
Dermoscopy as a Diagnostic and Evaluation Tools in Childhood
Alopecia Totalis
Nani Kumala Dewi
1*
, Nelva K. Jusuf
2
1
Department of Dermatology and Venereology, Faculty of Medicine Universitas Sumatera Utara, Jln. Dr. Mansur No. 66,
Medan, Sumatera Utara
2
Department of Dermatology and Venereology, Faculty of Medicine Universitas Sumatera Utara, Jln. Dr. Mansur No. 66,
Medan, Sumatera Utara
Keywords: Alopecia totalis, dermoscopy, alopecia areata, children
Abstract: Alopecia totalis is a manifestation of alopecia areata that is characterized by total scalp hair loss and have a
higher risk for poor prognosis and treatment failure. Early-onset, nail involvement, history of atopy, and
long duration of disease are associated with poor prognosis. Total scalp hair loss in alopecia totalis might
have a sudden onset or following partial alopecia. In the case of diffuse scalp hair loss, the clinical finding
might be similar to telogen effluvium and trichotillomania. Hence, a biopsy is required to establish the
proper diagnosis. However, this invasive diagnostic method is not favorable for most patients, especially
children. Several studies have been done to show the reliability of dermoscopy to diagnose alopecia areata.
Some dermoscopic features, such as yellow dots, black dots, broken hairs, tapering hair (exclamation
marks), and short vellus hairs, are known as characteristic findings in this disease. Sign of hair regrowth
might be detected earlier with this technique, and even it is not yet visible with naked eyes. We are
reporting an 8-year-old girl with a history of recurrent total scalp hair loss for four years and clinical
findings of diffuse hair loss on smooth surface scalp skin, nail pitting, and transverse leukonychia. Initial
dermoscopy evaluation revealed characteristic findings of alopecia areata and regrew of short vellus hair
was detected earlier with this examination. Therefore, we would like to report the use of dermoscopy as a
diagnostic and evaluation tools in childhood alopecia totalis.
1 INTRODUCTION
Alopecia areata is an autoimmune disease with
nonscarring hair loss that affects any hair-bearing
areas (Alkhalifah et al, 2010). However, the
pathogenesis is not known, and prevalence of this
disease varied from 0.7 – 3.8% around the globe
with similar gender proportion (Wasserman et al,
2007). Most patients have the onset of the first
lesion before 20 years old and 20% of cases found in
childhood (Nanda et al, 2002).
Clinical manifestation
may vary from single patch to multiple or diffuse
hair loss. In severe cases, the disease can extend to
total scalp hair loss or known as alopecia totalis, and
even including body hair in alopecia universalis
(Alkhalifah et al, 2010). Around 5% of alopecia
cases evolve into these long forms and associated
with poor prognosis, treatment failure, and high
relapse rate (Alkhalifah et al, 2010; Jang et al,
2017). Other prognostic factors such as early-onset
at a young age, long duration, ophiasis pattern, nail
abnormalities, history of atopy, other autoimmune
diseases, and family member are also related to a
poor outcome (Alkhalifah et al, 2010; Olsen, 2011).
In general, clinical diagnosis of alopecia areata is
made based on the typical pattern of hair loss with
the presence of characteristic exclamation mark hair
(Alkhalifah et al, 2010). However, in some cases,
the clinical diagnosis may not be straightforward
where biopsy evaluation may be required to confirm
the diagnosis, but this invasive technique is not
favored by most patients, especiallychildren. In the
case of alopecia totalis with diffuse or total scalp
hair loss, dermoscopy examination might be
necessary to exclude some differential diagnosis
such as telogen effluvium and trichotillomania.
Dermoscopy is a noninvasive diagnostic method
that allows evaluation of microstructures of the
epidermis, the dermo-epidermal junction, and the
papillary dermis which are not visible to the naked
430
Dewi, N. and Jusuf, N.
Dermoscopy as a Diagnostic and Evaluation Tools in Childhood Alopecia Totalis.
DOI: 10.5220/0009990904300435
In Proceedings of the 2nd International Conference on Tropical Medicine and Infectious Disease (ICTROMI 2019), pages 430-435
ISBN: 978-989-758-469-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

eye (Mane et al, 2011). Characteristic dermoscopic
features of alopecia areata are yellow dots, black
dots, broken hairs, tapering hair (exclamation
marks), and short vellus hairs (Mane et al, 2011; Inui
et al, 2008). In the remission phase, the white
perihilar sign might be found, and short vellus hair
becomes more prominent (Jha et al, 2017).
However, the proportion of these diagnostic features
might vary according to the literature. Therefore, we
would like to report the use of dermoscopy as a
diagnostic and evaluation tool in the case of
childhood alopecia totalis.
2 CASE
An 8-year-old girl came with a chief complaint of
diffuse scalp hair loss since the age of 4.
Approximately four years ago, parents incidentally
found a single hair loss patch on the scalp without
any associated symptoms. The year after, the patient
developed total scalp hair loss following an episode
of high fever and upper respiratory tract infection.
Eyebrows, eyelashes, and other hair-bearing areas
were not involved. Eventually, hair regrowth
observed after resolution of fever and infection.
However, relapses were noted at least twice a year,
following episodes of high heat and
tonsillopharyngitis. The parents denied any
application of topical or oral medications before the
onset of disease. The patient has a history of rhinitis
allergic (atopy). However, no prior history of
autoimmune diseases and a family history of hair
loss were noted. Parents brought her to a
dermatologist, and unknown topical medications
were given with good response. However, two
months prior to a consultation, the patient developed
a recurrent episode of total scalp hair loss three
weeks after hospital admission due to typhoid fever.
Even hair regrow already been noted,the frequent
relapses and lack of self-confidence prompted this
consult.
In physical examination, the patient was in
good general condition and nutritional status.All of
the vital signs were within the normal range.
Dermatologic examination revealed skin-colored
smooth surface macule with diffuse hair loss,
decreased of terminal hairs, and predominance of
vellus and broken hairs on the scalp. Furthermore,
multiple discrete lenticular hypopigmented macules
with indistinct borders and fine scales also observed
on the face. Small superficial pitting and transverse
leukonychia on fingernails were also noted.
Eyebrows and eyelashes were intact (Figure 1a – e).
From the initial assessment, the differential
diagnosis for the hair loss, in this case, are alopecia
totalis, telogen effluvium, and
trichotillomania.Wood's lamp examination on
hypopigmented facial lesions did not show any
enhancement and skin scrapping with KOH 10%
also failed to show fungal elements. These findings
are compatiblewith pityriasis alba. Serology
examination showed typical results for ANA, anti-
dsDNA, free T3, T4, TSH, which ruled out possible
associated autoimmune conditions such as lupus,
and thyroid disease. Initial dermoscopy evaluation
on the scalp revealed yellow dots, black dots,
exclamation hairs, broken hairs, and short vellus
hairs (Figures 1f – g). Based on these findings, the
diagnosis of alopecia totalis was established.
Hence, the patient was given topical minoxidil
2% solution applied once a day. On follow up visit
two months after treatment initiation, scalp hair has
regrown with 3 – 4 cm length in most areas.The
dermoscopic examination also showed signs of hair
regrowth with an increasing proportion of terminal
hairs and significant reduction of broken and short
vellus hair. Exclamation hair, yellow dots, and black
dots were not seen anymore (Figures 2). Therefore,
the patient was advised to continue the medications
until the scalp hair fully regrows with proper
compliance, do regular monthly visit, and avoid
triggering factors such as fever and infection.
3 DISCUSSION
Alopecia totalis is a manifestation of alopecia areata
that is characterized by total scalp hair loss and
associated poor prognosis and treatment failure with
only less than 10% of patients achieved complete
resolution. (Jang et al.,2017) Only limited data are
available regarding alopecia areata in children.
Prevalence of childhood alopecia is around 11.1% in
a multiethnic community of Singapore. Even though
there is a slight male predominance, the proportion
of severe alopecia is found higher in female patients.
In the case of extensive alopecia, such as alopecia
totalis and universalis, the onset usually is more than
six months, and most of them have recurrent
episodes.(Tan et al.,2002) As an autoimmune
disease with multifactorial etiologies, alopecia areata
can be triggered by microtrauma or destruction of
hair follicles, bacteria or virus infection, and
emotional stress.(Ito,203)Clinical manifestation of
alopecia totalis commonly found as sudden onset of
asymptomatic total scalp hair loss on the healthy and
smooth skin surface with the characteristic
Dermoscopy as a Diagnostic and Evaluation Tools in Childhood Alopecia Totalis
431
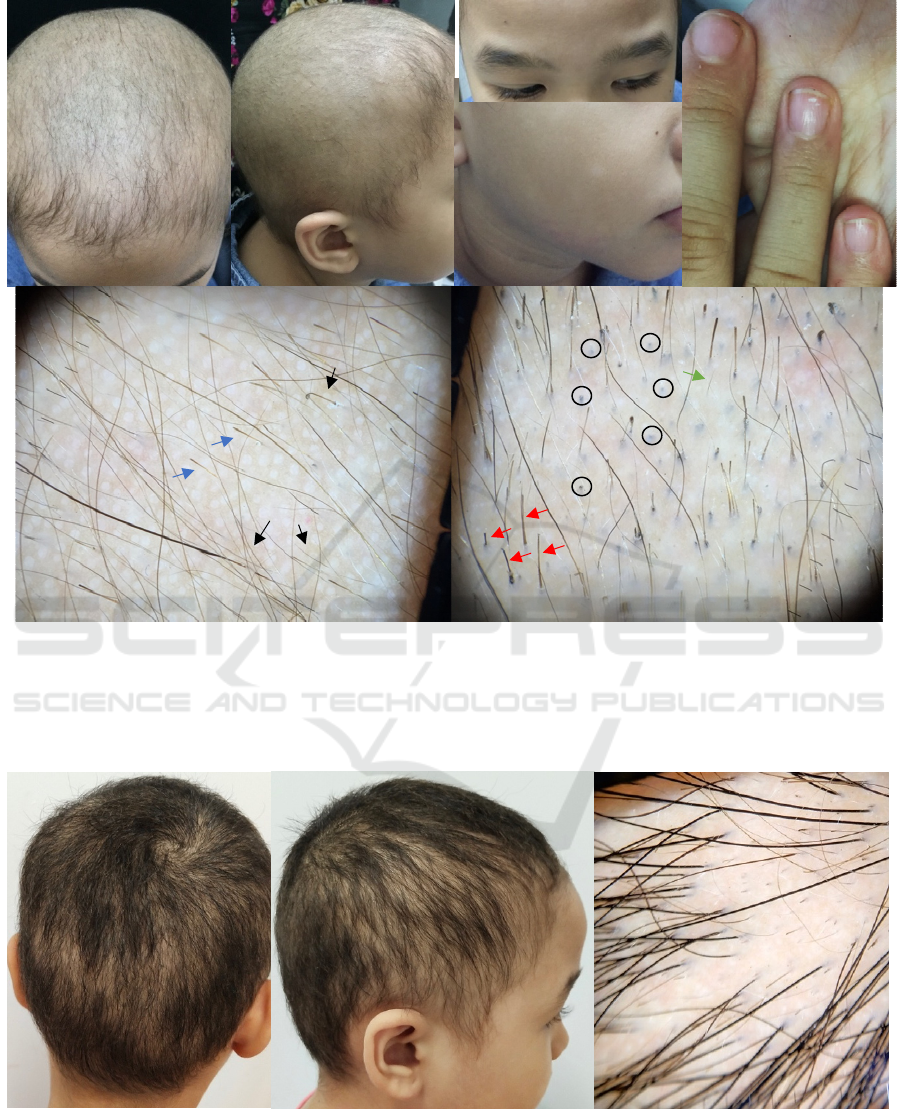
Figures 1. Dermatologic examination revealed (a), (b) diffuse scalp hair loss with decreased of terminal hairs and
predominance of vellus and broken hairs, (c) intact eyebrows and eyelashes, (d) multiple hypopigmented macules with fine
scales on the face which are consistent with pityriasis alba,and (e) multiple small superficial pitting and transverse
leukonychia on fingernails.(f), (g) Initial dermoscopic examination showed yellow dots(black arrow), exclamation hairs
(blue arrow), black dots (black circle), broken hairs (red arrow), and short vellus hairs (green arrow).
Figures 2. Follow up visit two months after therapy initiation, (a), (b) hair regrowth was observed in most areas of the scalp.
(c) The dermoscopic evaluation also revealed significantly increased of terminal hairs, reduction of broken and short vellus
hairs, but exclamation hairs, yellow dots, and black dots not seen.
(d)
(e)
(a) (b) (c)
(f)
(g)
(a)
(b)
(c)
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
432
exclamation hairs and sparing of white hairs. In some
cases, the onset might be following episodes of
partial alopecia. (Alkhalifah et al, 2010; Otberg et al.,
2012) Nail involvement found in 7 – 66% of cases,
with small superficial pitting as the most common
finding, followed by trachyonychia, Beau’s lines,
onychorrhexis, thinning or thickening of nails,
onychomadesis, koilonychia, punctate or transverse
leukonychia, and red lunulae. (Alkhalifah et al, 2010)
In childhood alopecia, 8.4% of patients developed
nail abnormalities, including pitting, trachyonychia,
and longitudinal ridging, which correlated with the
severity of the disease.(Tan et al.,2002) Furthermore,
history of atopy, autoimmune diseases such as
vitiligo, thyroid disease, lupus, and other conditions
are also associated with a higher incidence of
extensive alopecia (totalis and universalis).
(Alkhalifah et al, 2010)
Diagnosis of alopecia areata usually mostly made
based on clinical findings, and ancillary tests might
not be needed to confirm the diagnosis. However, in
some cases with diffuse hair loss, as seen in our
patients, the clinical appearance might be similar to
telogen effluvium and trichotillomania. (Alkhalifah
et al, 2010) Hence, histopathology evaluation from a
scalp biopsy is required to rule out other etiology.
The common histopathological findings are
generalized miniaturization, a marked increase in the
catagen and telogen hair follicles and peribulbar
lymphocytic infiltrate (a swarm of bees)as the
hallmark of the acute phase. These features help the
physician to confirm the diagnosis of alopecia
areata.
11
However, this invasive diagnostic method is not
favorable or routinely done, especially in children.
Therefore, there are many studies have done lately to
verify the diagnostic features of alopecia areata in
dermoscopy. In the literature, characteristic
dermoscopic findings of alopecia areata are yellow
dots, black dots, broken hairs, exclamation mark hair,
and short vellus hairs (Table 1).(Mane et al.,
2011;Inui et al., 2008;Mahmoudi et al., 2018)
Among these features, yellow dotscan be regarded as
a sensitive marker for alopecia areata due to its high
frequency.(Jha et al., 2017) According to Inui et al.,
yellow dots and short vellus hairs were the most
sensitive markers for the diagnosis, and black dots,
exclamation mark hairs, and broken hairs were the
most specific markers. (Inui et al., 2008)
Some
studies have tried to correlate these dermoscopic
findings with disease activity and severity.
Guttikonda et al. found that among those features,
black dots, broken hairs, and exclamation mark hairs
are correlated with disease activity.(Guttikonda et al.,
2016) Furthermore, black dots and yellow dots
correlated positively with the severity of the alopecia
areata. (Inui et al., 2008;Guttikonda et al., 2016)
On the other hand, short vellus hairs correlated
negatively with either disease activity or severity.
This feature is commonly found in patients under
treatment and indicates an early sign of disease
remission. Regrowth of short vellus hair after surgery
can be seen in dermoscopy even before they can be
perceived by the naked eye.(Guttikonda et al., 2016)
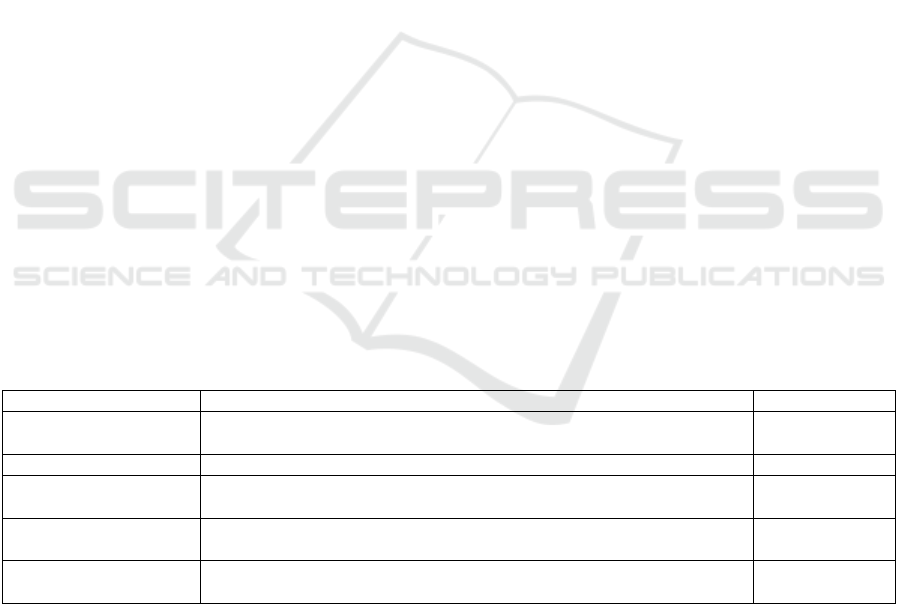
Table 1. Dermoscopic features of alopecia areata
Findings Definition Frequency
Yellow dots Round or polycyclic yellow to yellow-pink dots that represent distended
follicular infundibula filledwith sebum and keratin remnants
63,7 – 89,6%
Black dots The remnant of broken hair shafts inside follicular ostia 40,9 – 78,4%
Exclamation mark hair Broken hairs that tapered toward follicles (diameter of proximal hair
follicle < distal)
31 – 66,6%
Short vellus hair Thin, unpigmented hairs with length ≤10 mm may demonstrate early
disease remission
12,1 – 31,7%
Broken hair Fracture of dystrophic hair shafts or rapid regrowth of hairs that formerly
manifested as blackdots
9,5 – 55,4%
(references: Mahmoudi H, Salehi M, Moghadas S, Ghandi N, Teimourpour A, Daneshpazhooh M. Dermoscopic findings in
126 patients with alopecia areata: A cross-sectional study. International Journal of Trichology 2018;10(3):118-23.)
In contrast, other condition with prolonged
diffuse hair loss such as chronic telogen effluvium
did not show specific dermoscopic findings.
Diagnosis of telogen effluvium may be suspected
when empty hair follicles (sometimes appearing as
yellow dots) and short, dark, regrowing hairs with
standard thickness are present in the absence of the
characteristic features of other scalp disorders. In
trichotillomania, the presence of broken hair shafts
with different lengths and signs of plucking, such as
a bleeding point on the scalp may help to establish
the diagnosis. These findings are not usually seen in
alopecia areata and remain as the hallmark of this
psychiatric disorder.(Lacarrubba et al., 2015)
Dermoscopy as a Diagnostic and Evaluation Tools in Childhood Alopecia Totalis
433

Treatment of childhood alopecia areata has to be
pro-active due to its chronicity and high risk of
extensive involvement. However, only limited data
are available regarding treatment options in this
population. Topical medications such as
corticosteroid, minoxidil, anthralin, and
immunotherapy (diphenylcyclopropenone (DPCP),
squaric acid dibutyl ester (SADBE)) remains as first-
line therapy. The physician might use a combination
of 2 or 3 topicals as second-line therapy. Inadequate
data are available to support the use of systemic
treatment in childhood alopecia to prevent extensive
alopecia. Therefore, systemic medications remain
the last frontier in treatment options.(Cranwell et al.,
2018).
In our case, we found an 8-year-old girl with a 4-
year history of multiple episodes of sudden onset
total scalp hair loss which were triggered by fever
and infection. Nail abnormalities such as small
superficial pitting, transverse leukonychia, and
history of atopy (rhinitis allergic) and also a minor
feature of atopic dermatitis (pityriasis alba) are
found in this patient. Clinical findings of diffuse
scalp hair loss can be found not only in alopecia
totalis but also telogen effluvium and
trichotillomania. However, diagnosis of alopecia
totalis was established by characteristic dermoscopic
findings of yellow dots, black dots, exclamation
hairs, broken hairs, and short vellus hairs. Hence,
telogen effluvium could be ruled out. Furthermore,
trichotillomania was ruled out due to no signs of a
bleeding point on the scalp, and all the broken hairs
were within a similar length.
Sensitive markers for disease severity, black
dots, and yellow dots, are found in the initial
dermoscopy evaluation, which is compatible with
the clinical manifestation of extensive alopecia
totalis. Hence, our patient was immediately started
on topical minoxidil solution as the first-line therapy
in childhood alopecia. Within two months, scalp hair
has regrown in most areas and dermoscopic markers
for disease activity, including black dots, broken
hairs and exclamation mark hairs, were not found
anymore. Short vellus hairs, as the sign of remission
phase in alopecia, were found as the dominant
dermoscopic findings in the follow-up visit
andindicated good treatment response. These
findings are similar to several studies done
previously and prove the reliability of dermoscopy,
not only as a diagnostic tool but also for evaluation
of treatment.
4 CONCLUSION
Alopecia totalis in children is associated with long
duration, a severe progression of the disease, and
inadequate response to treatment. In the case with
diffuse scalp hair loss, the clinical findings might not
lead to a straightforward diagnosis of alopecia
totalis. Dermoscopy has been used to confirm a
diagnosis in which biopsy may not be visible. Black
dots, broken hairs, and exclamation mark hairs are
correlated with disease activity. While short vellus
hairs might be the early sign of remission, even the
hair regrowth sign has not been visible with naked
eyes. Therefore, dermoscopy is an advantageous and
practical non-invasive diagnostic and evaluation
method in childhood alopecia totalis.
REFERENCES
Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Saphiro
J. 2010. Alopecia areata update: Part I. Clinical
picture, histopathology, and pathogenesis. J Am Acad
Dermatol. 62:177-88.
Cranwell WC, Lai WYV, Photiou L, Meah N, Wall D,
Rathnayake D, et al. 2018. Treatment of alopecia
areata: An Australian expert consensus statement.
Australian Journal of Dermatology. Nov.
DOI:10.1111/ajd.12941.
Guttikonda AS, Aruna C, Ramamurthy DVSB, Sridevi K,
Alagappan SKL. 2016. Evaluation of clinical
significance of dermoscopy in alopecia areata.
International Journal of Dermatology. 61(6):628-633.
Inui S, Nakajima T, Nakagawa K, Itami S. 2008. Clinical
significance of dermoscopy in alopecia areata:
Analysis of 300 cases. Int J Dermatol. 47:688-693.
Ito T. 2013. Recent advances in the pathogenesis of
autoimmune hair loss disease alopecia areata.
Clinical and Developmental Immunology.
2013:348546.
Jang YH, Hong N-S, Moon SY et al. 2017. Long-term
prognosis of alopecia totalis and alopecia universalis:
a longitudinal study with more than 10 years of
follow-up: better than reported. Dermatology.
233:250–6.
Jha AK, Udayan UK, Roy PK, Amar AKJ, Chaudhary
RKP. 2017. Dermoscopy of alopecia areata in
restrospective analysis. Dermatol Pract Concept.
7(2):12.
Lacarrubba F, Micali G, Tosti A. Scalp dermoscopy or
trichoscopy. In: Ioannides D, Tosti A (eds). 2015.
Alopecias – Practical Evaluation and Management.
Basel: Karger. vol 47, p21-32.
Mahmoudi H, Salehi M, Moghadas S, Ghandi N,
Teimourpour A, Daneshpazhooh M. 2018.
Dermoscopic findings in 126 patients with alopecia
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
434

areata: A cross sectional study. International Journal
of Trichology 10(3):118-23.
Mane M, Nath AK, Thappa DM. 2011. Utility of
dermoscopy in alopecia areata. Indian J Dermatol.
56:40711.
Nanda A, Al-Fouzan AS, Al-Hasawi F. 2002. Alopecia
areata in children: a clinical profile. Pediatr Dermatol.
19:482-5.
Olsen EA. 2011. Investigative guidelines for alopecia
areata. Dermatol Ther. 24:311–319.
Otberg N, Saphiro J. 2012. Hair Growth Disorders. In
Goldsmith LA, Katz S.I., Gilchrest B.A., Paller A.S.,
Leffell D.J., Wolff K. (Eds.): Fitzpatrick’s
Dermatology In General Medicine. 8
th
edition. New
York: McGraw-Hill Companies. p 979-1008.
Tan e, Yong KT, Yoke CG. 2002. A clinical study of
childhood alopecia areata in Singapore. Pediatr
Dermatol. 19(4):298-301.
Wasserman D, Guzman-Sanchez DA, Scott K, McMichael
A. 2007. Alopecia areata. Int J Dermatol. 46:121-31.
Dermoscopy as a Diagnostic and Evaluation Tools in Childhood Alopecia Totalis
435
