tangerang, Banten, Indonesia
2
Center for Plant Conservation Botanic Gardens-LIPI Jakarta, Bogor, Indonesia
3
Department of Industrial Engineering, Nahdlatul Ulama Indonesia University, Jakarta, Indonesia
4
Department of Chemical Engineering, Pamulang University, South Tangerang, Banten, Indonesia
5
Center for Physics, Indonesian Institute of Sciences, Puspiptek, South Tangerang, Banten, Indonesia
6
Center for Innovation, Indonesian Institute of Sciences, Cibinong, Indonesia
7
Center for Pharmaceutical and Medical Technology, Agency for the Assessment and Application of Technology,
Puspiptek, South Tangerang, Banten, Indonesia
lusianatoga28@gmail.com, syahrizal.maulan@ymail.com, etik@nano.or.id, nurul@nano.or.id
Keywords: Nano Mangosteen Pericarp, Solubility, Potential Zeta, Phenolic Content, Functional Group.
Abstract: Garcinia mangostana, commonly known as mangosteen is a tropical fruit that grows in Asian region. Nano
Mangosteen’s Pericarp have successfully been made by ball milling method with variations in milling time
(30, 90, 150, 210 minutes), and non milling as a comparison. Mangosteen pericarp was dried in an oven at
70 ˚C for 12 hours, then continued by grinding and sieving using mesh 80, then milled. The effect of
variations in milling time on the functional groups of nano mangosteen pericarp, also the correlation on total
phenolic content and antioxidant activity in previous research were investigated. The morphology of the
mangosteen pericarp shows that the grain size of mangosteen pericarp is getting finer along with increasing
milling time. It can provide a clear reason for explaining the increasing solubility value of the samples. The
zeta potential data shows that after being milled the mangosteen pericarp becomes unstable, thus it is easy to
agglomerate. It was obtained that the total phenolic content and the antioxidant activity increased followed
by longer milling times. The FTIR analysis indicated that the enhancement in total phenolic content is not
due to transformation in functional groups of phenolic compounds.
1 INTRODUCTION
Free radicals are atoms or molecules that have
unpaired electron. The unpaired electron cause free
radicals to be very reactive then take electron from
other compounds such as proteins, lipids,
carbohydrates, and DNA to neutralize themselves
(Liochev, 2013). The negative effects of free
radicals on the body can be prevented by compounds
called antioxidants. Antioxidants have the ability to
give electron, bind, and end free radical chain
reactions (Halliwell, 2012). Antioxidants are
electron-giving compounds (donor electrons), which
are able to inactive the development of oxidation
reactions by preventing radical formation.
Antioxidants are compound that can slow or
prevent the oxidation process. This compound can
significantly slow down or inhibit the oxidation of
substances that are easily oxidized even in low
concentrations. Based on (Buck, 1991) sources of
antioxidants are divided into two, namely natural
antioxidants and synthetic antioxidants. Synthetic
antioxidants are antioxidants obtained from the
synthesis of chemical reactions and are produced for
commercial purposes. Examples of synthetic
antioxidants include Butyl Hydroxy Anisol (BHA),
Butyl Hydroxy Toluene (BHT), Propyl galate, Tert-
Butyl Hydroxy Quinon (TBHQ), Tocopherol and
others. Natural antioxidants can generally be
obtained from phenolic compounds or plant
polyphenols which can be in the form of alkaloids,
322
Nugroho, D., Daratika, D., Agustin, E., Kamila, M., Rifada, M., Togatorop, L., Widayatno, W., Maulana, S., Setyawati, D., Mardliyati, E. and Rochman, N.
Characteristic of Garcinia Mangostana’s Pericarp Prepared by Mechanical Milling.
DOI: 10.5220/0009991700002964
In Proceedings of the 16th ASEAN Food Conference (16th AFC 2019) - Outlook and Oppor tunities of Food Technology and Culinary for Tourism Industry, pages 322-329
ISBN: 978-989-758-467-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

flavonoids, saponins, quinones, tannins, sterids/
triterpenoids (Gordon, 1994). Natural antioxidants
are generally more desirable than synthetic
antioxidants. Many natural antioxidants are plants,
vegetables and fruits (Winarsi, 2007). Among fruits
that contain a lot of antioxidant compounds are
mangosteen fruit, especially on the mangosteen
pericarp. Based on phytochemical research,
mangosteen pericarp contains phenolic antioxidant
compounds. These phenolic compounds are
xanthones, anthocyanins, tannins, epikatekin and
other phenolic acid compounds (Zadernowski et al.,
2009).
One of the problems that occur in the utilization
of mangosteen pericarp waste, that is the active
compound, the source of natural antioxidants tends
to be less practical, has instability to the color, low
solubility, and bioavailability that still tends to be
low. Currently, nanotechnology is developed in food
and drug products that can accelerate the rate of
release of compounds of active compound, increase
solubility and increase absorption in the body.
(Ningsih et al., 2017).
In the previous study, it was observed the effect
the time of milling of mangosteen pericarp powder
with a variation of time 0, 30, 90, 150 and 210
minutes using ballmill on its effect on antioxidant
activity and total phenolic. It was reported that the
particle size of mangosteen pericarp powder affects
levels of total phenol and antioxidant activity seen
from IC
50
values. Mangosteen pericarp with the
smallest particle size has the largest total phenol
content and the smallest IC
50
. The largest total
phenol content indicates the content of polyphenol
compounds in samples with a large amount of
content. IC
50
values describe the total antioxidants
needed to capture free radicals as much as 50%. The
results of Daratika et al., 2018 reported that there
was an increase in total phenol content along with an
increase in milling time.
According to (Garcia's, 1999) study, the relative
ability of flavonoids from olive leaves to absorb
radical cations using the ABTS + method is
influenced by the presence of different functional
groups, especially in the number and position of free
hydroxyl groups in their structure. This is possible
because of changes in functional groups along with
changes in particle size. FTIR testing is carried out
to analyze whether there is a change in functional
groups in the organic compounds of mangosteen
pericarp powder. This research are in accordance
with the result that there was a positive correlation
between flavanoid content and particle size in rice
accession (Shen, 2009). There is also a study in
(Luthria, 2008), that the value of total phenol
content extracted from parsley leaves is obtained
when the particle size of the extraction results is
getting smaller.
In addition, it was reported that agglomeration in
the morphology of mangosteen pericarp powder
samples. According (Darusman, 2014) study, there
was clumping or agglomeration of pure GMP
(Glimepirid) drug particles which caused GMP to be
hydrophobic so it was difficult to dissolve in water.
Therefore, in this paper we will discuss potential
zeta in each sample to find out its relation to
solubility. The greater the potential zeta value, the
better the stability of the solution will be to reject
aggregation (Sari et al., 2013). The purpose of this
study was to characterize mangosteen pericarp
samples including zeta analysis of potential
mangosteen peel nano powder which might be
influenced by the process when milling and identify
the increase in the value of total phenol content with
the hypothesis influenced by changes in functional
groups.
2 MATERIAL AND METHODS
The raw material for grade A mangosteen is taken
from Purwakarta, West Java. The material used
consists of water, potassium bromide (KBr), Ethyl
Alcohol. Whereas the tools used consist High
Energy Milling Machine- Ellipse 3 Dimension
(HEM-E3D), Bruker Tensor 37 FTIR Spectroscopy,
Delsa Nano C-Particle Size Analyzer, and FEI
Quanta 650-Scanning Electron Microscopy.
2.1 Preparation of Mangosteen
Pericarp
Preparation of mangosteen pericarp powder samples
was carried out in according with previous studies.
In Daratika’s et al,. research 2018, the raw material
for mangosteen fruit is obtained from Purwakarta,
Indonesia plantations. The pericarp of the
mangosteen is boiled at a temperature of 200-250 °C
for 15 minutes. Then the soaking process is done by
using cold water to accelerate the cooling of the
sample. Furthermore, the mangosteen pericarp
slashed into small sizes around 50 mm in size. Then
the mangosteen pericarp is dried at 65 °C. According
to Afifah and Niwat, 2015 drying samples at
temperatures below 75 °C can protect the damage of
polyphenols. The mangosteen pericarp is then milled
and sifted on 80 mesh. The process of removing the
mangosteen powder particles in this study is the top
Characteristic of Garcinia Mangostana’s Pericarp Prepared by Mechanical Milling
323

down method using the HEM-Ellipse 3 Dimension
machine. Variations in milling time were observed
as parameters to determine the relationship between
milling time and particle size.
2.2 Characterization of Mangosteen
Pericarp
Mangosteen pericarp powder was characterized by
particle size and potential zeta using Delsa Nano C-
Particle Size Analyzer. The sample was dissolved
with ethyl alcohol solvent and measured at
temperature 25 °C with refractive index 1.3611,
liquid viscosity 1.1015 cPoise and scattering
intensity 8238 cps. While potential zeta is analyzed
to determine the nature of nanoparticle surface loads
and distribution potential particle samples. The
sample was dissolved in a water solvent then 0.9 mL
of the sample was measured under conditions of
temperature 24.8 °C, refractive index 1.3328,
viscosity 0.8919 and dielectric constant 78.4.
SEM testing is also done to find out information
about surface topography, composition, and other
characteristics such as electrical conductivity. Tests
are carried out using Scanning Electron Microscopy-
FEI Quanta 650. To determine the solubility, the
method of Al-Kahtani and Hassan (1990) was
applied. By10 grams of sample and 100 mL of
distilled water was put into a beaker glass. Rotate
using magnetic bar at a speed of 200 rpm at room
temperature 25 ° C (Jittanit, 2011).
2.3 Fourier Transform Infrared
Spectroscopy- Bruker Tensor 37
Making pellets is done by entering 200 mg KBr into
the mortar and mixing with 2 mg of sample. Mix
until homogeneous and done quickly. The
homogeneous mixture then made pellet with a mini
hand press tool and pay attention to the process. The
resulting pellets are stored in a dry place.
3 RESULT AND DISCUSSION
The morphology of the mangosteen pericarp
samples measured using Scanning Electron
Microscopy (SEM) can be seen in Fig. 1 and 2
indicating that the grain size getting finer along with
increasing holding time of milling. Daratika et al.,
(2018) reported that non milling mangosteen
pericarp (escaped mesh 80) with a particle size of
438 nm had grain sizes around 82,142 nm, milled for
90 minutes with a particle size of 257 nm had grain
sizes around 21,613 nm, and milled for 210 minutes
with a particle size of 205 nm had grain sizes around
1,855 nm.
Naturally, the process of milling on a material in
order to produce nanoparticles has two
consequences, namely fracture and agglomeration.
The first possible condition that occur when milling
is fracture of the particle as a result of a sufficiently
high stress field inside the particle which buildup
during the impact between the media (Knieke C.,
2012). The second possible condition is
agglomeration, when the particle size are below 1
µm, the particles tend to agglomerate because of
Browns’s motion increased and smaller interparticle
distances. Both of them enhancing the collision rate
of the particles (Knieke C., 2012).
In mangosteen pericarp samples before milling
(Fig. 1) and mangosteen pericarp samples which
were milled 90 minutes and 210 minutes (Fig.2)
there was a difference that the morphology of the
mangosteen pericarp samples before milling did not
occur between small particles and larger particles.
Whereas in the mangosteen pericarp sample after
milling there is a clumping or collision between
smaller particles and larger particles. This indicates
that there is agglomeration. Particle agglomeration
can be interpreted as forming a collection of
particles in solution and is one of the mechanisms
that causes destabilization of colloids. There are
several things that cause agglomeration, including
the smaller particle size.
In DLVO theory, (Derjaguin and Landan, 1941),
the agglomeration and stability of particle dispersion
are determined by the sum of the attractive and
repulsive force between individual particle. The
attraction between particle is due to the van der
Waals force. The interaction of the electrical double
layer surrounding each particles is called
electrostatic repulsive force. This theory indicate
that agglomeration is related to the potential zeta
value. The potential zeta value is used to
characterize the characteristic of particle surface
charge associated with nanoparticle electostatic
interactions (Couvreur et al., 2002). Electrostatic
interactions are resistive repulsive forces between
particles that affect stability in the suspension so that
they can prevent particle aggregation.
16th AFC 2019 - ASEAN Food Conference
324

(a) (b) (c)
Figure 1: The morphologies of mangosteen pericarp (non milling) mag 250x and 500x (a); milling 90 minutes (b); milling
210 minutes (mag. 250x and 1500x) (c).
Reflect the electrical potential of particle and is
influenced by the composition of the particle and the
medium in which it is dispersed (Singh et al., 2009).
The greater the electrostatic ability of a charge, the
more stable it is rejecting aggregation. Conversely
the smaller the electrostatic ability, the weaker it is
to resist aggregation.
Table 1 shows the solubility time of the
mangosteen pericarp milled in a certain time
variation (30, 90, 150, and 210 minutes) and non
milling as a comparison. Along with the addition of
milling time in the mangosteen pericarp samples
dissolved in certain water with a temperature of 25
ºC. It can be understood because the longer milling
time of mangosteen pericarp samples has a smaller
grain size, also followed by the smaller particle size.
Solubility of a substance will increase along with the
reduces particle size of substance.
Table 1: Solubility time and zeta potential of mangosteen
pericarp samples.
Sample
Measurement Method
Solubility Time
(s)
Zeta Potential
(mV)
Before milling 312.67±9.50 118.96
After milling
30 minutes 196.33±2.08 -18.03
90 minutes 58.03±3.50 -16.56
150 minutes 44.73±1.95 -20.3
210 minutes 33.4±2.19 -14.08
30 minutes 196.33±2.08 -18.03
Zeta potential is a parameter of electrical charge
between particles in colloids. The magnitude of zeta
potential provides information about stabilization of
the samples Patel et al., (2011) informing about the
guidelines for classifying nanoparticles dispersions
with zeta potential value (±) 0 – (±) 10 mV are very
unstable, (±) 10 – (±) 20 mV are relatively stable,
(±) 20 – (±) 30 mV are quite stable, and > (±) 30 mV
are very stable. The higher zeta potential value, the
more it will prevent flocculation. Zeta potential of
mangosteen pericarp samples can be observed in
Table 2. Non-milling mangosteen pericarp have a
very high zeta potential value, which shows the
repulsive forces of the particle are stronger, then the
samples have a high stability to resist aggregation.
While, for mangosteen pericarp samples that are
milled at a certain time variation (30, 90, 150, and
210 minutes) have a potential zeta value of less than
30 mV. The low potential zeta represents the
attractive forces between the dispersion particles
exceeding the repulsive forces.This causes these
particles to be easy to agglomerate, further causing
flocculation.
In the previous work, the correlation between
total phenolic content and antioxidant activity
against particle size of Garcinia mangostana’s
Pericarp has obtained that non milling sample with a
particle size had total phenolic content around 14.52
x 104 µg GAE/g samples, while the sample which is
milled for 210 minutes having the highest total
phenolic content (17.44 x 104 µg GAE/g samples)
accompanied by the lowest IC
50
value (254.84
µg/ml) that shows strong antioxidant activity
(Daratika et al., 2018). It can be observed that by
applying milling to reduce the particle size of the
sample produces a different total phenolic content.
In the initial hypothesis, it is caused by the
transformation of functional groups from phenolic
compounds. A functional group is defined as a group
of atoms joined in a specific manner, that gives the
chemical properties of the organic compound and
are the centers for chemical reactivity.
Characteristic of Garcinia Mangostana’s Pericarp Prepared by Mechanical Milling
325
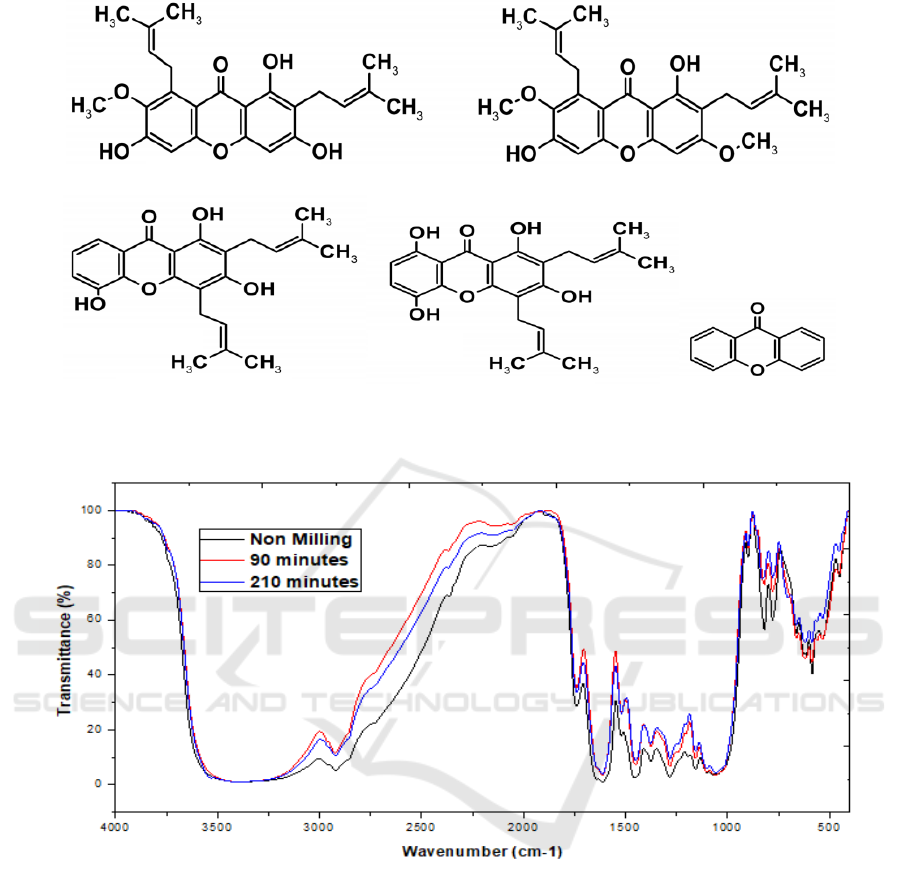
Figure 2: Chemical structure of xanthones and their derivatives: α-mangostin (a); β-mangostin (b); gartanin (c); 8-
desoxygartanin (d); xanthone (e) (Walker E. B., 2007).
Figure 3: FTIR spectra of mangosteen pericarp.
In this study, Fourier-transform infrared
spectroscopy (FTIR) measurement accomplished to
obtain the functional group of mangosteen pericarp.
The FTIR spectra were collected in transmission
mode and covering the spectral range from 400 to
4000 cm-1 using Bruker Tensor 37 FTIR
spectrophotometer. The band intensities in different
regions of the spectrum for non-milling mangosteen
pericarp as a control and milled samples (90 and 210
minutes) were analyzed and are shown in Figure 3.
Naturally, mangosteen fruit is a rich source of
phenolic compound such as xanthones,
proanthocyanidins, anthocyanins, and phenolic acids
(Naczk et al., 2011). Mangosteen pericarp contains
of α-, β-, γ- mangostin, 8-deoxygartanin,
mangostinone a and b, gartanin, garcinone b and
mangostanol (Muchtaridi et al., 2016), (Jung et al.,
2006) reported that mangosteen pericarp conceived
xanthones as the major phenolic, with the chemical
structures can be observed in Figure 2.
The analysis using FTIR spectrophotometer
shows the presence of C-H, C=C aromatic, C-O eter,
O-H fenol. This functional group is appropriate with
functional groups in compounds xanthone, and their
derivatives with the structure as in Figure 2.
Xanthones is a cyclic polyphenol ketone compound
with the chemical formula C
13
H
8
O
2
, basic structure
xanthones consists of three benzene with one
benzene in the middle which is a ketone.
(a)
(b)
(e)
(d)
(c)
16th AFC 2019 - ASEAN Food Conference
326

Table 2: The functional groups of mangosteen pericarp.
Wavenumber (cm-1) Bond
Functional
Group
0' 90' 210' 0' 90' 210'
780, 45 780,61 780,11 C-H C-H C-H
Aromatic ring 817,58 816.76 830.79 C-H C-H C-H
899,78 898.54 898.51 C-H C-H C-H
1073,82 1053.20 1053.87 C-O C-O C-O
Eter
1156,42 1156.50 1156.62 C-O C-O C-O
1281,6 1282.29 1282.03 C-O C-O C-O
1375,12 1374.97 1376.73 C-H C-H C-H
Alkana
1453,33 1451.53 1450.67 C-H C-H C-H
1505 1518.83 1518.88 C=C C=C C=C Aromatik ring
1610,97 1612.02 1612.58 C=C C=C C=C Alkena
1740.14 1738.86 1739.18 C=O C=O C=O Keton
2922.69 2925.99 2923.37 C-H C-H C-H Alkana
3396.31 3397.68 3396.73 O-H O-H O-H Phenol
Almost all derivative molecules of xanthones
have a phenolic group in consequence xanthones
often called as polyphenols. Thus, it can be seen that
these compounds are polyphenols compounds
contained in the mangosteen pericarp (Muchtaridi et
al., 2016) and have antioxidant activity. The results
of FTIR data analysis showed that the functional
groups of the samples (non milling and milled with
variations of time 90 and 210 minutes) did not
indicate a significant difference in their spectra.
Therefore, it can be seen that there is no functional
group transformation in the mangosteen pericarp
samples. It can be understood by reason because
there is no chemical treatment that causes changes in
functional groups.
(Zhou et al., 2004) have reported that micro-
milled aleurone of Switzerland red wheat extracted
with 50% acetone showed significantly higher
sovent-extractable phenolic content compared to
untreated counterpart, which each individual
phenolic compound is examined increased for
different extents. Recent work carried out (Rosa et
al., 2013) reported that wheat bran’s antioxidant
activity was linearly correlated with specific surface
area, the medium particle size, and the proportion
particles smaller than 50 µm in diameter. Particle-
size reduction processes using ball milling, nano-ball
milling, and ultra-fine grinding have been shown
increase the accessibility of phenolic compounds to
extraction solvents (Wang et al., 2014). The basic
principles of engineering nanoparticle materials is
by utilizing the influence of particle size, the effect
of surface area, and the interaction between
nanoparticles and other materials.
In the previous study, it was found that the
increase in milling time on mangosteen pericarp
sample produces higher total phenolic content
accompanied by stronger antioxidant activity
(Daratika et al., 2018). The fact obtained from the
FTIR analysis stated that the difference values of
total phenolic content that are milled with a certain
time variation is not caused by the transformation of
functional groups so that there are changes in the
type and amount of phenolic compounds which
present in the samples. However, there are physical
processes that occur when reducing particle size.
The small size of nanoparticles has a greater
comparison between surface area and volume when
compared to similar particle with the larger size.
Consequently, there will be more atoms on the
surface of the nanoparticle material that come in
direct contact with material, causing this
nanoparticle to be more reactive. For this reason, an
increase in antioxidant activity and total phenol
content is caused by the better exposition of
phenolic compounds when samples milled, thereby
improving the accessibility of hydrogen to bind free
radicals from DPPH as synthetic free radicals to
form complex antioxidants that are stable.
4 CONCLUSIONS
Mechanical treatment of milling results in finer
particles and smaller zeta potential than samples
without milling. However, the milling treatment did
not change the functional group of mangosteen
Characteristic of Garcinia Mangostana’s Pericarp Prepared by Mechanical Milling
327

pericarp. Characterization using SEM shows that the
mangosteen pericarp that is milled in a longer time
have a finer grains which causes agglomeration.
This has an effect on increasing the solubility time
along with the small particle size. This has an effect
on increasing the solubility time along with the
small particle size. Besides the smaller particle size
also affects the total phenol content and antioxidant
activity as in previous studies.
ACKNOWLEDGEMENTS
This research was partly supported by The National
Innovation System Research Incentive program, The
Ministry of Research, Technology, & Higher
Education, Indonesia, 2018.
REFERENCES
Afifah, R.A., Niwat, C., 2015. The Use of Mangosteen
Pericarp (Garcinia mangostana L.) Extract to Fortify
the Green Tea Drink Enchanced Antioxidant Activity.
KKU Research Journal: 20(3) : 305-313.
Al-Khatani Hassan, Hassan B.H., 1990. Spray Drying of
Roselle (Hibiscus Sabdariffa L.) Extract. Journal of
Food Science Volume 55, No. 4.
Buck DF. Antioksidant. J. Smith (eds)., 1991. Food
Additive User’s Handbook. Galsgow-UK : Blakie
Academic & Profesional.
Daratika Dyah Ayu, Kamila Muthia, Nugroho D.W,
Widayatno W.B, Maulana Syahrizal, Mardliyati Etik,
Rochman Taufiqu Nurul., 2018. The Correlation
between Total Phenolic Content and Antioxidant
Activity Against Particle Size of Garcinia
mangostana’s Pericarp. Proceedings of The 2nd
International Seminar and Workshop of Plant Industri.
Jember, Indonesia 1-2 November 2018.
Derjaguin BV, Landau LD., 1941. Theory of the stability
of strongly charged lyophobic sols and of the adhesion
of strongly charged particles in solutions of
electrolytes. Acta Physicochim URSS 14:733-762.
Garcia O.Benavente, Castillo J, Lorente .J, Ortuno .A, Rio
J.A.D., 1999. Antioxidant Activity of Phenolics
Extracted From Olea Europaea L. Leaves. Food
Chemistry,68, 457-462.
https://doi.org/10.1016/S0308-8146(99)00221-6.
Gordon L. 1994. Functional Food, Food Design,
Pharmafood, Newyork : Champman and Hall.
Jittanit W, Khuenpet K, Charoenjarasrerk N, Jaijit S,
Arayapoonpong S.M., 2016. Agriculture and Natural
Resources Journal.Vol. 50, Pages 139-145.
Jung, H.A., Su, B.N., Keller, W.J., Mehta, R.G.,
Kinghorn, D., 2006. Antioxidant Xanthones from
pericarp of Garcinia mangostana (Mangosteen). J.
Agric. Food. Chem. 54, 2077-2082.
Knieke, Catharina., 2012 Fracture at the Nanoscale and
the Limit of Grinding. Cuviller Verlag; 20.
https://books.google.co.id/books/about/Fracture_at_th
e_Nanoscale_and_the_Limit.html.
Luthria DL., 2008. Influence of experimental conditions
on the extraction of phenolic compounds from parsley
(Petroselinum crispum) flakes using a pressurized
liquid extractor. Food Chem. 107(2):745-752.
Liochev, S.I., 2013 Reactive oxygen species and the free
radical theory of aging. Free Radical Biology and
Medicine,60,14.doi:10.1016/j.freeradbiomed.2013.02.
01.
Naczk, M., Towsend, M., Zadernowski, R., Shahidi, F.
2011. Protein-binding and antioxidant potential of
phenolics of mangosteen fruit (Garcinia mangostana).
Food Chem. 128, 292–298.
https://doi.org/10.1016/j.foodchem.2011.03.017.
Ningsih Nirmala, Yasni Sedarnawati, Yuliani Sri., 2017.
Nanoparticle of red mangosteen peel extract synthesis
and the functional characteristics of its encapsulated
product. IPB, Technology Journal and food industry
vol. 28(1):27-35.
Muchtaridi, Qosim W.A., 2016. Quantitative analysis of
A-mangostin in mangosteen (Garcinia mangostana L.)
pericarp extract from four district of West Java by
HPLC method. International Journal of Pharmacy and
Pharmaceutical Sciences Vol 8, Issue 8, 232-236.
Rosa, N. N., Barron, C., Gaiani, C., Dufour, C., & Micard,
V., 2013. Ultra-fine grinding increases the antioxidant
capacity of wheat bran. Journal of Cereal Science, 57,
84–90.
Sari, T. P., Mann, B., Sharma, R., & Kumar, R., 2013.
Process Optimization for the Production of
Nanoencapsulated Curcumin and Analysis for
Physicochemical Characteristics and Antioxidant
Mechanism. International Journal of Biotechnology
and Bioengineering Research, 4(6), 581–586.
Shen Yun, Jin Liang, Xiao Peng, Lu Yan, Bao Jinsong.,
2009. Total phenolics, flavonoids, antioxidant capacity
in rice grain and their relations to grain color, size and
weight. Zhejiang University; Journal of Cereal
Science 49, 106–111.
Couvreur P, Barratt G, Fattal E, Legrand P, Vauthier C.,
2002. Nanocapsule technology: a review. Crit Rev
Ther Drug Carrier Syst. 19:99-134.
V.R. Patel, Y.K., 2011. Agrawal. Nanosuspension: an
approach to enhance solutibility of drugs. J. Adv.
Pharm. Technol. Res. 2. 81-87.
Wang Tao, He Fuli, Chen Guibing., 2014. Improving
bioaccessibility and bioavailability of phenolic
compounds in cereal grains through processing
technologies: A concise review. Journal Of Functional
Foods 7 101–111.
http://dx.doi.org/10.1016/j.jff.2014.01.033.
Winarsi Hery., 2007. Natural Antioxidants and Free
Radicals. Yogyakarta: Kanisius. Page. 189-90.
Zadernowski, R., Czaplicki, S. and Nacz, M., 2009.
Phenolic acid profiles of mangosteen fruits (Garcinia
mangostana). Food Chemistry 112: 685-689.
16th AFC 2019 - ASEAN Food Conference
328

Zhou Kequan, Laux J.J, Yu Liangli., 2004. Comparison of
Swiss Red Wheat Grain and Fractions for Their
Antioxidant Properties. Journal of Agricultural and
Food Chemistry, Vol. 52, No. 5. Doi:
10.1021/jf030640w
Characteristic of Garcinia Mangostana’s Pericarp Prepared by Mechanical Milling
329
