
The Development of Functional Food in Indonesia: Based on
Regulation Compared to Other Countries
Muhammad Zulhamdani, Ria Hardiyati, Indah Purwaningsih, Chichi Shintia Laksani and Yan Rianto
Indonesian Institute of Sciences, Jakarta, Indonesia
Keywords: Comparative Study, Food Industry, Functional Food Policy, Food Regulation.
Abstract: The trend of the prevalence of degenerative diseases is increasing nowadays including in Indonesia. As the
level of education and the level of income of the Indonesian people grow, consumers' desire for food products
is increasingly complex. In choosing food products, people today do not only choose foods that have a
satisfying effect but are looking for products with other advantages, one of which is functional value for
health. In developing functional food products in the food industry, many aspects must be considered and
influenced for development. One of these aspects is the regulation governing the functional food. With the
existence of clarity of regulation in the registration of functional food or food with claims to be an opportunity
for the food industry in Indonesia to produce healthy food. however, many regulations governing food have
caused the growth of the food industry in Indonesia to begin to decline. This paper examines the development
of functional food based on regulation in Indonesia and compared to other countries such as Canada, India,
Thailand, and Malaysia. Regulations regarding functional food issued by Indonesia are not much different
from countries that have issued similar regulations. The food industry that will sell food products with health
or other functional claims must provide scientific evidence and clinical trials to humans. However, this is
burdensome for the food industry because clinical trials on food that are applied are almost the same as clinical
trials on medicinal products.
1 INTRODUCTION
At this time, Indonesia faces a prolonged problem
related to food that has an impact on nutrition and
health. Consumption patterns and unhealthy lifestyles
have caused malnutrition and health problems. This
is indicated by the results of basic health research in
2018 (National Institute of Health Research and
Development, 2019) which states that the trend of
non-communicable diseases in Indonesia is
increasing such as diabetes mellitus, hypertension and
obesity compared to the results of health basic
research in 2013 (Figure 1). In the end, the Indonesian
people began to realize this condition, which led to a
shift in consumption patterns that began to realize the
importance of healthy living. Along with the
increasing public awareness of food, the demand for
healthy food is also increasingly shifting. Food that is
now starting to be in great demand by consumers is
functional food that is food that not only has good
nutritional composition and attractive appearance and
taste, but also has certain physiological functions for
the body, for example to lower blood pressure, reduce
cholesterol levels, reduce levels blood sugar, increase
the absorption of calcium, and others.
Figure 1: Trend of non-communicable diseases. Source:
National Institute of Health Research and Development,
2019.
In Asia, where functional foods have been
regarded as an integral part of the culture for many
years, there is a firm belief that foods and medicine
come from the same source and serve the same
purpose. However, functional foods actually have a
Zulhamdani, M., Hardiyati, R., Purwaningsih, I., Laksani, C. and Rianto, Y.
The Development of Functional Food in Indonesia: Based on Regulation Compared to Other Countries.
DOI: 10.5220/0009983400002964
In Proceedings of the 16th ASEAN Food Conference (16th AFC 2019) - Outlook and Opportunities of Food Technology and Culinary for Tourism Industry, pages 153-165
ISBN: 978-989-758-467-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
153

quite long history. In China, Japan and other Asian
countries, many types of foods have traditionally
been associated with specific health benefits
(Weststrate et.al, 2002). In Japan, research on
functional foods started in the early 1980s. In 1991, a
specific regulatory framework concerning Food for
Specific Health Use (called FOSHU) was introduced,
which made it possible to make limited health claims
after receiving approval from the Ministry of Health.
The key to success for food manufacturers is to
develop products that are accepted by consumers and
are consistent with the consumer’s understanding and
appreciation of functional foods within the existing
culture. However, scientists and regulators have only
recently begun to agree that the functionality of
functional foods should be found in whole foods
rather than in their individual components
(Verschuren, 2002). After the introduction of the
FOSHU regulation, the number of functional food
products increased, especially from 1997 to 2007,
according to consumer demand, the net sales of the
FOSHU products were the highest in 2007 at 6.2
billion dollars (110 JPY/USD) (Iwatani and
Yamamoto, 2019). In Canada there is also no
universally accepted definition of functional foods,
but 60% of the people select foods they believe are
functional. Canada also has undertaken initiatives to
establish nutrient and health claims regulations.
These claims describe the relationship between a food
component and a disease or health-related condition.
The approval of claims has been based on an
extensive review of existing scientific literature in the
form of an authoritative statement of a scientific body
(Verschuren, 2002).
Functional food manufacturers follow specific
processes to assure the creation of products with true
values. Many regulatory authorities around the world
craft strict and detailed regulations and standards to
ensure the efficiency and safety of the foods. The
regulatory bodies also may study if the products
provide true value for consumers. Regulations vary
from country to country, which makes exporting
functional foods a challenging task, especially if the
regulations are not clear, or if the products have not
been customized to suit the regulation of the
importing country (Farid et.al, 2019).
Indonesia itself has huge opportunities for the
development of the functional food industry.
Currently, there has been a great deal of attention
given to the functionality of foods that are endemic in
Indonesia. Indonesia's traditional food wealth is very
diverse and is believed to have certain health benefits,
tempeh, honey, turmeric, ginger, aromatic ginger
(kencur), Curcuma zanthorrhiza (Javanese turmeric),
Javanese tamarind juice and so forth. Several types of
Indonesian local foodstuffs whose prospects have
been assessed for functional foodstuff include uwi
(Dioscorea alata) (Hapsari, 2014), microalgae (Nur,
2014), and sweet potatoes (Ginting et al, 2011).
Javanese tradition in the development of herbal
medicine, is a traditional wealth that has the potential
to develop functional foods typical of Indonesia.
To support the development of functional food
industry in Indonesia, there are several policies that
has been issued by government. However, these
regulations have not had a significant impact on the
development of functional food products or food with
claims in Indonesia. Functional food in Indonesia is
more dominated by processed food sourced from
fibre, milk and flour. In addition, the growth of the
food and beverage industry in Indonesia in 2018 has
decreased which is only about 7.91% compared to
2017 where the growth of 9.23% (Ministry of
Industry, 2019). Therefore, this paper explains the
development of functional food in Indonesia based on
the functional food registration process in accordance
with policies issued by Indonesia compared to other
countries such as Canada, India, Thailand and
Malaysia.
2 METHOD
This paper is based on the results of a comparative
study with scientific review of the relevant literature
and regulations or policies issued by authorized
officials in their respective countries (Canada, India,
Thailand and Malaysia) related to functional food
regulation. The selection of the countries to be studied
is based on the similarities of those countries in the
regulation of functional food.
The comparative study reveals the information
about the specific regulation, year of implementation,
the regulatory agencies, the definition of functional
food, claims, and requirements and systems for
clinical trials in each country.
3 THE DEVELOPMENT OF
FUNCTIONAL FOOD
REGULATION IN INDONESIA
Regulation on functional food in Indonesia began
since Head of Badan Pengawas Obat dan Makanan
(the national authority on Food and Drugs in
Indonesia) has issued basic provisions on functional
food supervision No.HK.00.05.52.0685. However,
16th AFC 2019 - ASEAN Food Conference
154
the food fortification program that was launched in
1996 to improve the nutritional status of the
community in line with functional foods which
classified as dietary foods. The most popular
constituents of functional foods, promoted by food
industries for young children, are the very long
essential polyunsaturated fatty acids, such as omega-
3 (eicosapentaenoic acid and docosahexaenoic acid)
and omega-6 fatty acids as well as calcium
(Zawistowski, 2014). Functional foods in Indonesia
can be marketed using the same regulatory system
that is applied for conventional foods, as long as this
category of foods contains.
This regulation defined a functional food as
processed food that contains one or more functional
components which, based on scientific studies, have
certain physiological functions, are proven to be
harmless and beneficial to health. On the food label,
permitted claims are nutrition content claims and
nutrition function claims, while health claims are
prohibited.
In 2011, BPOM revised the regulation about Basic
provisions on functional food supervision in 2005
with Regulation of the head of BPOM regarding
claims in labels, and processed food advertising
supervision. In this regulation, functional food
definition is still being used. It regulates about
processed food with claim. There are three claims that
approved by the government to registered functional
food. Three claims consist of nutrition claim, health
claim and glycaemic index claims. There are 2
additional claims that did not exist in the previous
regulation, which is health claims and glycaemic
index claims. In nutrition claim consist of nutrition
claim and nutrient function claims. Then, in health
claims consist of nutrition function claims, other
function claims, and disease risk reduction claim.
Based on regulation, functional food must meet
the following requirements:
a. contains types of food components in the
amount according to the limits specified as set out
in Table 1 about other functional claims and Table
2 about claims for disease risk reduction;
b. has sensory characteristics such as appearance,
colour, or texture consistency and taste acceptable
to consumers; and
c. served and consumed as food or drinks.
Function claim and disease risk reduction claim
must be based on the results of human studies that
meet applicable scientific principles (experimental
randomized controlled trials (RCT) or observational
studies if experimental studies are not possible). In
vitro and animal research can be submitted to support
the application.
In this regulation, a list of diseases that can be
prevented through the content of functional food
components has been established in it as listed in
Table 2.
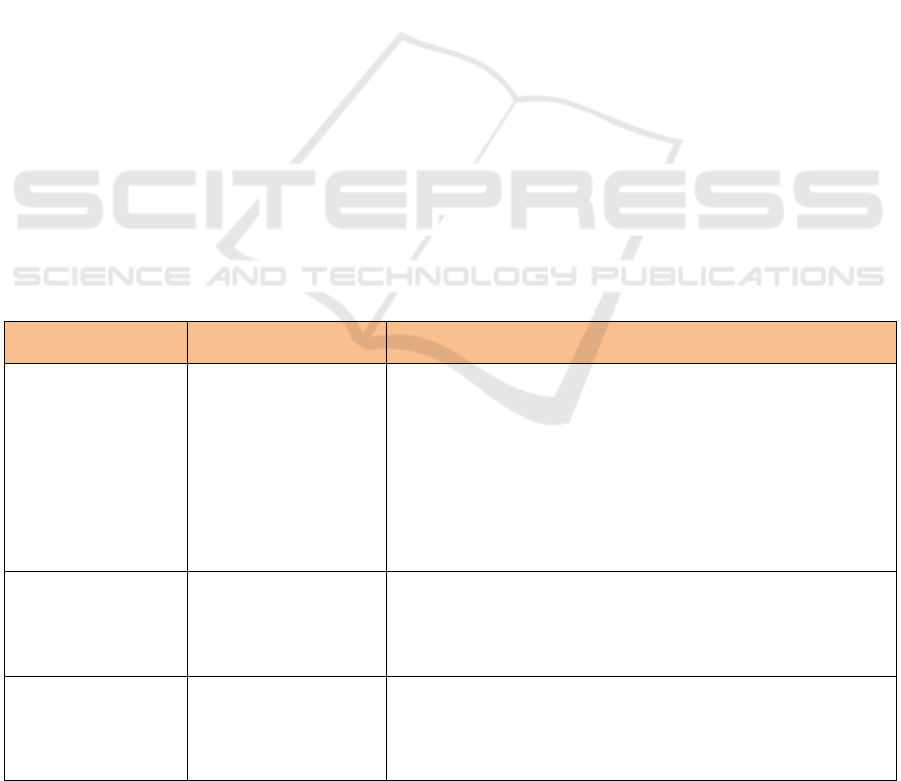
Table 1: Types of Food Component for Other Functional Claim.
Types of Food
Component
Claims
Requirements
Soluble dietary fibre
(Psyllium, beta glucan
from oats, inulin from
chicory and pectin
from fruits)
help reduce blood
cholesterol levels if
accompanied by a diet
low in saturated fat and
low in cholesterol.
a. Must include the constituent components and their sources;
b. Containing fibre at least 3 g per serving
c. total fat as much as 3 g per serving, or if the serving is less than
50 g then the total fat content is as much as 3 g per 50 g;
d. saturated fat as much as 1 g per serving and calories derived from
saturated fat as much as 15%, if the amount of serving is less than
100 grams, then the saturated fat content is as much as 1 gram per
100 grams and calories derived from saturated fat 10% maximum;
e. cholesterol as much as 20 mg per serving, or if the serving is less
than 50 g, the cholesterol content is as much as 20 mg per 50 g.
Soluble food fibre
(Psyllium, beta glucan
from oats, inulin from
chicory and pectin
from fruits)
Maintain the functioning
of the digestive tract.
a. Must include the constituent components and their sources;
b. Containing fibre at least 3 g per serving
Insoluble food fibre
Facilitate bowel
movements (laxatives)
and accompanied by
drinking enough water
a. Must include the constituent components and their sources;
b. Containing fibre at least 3 g per serving
c. Soluble fibre (beta glucan) oats of at least 3 grams or more per
day.
d. Soluble fibre from psyllium seed husk of at least 7 grams per day.
The Development of Functional Food in Indonesia: Based on Regulation Compared to Other Countries
155
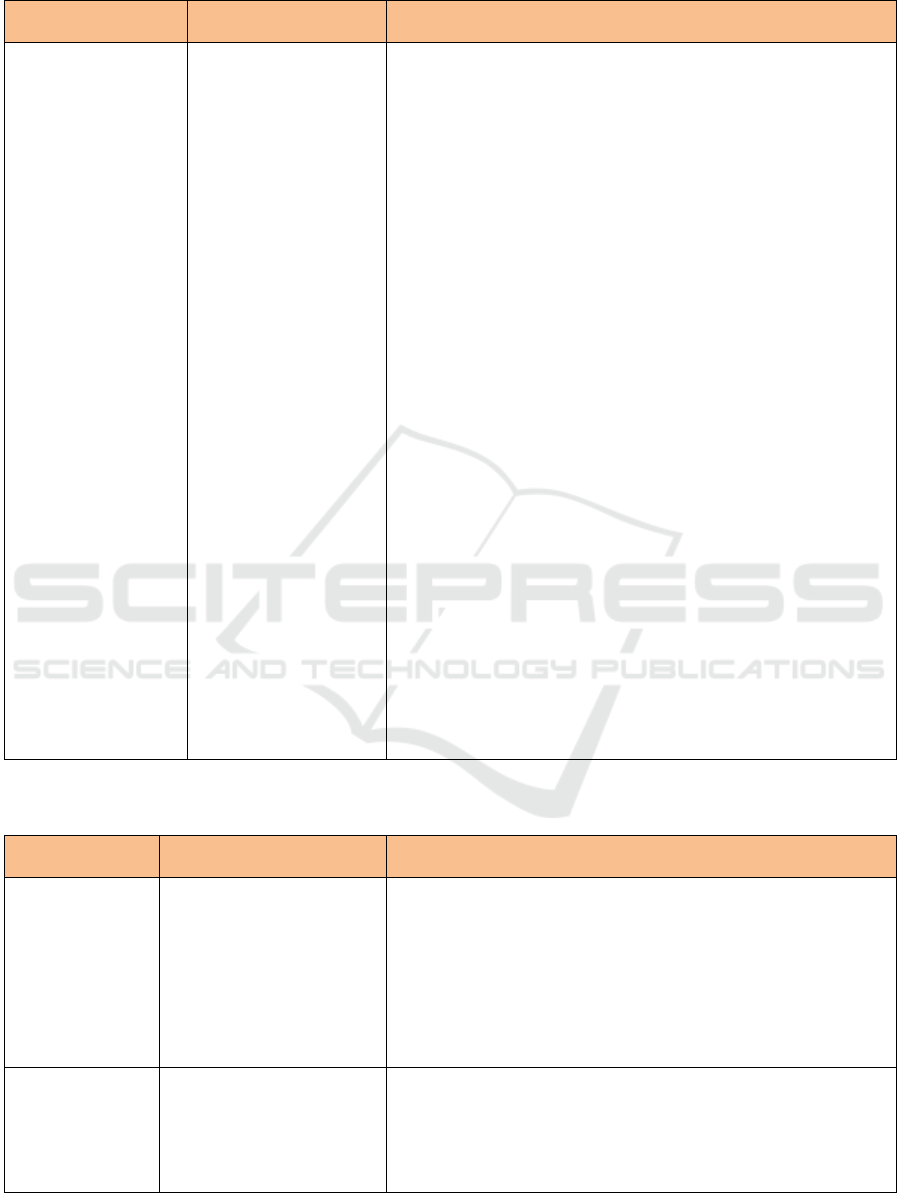
Table 1: Types of Food Component for Other Functional Claim. (Cont.)
Types of Food
Component
Claims
Requirements
Phytosterols and
Phyto stanols
Reduce the absorption
of cholesterol from food
in the intestine if
accompanied by a diet
low in fat, low in
saturated fat, and low in
cholesterol.
• Esterifying a mixture of phytosterols from edible oil with food
grade fatty acids. A mixture of phytosterols must contain at least
80% beta-sitosterol, camp sterol and stigmasterol (combined
weight).
• Esterifying a derivative of a Phyto stanols mixture from edible oil
or a by-product of the process of making craft paper pulp with
food grade fatty acids. The phyto stanols mixture must contain at
least 80% cytostanol and campestanol (combined weight).
• at least 0.65 grams phytosterols per serving for spread and salad
dressing or;
• at least 1.7 grams phytostanols per serving for spreads, salad
dressing, snack bars, pickled milk;
• Only apply for types of food that do not require high heating in its
preparation;
• Must meet following requirements:
a. total fat as much as 3 g per serving, or if the serving is less than
50 g then the total fat content is as much as 3 g per 50 g;
b. saturated fat as much as 1 g per serving and calories derived
from saturated fat as much as 15%, if the amount of serving is
less than 100 grams, then the saturated fat content is as much as
1 gram per 100 grams and calories derived from saturated fat
10% maximum;
c. cholesterol as much as 20 mg per serving, or if the serving is
less than 50 g, the cholesterol content is as much as 20 mg per
50 g.
• For food products that contain vegetable oil, replacement of the
word "phytosterols" and " phyto stanols " to "sterol esters and
vegetable oil stanols esters" allowed the origin of the vegetable oil
is the only source of sterol ester / stanols in the food product.
• Fat content may exceed 3 g per 50 g by adding the statement: "see
nutrition information for fat content value", but still contains 0.65
g or 1.7 g phyto stanols phytosterols per 50 grams of food
especially for product spreads and salad dressings.
• Meet minimum nutrient requirements, except for salad dressing.
Source: Appendix IV in Regulation of Head BPOM, 2011.
Table 2: Types of Food Component for Disease Risk Reduction Claims.
Types of Food
Component
Claims for Disease Risk
Reduction
Requirement
Folic acid
• Reduce the risk of failure
of neural tube defects in
the fetus, with a balanced
nutritional diet.
• Reduce the risk of failure
of neural tube defects in
the fetus with
accompanied by a
balanced nutritional diet.
• Consumption of these foods a day can meet 100% RDA of folic
acid.
• The food must not contain vitamin A in the form of retinol or pro
vitamin A and vitamin D more than 100% AKG a day.
• The label should include a recommendation regarding product
preparation, which is: "The product should be dissolved in boiled
water which has a maximum temperature of 40° C, because at
high temperatures folic acid will be damaged".
Calcium
Can help slow the
occurrence of osteoporosis
in the future if accompanied
by regular physical exercise
and balanced nutritional
consumption.
• Must contain at least 75% of the RDA per day according to age
group.
• Phosphorus levels should not exceed calcium levels.
• Calcium should not be associated with height increase (bone
length).
16th AFC 2019 - ASEAN Food Conference
156
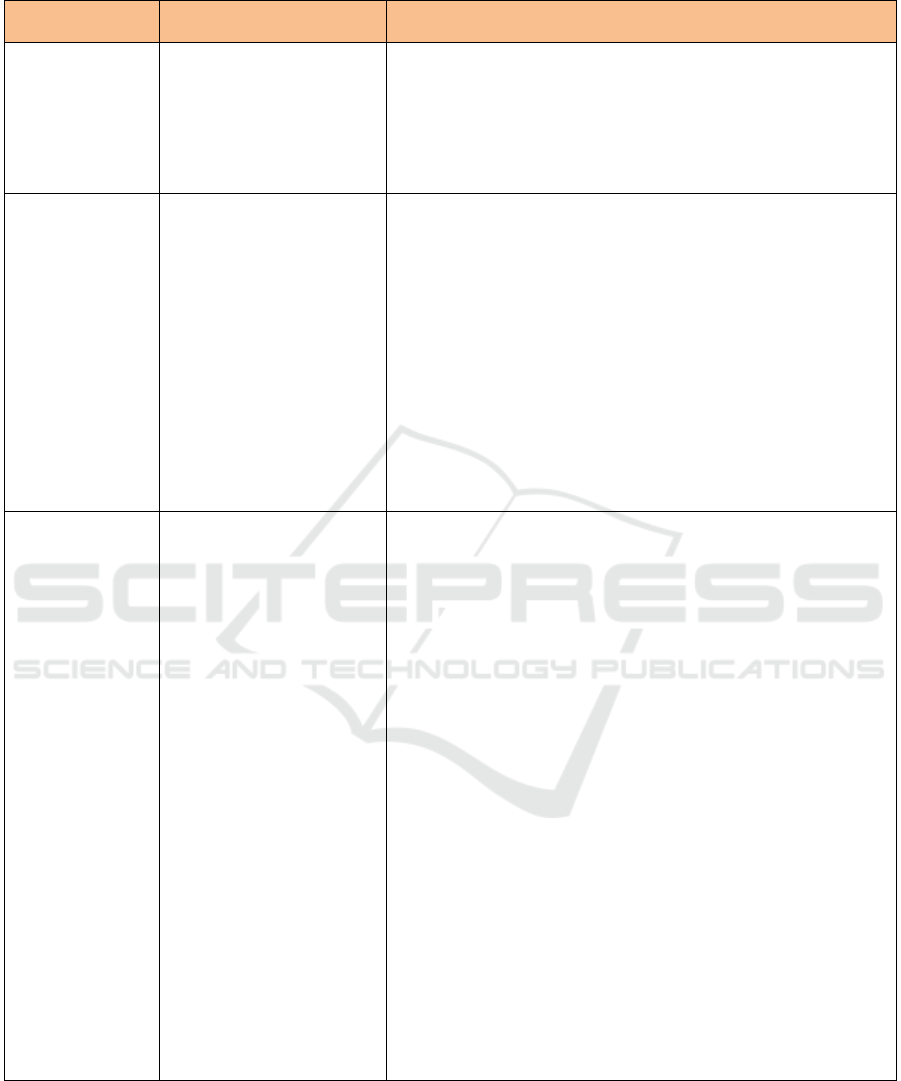
Table 2: Types of Food Component for Disease Risk Reduction Claims. (Cont.)
Types of Food
Component
Claims for Disease Risk
Reduction
Requirement
Sugar Alcohol/
Polyol
Can help reduce the risk of
dental caries, when
associated with lifestyle
habits.
• Does not contain mono- and di-saccharides.
• Possible using the following shortened claim: "This food can help
reduce the risk of dental caries".
• Must not mention the degree of reduction in the risk of dental caries
due to consumption of food containing polyols.
• Shall not be mentioned that the consumption of food containing
polyols are the only way to reduce the risk of dental caries.
Dietary fiber
soluble (psyllium,
beta glucan from
oats, inulin from
chicory and pectin
from fruit, inulin
from chicory and
pectin from fruit)
• Can help reduce the risk
of coronary heart disease,
a disease associated with
multi factors.
• Can help control blood
sugar in patients with
diabetes mellitus type II.
• Meet the following requirements:
a. As much as 3 g total fat per serving, or if the serving is less
than 50 g, the total fat content is as much as 3 g per 50 g;
b. As much as 1 g saturated fat per serving and calories derived
from saturated fat as much as 15%, if the amount of serving is
less than 100 grams, then the saturated fat content is as much
as 1 gram per 100 grams and calories derived from saturated
fat 10% maximum;
c. as much as 20 mg cholesterol per serving, or if the serving is
less than 50 g, the cholesterol content is as much as 20 mg per
50 g.
• At least 0.6 g soluble food fibre per serving;
• Prohibited to include statements related to colon cancer.
• At least 3 g soluble fibre (beta glucan) oats or more per day.
• At least 7 g soluble fibre from psyllium seed husk per day.
Phytosterols and
phytostanols
• Can help reduce the risk
of coronary heart disease.
• Food contains phytosterols of at least 0.65 grams per serving for
spread and salad dressing or;
• Food contains phytostanols of at least 1.7 grams of phytostanols
per serving for spreads, salad dressing, snack bars, pickled milk;
• Only apply for types of food that do not require high heating in its
preparation;
• Must meet following requirements:
d. total fat as much as 3 g per serving, or if the serving is less than
50 g then the total fat content is as much as 3 g per 50 g;
e. saturated fat as much as 1 g per serving and calories derived
from saturated fat as much as 15%, if the amount of serving is
less than 100 grams, then the saturated fat content is as much as
1 gram per 100 grams and calories derived from saturated fat
10% maximum;
f. cholesterol as much as 20 mg per serving, or if the serving is
less than 50 g, the cholesterol content is as much as 20 mg per
50 g.
• For food products that contain vegetable oil, replacement of the
word "phytosterols" and " phytostanols " to "sterol esters and
vegetable oil stanols esters" allowed the origin of the vegetable oil
is the only source of sterol ester / stanols in the food product.
• Fat content may exceed 3 g per 50 g by adding the statement: "see
nutrition information for fat content value", but still contains 0.65
g or 1.7 g phyto stanols phytosterols per 50 grams of food
especially for product spreads and salad dressings.
• Meet minimum nutrient requirements, except for salad dressing.
The Development of Functional Food in Indonesia: Based on Regulation Compared to Other Countries
157
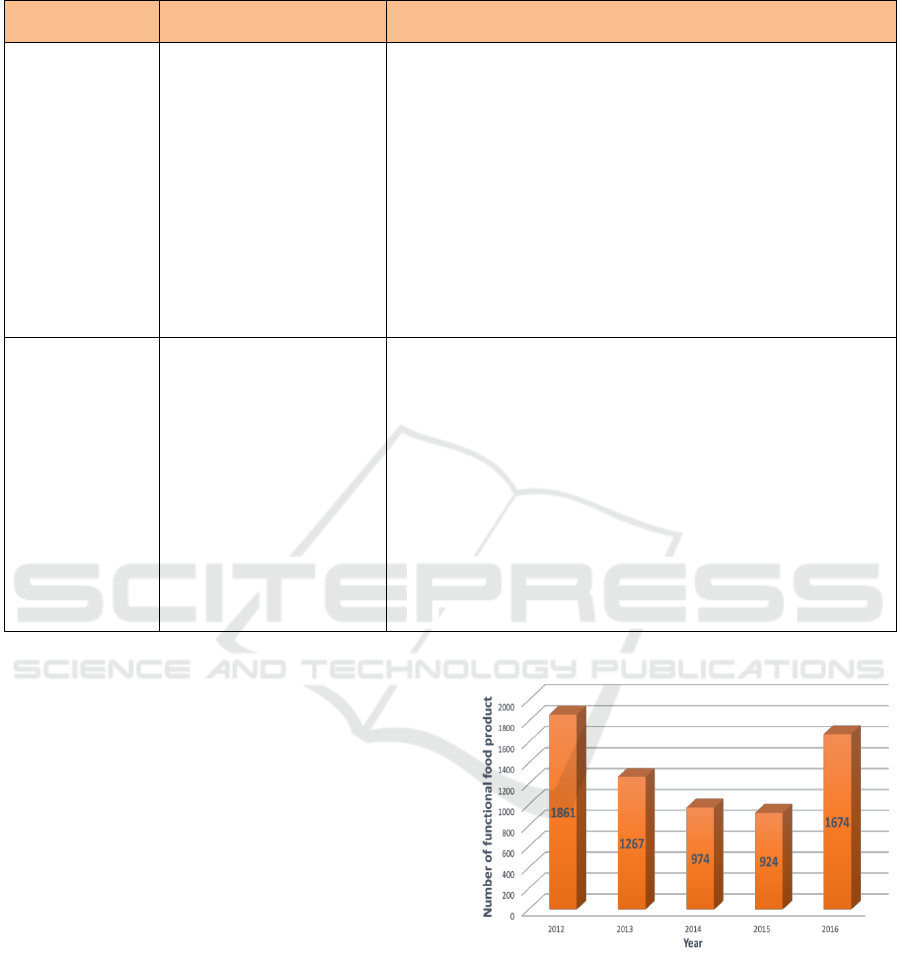
Table 2: Types of Food Component for Disease Risk Reduction Claims. (Cont.)
Types of Food
Component
Claims for Disease Risk
Reduction
Requirement
Peptides and
Certain Proteins
(Soybeans)
Can help reduce the risk of
coronary heart disease.
• Contains at least 6.25 g of soy protein per serving;
• Must meet the requirements:
a. as much as 120 mg sodium;
b. as much as 3 g total fat per serving, or if the serving is less than
50 g then the total fat content is as much as 3 g per 50 g;
c. as much as 1 g saturated fat per serving and calories derived
from saturated fat as much as 15%, if the amount of serving is
less than 100 grams, then the saturated fat content is as much as
1 gram per 100 grams and calories derived from saturated fat
10% maximum;
d. as much as 20 mg cholesterol per serving, or if the serving is
less than 50 g, the cholesterol content is as much as 20 mg per
50 g.
• Must specify the amount of soy protein per serving.
Soy Isoflavones
(daidzein, daidzin,
genistein,
genistin)
Can help reduce cholesterol
levels in the blood, so that it
can help reduce the risk of
atherosclerosis and coronary
heart disease".
• Must contain soy protein or peptide (as a non-isoflavone
component) and not be a pure isoflavone;
• Must contain at least 5 mg isoflavones per serving;
• Meet the following requirements:
a. as much as 3 g total fat per serving, or if the serving is less than
50 g then the total fat content is as much as 3 g per 50 g;
b. as much as 1 g saturated fat per serving and calories derived
from saturated fat as much as 15%, if the amount of serving is
less than 100 grams, then the saturated fat content is as much as
1 gram per 100 grams and calories derived from saturated fat
10% maximum;
c. as much as 20 mg cholesterol per serving, or if the serving is
less than 50 g, the cholesterol content is as much as 20 mg per
50 g.
Source: Appendix IV in Regulation of Head BPOM, 2011.
After the issuance of revised regulation in 2011,
trend of product registration on food with claim in
Indonesia was declined until 2015. In 2016, trend of
product registration was increase again as shown in
Figure 2. This indicates that the regulations issued by
the government cannot all be understood by the
applicants for registration of food products with
claims. This is caused by a change in the type of claim
that is permitted and the procedure for obtaining
approval of the claim.
In 2016, the National Agency of Drug & Food
Control (BPOM), revised the regulation. There are
some changes in the regulation of which is the
elimination of the definitions of functional foods and
its criteria. The regulation states that the definition of
functional food is replaced by food with claims.
However, this regulation increases the number of
claims in processed food.
Claim in processed food that approved by
National Agency of Drug and Food Control are
nutrition claim, health claim and other claims.
Nutritional claim consists of nutrient content claim,
and comparative nutrient claim. Health claims consist
of nutritional function claims, other functional claims,
Figure 2: Trend of Product Registration on Food with Claim
in Indonesia. Source: The National Agency of Drug & Food
Control (NADFC/BPOM), 2019.
and disease risk reduction claim. Other claims consist
of isotonic claim, no sugar addition claim, lactose
claim, and gluten claim.
Another revision that is in the new regulations is
the removal of the list of diseases (that shown in Table
2) that can be prevented through disease risk
reduction claim and replaced with legislation that any
16th AFC 2019 - ASEAN Food Conference
158

disease risk reduction claim submission by writing to
the Head of the National Agency of Drug & Food
Control (BPOM) to do the assessment.
4 REGULATION COMPARISON
TO OTHER COUNTRIES
In this section, we explore the regulation around the
world who have similarities regulation in Indonesia.
Functional food is regulated by the rules to protect
people from consumption of wrong claimed food.
4.1 Canada
In Canada, there are no regulations dealing
specifically with nutraceuticals or functional foods.
All foods and drugs fall under the provisions of the
Food and Drugs Act and Regulations. Food is under
the governance of the Food Directorate branch of
Health Canada and all health claims applications are
assessed using the Food and Drug Regulations under
the Canada Food and Drug Act. In 1953, The
Canadian Food and Drugs Act and Regulations was
passed into law. In this regulation, definition of food
is any article manufactured, sold or represented for
use as food or drink for human beings, chewing gum,
and any ingredient that may be mixed with food for
any purpose whatever. In the summer of 1996, the
Food Directorate of the Health Protection Branch of
Health Canada began deliberations, resulting in a
policy options paper and in which the proposed
definition for a nutraceutical was a product that has
been isolated or purified from foods and is generally
sold in medicinal forms not usually associated with
food (Fitzpatrick, 2004). This institution helps people
make informed decisions about food choices
provided they are truthful and not misleading. They
assess whether health claims are truthful and not
misleading by reviewing mandatory and voluntary
pre-market submissions. Depending on the novelty of
the substance that is the subject of the health claim,
the food product may also be subject to safety
assessment if it is considered a novel food.
Health Canada in 2001 created the Natural Health
Product Directorate (NHPD) to resolve the
inconsistencies in the system. The directorate
introduced a drafted regulation that addressed issues
relating to product licensing, site licensing, good
manufacturing practices, labelling and packaging,
clinical trials, and adverse reaction reporting. The
NHP regulation in Canada came into effect in 2004.
Previously, the status quo practice was that natural
health products were not defined by the Canada Food
and Drug Act (nutraceuticals were only defined)
(Malla, et.al, 2013). Since 2004, Health Canada has
authorized more than 61,000 NHPs for sale in Canada
(as of February 27, 2013) as well as 1250
manufacturing sites for these products (Harrison &
Nestmann, 2014).
NHPs are typically sold as capsules, tablets, or
liquid preparations, like pharmaceutical products.
When these ingredients are incorporated into food
products, they are generally not classified as NHPs.
But in 1998, Health Canada clearly distinguishes
between functional food and nutraceuticals.
Nutraceuticals are isolated or purified nutrients sold
in medicinal form (e.g. pill form, or more broadly, in
doses); nutraceuticals must have a health effect. A
functional food is similar in appearance to, or may be,
a conventional food; is consumed as part of a usual
diet and is demonstrated to have physiological
benefits and/or reduce the risk of chronic disease
beyond basic nutritional functions (Health Canada
1998). Functional foods are regulated not by the NHP
guidelines but rather by the Food Directorate of
Health Canada. Finally, a nutraceutical is a product
isolated or purified from foods that is generally sold
in medicinal form, not usually associated with food;
is demonstrated to have a physiological benefit or
provide protection against chronic disease (Walji &
Boon, 2008).
Canada lacks a comprehensive statutory
framework to deal with functional food claims, and
there is currently confusion between the classification
of natural health products (NHPs) and functional
foods. NHPs and foods are both regulated under the
Canadian Food and Drugs Act (Act), in accordance
with the applicable provisions of the Food and Drug
Regulations or the Natural Health Product
Regulations (NHP Regulations). A product may be
classified as both a food and an NHP, in which case,
the product is subject to the NHP Regulations, but is
exempt from the provisions of the Act and the Food
and Drug Regulations as they specifically relate to
food. A guidance document, “Classification of
Products at the Food-Natural Health Product
Interface” was released in 2009 to assist in
determining whether products that share
characteristics of both foods and natural health
products would be classified as food or NHPs food
products that are not NHPs must comply with all the
quality and safety requirements of the Food and Drug
Regulations. Functional food claims can be broadly
grouped into three main categories: nutritional
claims, general health claims and risk-reduction
claims. The labelling restrictions and requirements
The Development of Functional Food in Indonesia: Based on Regulation Compared to Other Countries
159

for functional foods depend primarily on the type of
functional claim that is being asserted. (McMahon &
Reguly, 2010).
There are two types of food health claims, namely
disease risk reduction claims and function claims. A
disease risk reduction claim is a statement that links a
food or constituent of a food to reducing the risk of
developing a diet-related disease or condition. A
function claim is a statement about the specific
beneficial effects that the consumption of a food or
food constituent has on normal functions or biological
activities of the body. (Health Canada, 2019). Health
claims and nutrient content claims are permitted
under the Food and Drug Regulations (FDR) Act. A
health claim is any claim that relates the consumption
of a food or ingredient and health. Disease risk
reduction claims relate the consumption of food (or a
food component) and the risk of developing a diet-
related disease. There are nine disease risk reduction
claims currently permitted in Canada (Malla et.al,
2013).
1. Low sodium and high potassium and reduced risk
of high blood pressure,
2. Adequate Vitamin D and calcium intake and
reduced risk of osteoporosis,
3. A diet low in saturated and trans fatty acids and
reduced risk of heart disease,
4. Consumption of fruit and vegetables and reduced
risk of some kinds of cancer,
5. Maximal fermentable carbohydrates in gum and
reduced risk of dental caries or cavities,
6. Phytosterols and lowering of cholesterol,
7. Oat fibre and reduced risk of heart disease,
8. Barley products and blood cholesterol lowering,
9. Unsaturated fat and blood cholesterol lowering.
Disease risk reduction claims must not be
misleading. As such, they must be based on adequate
scientific evidence and it should be reasonable and
feasible for an individual to consume an effective
amount of the food in the context of a healthy diet.
Except for a few exempt foods (“local,” “test
market,” or “specialty” foods), claims must appear in
both English and French. When the disease risk
reduction claim is made, the food must also declare a
Nutrition Facts table, including the amount of
nutrient, mineral, or vitamin that has the disease risk
reducing effect (Malla, et.al, 2013).
4.2 Thailand
In Thailand, food regulatory system is under the
authority of the Food and Drug Administration
(FDA), which is a sub department of the Ministry of
Public Health (MOPH). This bureau controls food
business in Thailand. And regulation for food is under
the Food Act B.E. 2522 (Ratanakorn, 2016). In
Thailand, Food innovation has the potential to grow
the Thai food industry. As increase in the population
of elderly people and lifestyle-related diseases
eventually caused the necessity for positioning the
foods not only for function as nutrition, but also for
sensory/satisfaction and health, there was increasing
interested in the functional food. From literatures and
documents found that Thai functional foods are
categorized as group of foods for special dietary uses
and fortified food with health claims. Rules and
regulations governing the labelling of functional food
products is an important to commercialization of
functional foods in Thailand too. Thailand’s health
food market is 160,000 million Baht or 4.58 billion
U.S. dollars in 2014. The market growth is about 6.1
percent per year and is growing at 6.0 percent per year
until 2016. From that market, the top place at
Thailand’s health food market was functional food.
(Supachaturat, 2017).
Based on Thai Food and Drug Administration,
functional food refers to food with special dietary
uses and has a similar appearance with conventional
food. It is consumed as part of a normal diet, and
exhibit physiological benefits such as the possibility
of reducing the risk of chronic diseases (Nor et.al.,
2016). In Thailand, nutritional labelling is mandatory
only for the following categories of foods: (1) foods
with nutrition claim, comparative or nutrient function
claim, (2) foods with claims of specific benefits or
functions to the body or specific ingredients, (3) foods
for specific target groups, for instance, school
children and the elderly, (4) other foods prescribed by
the Food and Drug Administration Office. In order to
make these claims, the nutrient must be present in
food in certain quantities. Health claims are not
permitted under current food regulations; however,
the health authorities are examining the draft Codex
document on health claims and developing claims
that can be used in support of functional foods
(Zawistowski, 2014). For other claims, the FDA must
approve such claims by reviewing all supporting
evidence, including the safety data (Ratanakorn,
2016).
In 2015, the Bureau of Food, The Food and Drug
Administration of Thailand has published regulation
about Public Manual Requesting for assessment of
health claim. This regulation revised that health
claims are permitted for food label. The claimed
benefit should arise from the consumption of a
reasonable quantity of the food or food constituents
in the context of a healthy diet and shall not depend
16th AFC 2019 - ASEAN Food Conference
160

on benefit from consumption together with other food
although it is normal practice or having intention to
consume together such as breakfast cereal is
consumed with milk. Health claims for nutrient claim
shall be based on scientific evidences to substantiate
the types of health claims as follows with systematic
review of Literature and Meta-analysis that
published in reliable journals or Recognized and
reliable technical opinions from international
recognized agencies, organizations ,or expert
committee or, and full copy of well-designed
human intervention study or other reasonable human
intervention study which number of samples and
sufficient preliminary study for consideration that
published in reliable journals as Full Text. For
diseases disk reduction, shall be has requirement
same as nutrient claims, with adding supporting
documents include peer-reviewed published articles,
animal study in vivo, ex vivo, or in vitro,
observational evidence of epidemiological study
which given its result consistent with the number of
well-designed study, evidence-based reference texts,
or other recognized and reliable texts (if any).
Adequacy of scientific evidence document depends
on quality of evidence that supported claims on
efficacy of food or constituents especially shall be
consistent with recommended use, objective of health
claims, dosage form, recommended intake duration of
intake and risk information (FDA, MPOH, 2016).
4.3 Malaysia
In Malaysia, the definition of functional foods and
nutraceuticals is still inconclusive. The term
“functional foods” is not used in the regulatory
system and there is no official definition of the term.
Food products are regulated under the Malaysian
Food Regulations 1985. A new regulation on
nutrition labelling and claims for foods was gazetted
on March 31, 2003 (Tee et.al, 2004). Nutraceuticals
and functional foods in Malaysia are distributed under
the food supplement category and not covered by the
Food and Drug Act. In trying to control the quality
and safety of these products to the consumers, the
Malaysian government under the jurisdiction of
Ministry of Health has assigned three bodies to
participate in the implementation of laws concerning
nutraceuticals and functional foods. The three
institutions consist of the National Pharmaceutical
Bureau, the Department of Food Quality, and
Malaysian National Codex Committee (Arshad,
2003).
So, there are no specific regulations for health
foods or functional foods. Currently, there are only
regulations on nutrition labelling and claims.
However, the government has appointed the Drug
Control Authority (DCA) and the National
Pharmaceutical Control Bureau to formulate a
separate regulation for dietary supplements (Lau,
2014). The Food Safety and Quality Division
(FSQD), Ministry of Health Malaysia has established
a regulatory framework to review applications for
health claims by food industry. An Expert Working
Group on Nutrition, Health Claims and
Advertisement meets regularly to evaluate
applications. Information required for applications
are clearly spell out (Tee, et.al, 2018).
In December 2010, Guide to Nutrition Labelling
and Claims for food industry has published by Expert
Committee on Nutrition, Health Claims and
Advertisement (Siong, T.E. et all, 2010). According
to Guide Book, there are several nutrition claims that
could be permitted in Malaysia as followed:
• Nutrient content claim
• Nutrient comparative claim
• Nutrient function claim and other function claim
• Claim for enrichment, fortification, or other
words of similar meaning
In Malaysia, the market for functional food is
enormous and still growing. The product considered
competitive products, whereby various products
under this category are often developed and
introduced into the market. There are three segments
in the functional food category were identified as the
main focus or strength, namely functional beverages,
dairy and soft drinks (Nor et.al., 2016). In conclusion,
regulation in Malaysia on functional food still have
restricted for claim such as claims for disease risk
reduction.
4.4 India
India has recently passed the Food Safety and
Standard Act 2006, a modern integrated food law to
serve as a single reference point in relation to
regulation of food products including nutraceutical,
dietary supplements and functional food. The Indian
Food Safety and Standard Act came into enforcement
in 2006 with two main objectives: to introduce a
single statute relating to food and to provide for
scientific development of the food processing
industry. The Food Safety and Standards Act 2006
consolidates the eight laws governing the food sector
and establishes the Food Safety and Standards
Authority (FSSA) to regulate the sector and other
allied committees. FSSA will be aided by several
scientific panels and a central advisory committee to
lay down standards for food safety. These standards
The Development of Functional Food in Indonesia: Based on Regulation Compared to Other Countries
161

will include specifications for ingredients,
contaminants, pesticide residue, biological hazards,
labels and others. The responsibility of framing and
regulating standards for nutraceuticals rests with the
Food Safety and Standards Authority of India
(FSSAI) as outlined in the Food Safety Act 2006.
This authority will be in charge of categories like
functional foods, nutraceuticals, dietetic products and
other similar products (Keservani, et.al, 2014).
Functional food is a relatively new concept to
Indian consumers. Food Safety and Standard Act
(FSSA) is the single reference point in relation to
regulation of functional foods in India. However,
Food Safety and Standards Authority of India
definition is relevant in Indian context. Broadly
“Functional food” may be defined as a food which
influences specific functions in the body that may
provide added health benefits or remedy from some
diseased condition following the addition/
concentration of a beneficial ingredient, or removal/
substitution of an ineffective or harmful ingredient
(Samal & Mohan, 2015). Health Claim that could be
approved by Indian Authority as followed (Sharma,
et.al, 2013, Verma & Popli, 2018):
• Nutrient function claim,
• Other function claims,
• Disease risk reduction claims.
In December 2016, Ministry of Health and Public
Welfare by the Food Safety and Standards Authority
of India has publish regulation that called the Food
Safety and Standards (Health Supplements,
Nutraceuticals, Food for Special Dietary Use, Food
for Special Medical Purpose, Functional Food and
Novel Food) Regulations. In this regulation, approval
claims are nutritional claim and health claim. The
health claim consists of two essential components,
namely nutrient or nutritional ingredients and health
related benefits. The health claim may include the
following types, but not limited to:
• Ingredient (nutrient or nutritional) function
claims,
• Enhanced function claims,
• Disease risk reduction claims,
• Health maintenance claims,
• Immunity claim – increased resistance (excluding
vaccines), and
• Anti-ageing claims.
In order to enhanced function claims and disease
risk reduction, regard shall be had to requirement of
availability of scientific literature including official
traditional texts and post market data or consumer
studies or cohort or retroactive studies based on
eating pattern and health benefits, epidemiological
international and national data, and other well
documented data, consensual, congruent and
concurrent validity studies, Health promotive and
disease risk reduction based on proof from literature
and human data of efficacy and safety of the nutrient,
not only controlled clinical trials for efficacy and
safety data, but also Nutra epidemiological data, and
qualified structure function claims for specific organ
or function which are comprehensible to consumer.
For the product led claims in respect of an article of
food based on human studies with evidence-based
data, regard shall be had to valid data and suitable
statistical design proving the benefit for disease risk
reduction, that is, human intervention studies; and
ingredient, that is, nutrient or nutritional.
For the health claim is prohibition of implied
claims for curing disease or claims of drug like
efficacy such as “Prevents bone fragility in post-
menopausal women”, and Prohibition of implied cure
for disease or claims by name of product such as
cancer cure or through pictures, vignettes or symbols,
namely, electrocardiogram tracing, lipid profile.
The FDA still allowed for industry that apply for
product with health claims without scientific support,
or if a novel ingredient is to be introduced, there shall
be a prior approval of the Authority which shall be
based on adequate scientific evidence.
4.5 Compared to Indonesian
Regulation
If compared to Indonesian Regulation, Regulation in
Canada, Thailand, Malaysia, and India has
similarities especially in approval claims. However,
Malaysia does not have permitted for health claim in
disease risk reduction. Another difference in
regulations in Indonesia compared to other countries
is the definition of functional food that has been
omitted in the latest regulations. This follows the
rules of the Codex Alimentarius which do not
regulate functional food but rather food with claims.
The latest regulations in Indonesia also state that
food with functional claims and health claims must
go through a series of experimental studies or
Random Clinical Trials. The application of RCTs is
treated the same as clinical trials on drugs. This is an
obstacle to the development of functional food
innovation, especially in clinical trials. As compared
to other countries, that to submit a health claim is
sufficient with scientific assessment evidence and in
accordance with research standards.
Table 3 shown a comparison of regulation
between Indonesia, Canada, Thailand, Malaysia and
India. From that table, Indonesia, Malaysia and India
16th AFC 2019 - ASEAN Food Conference
162
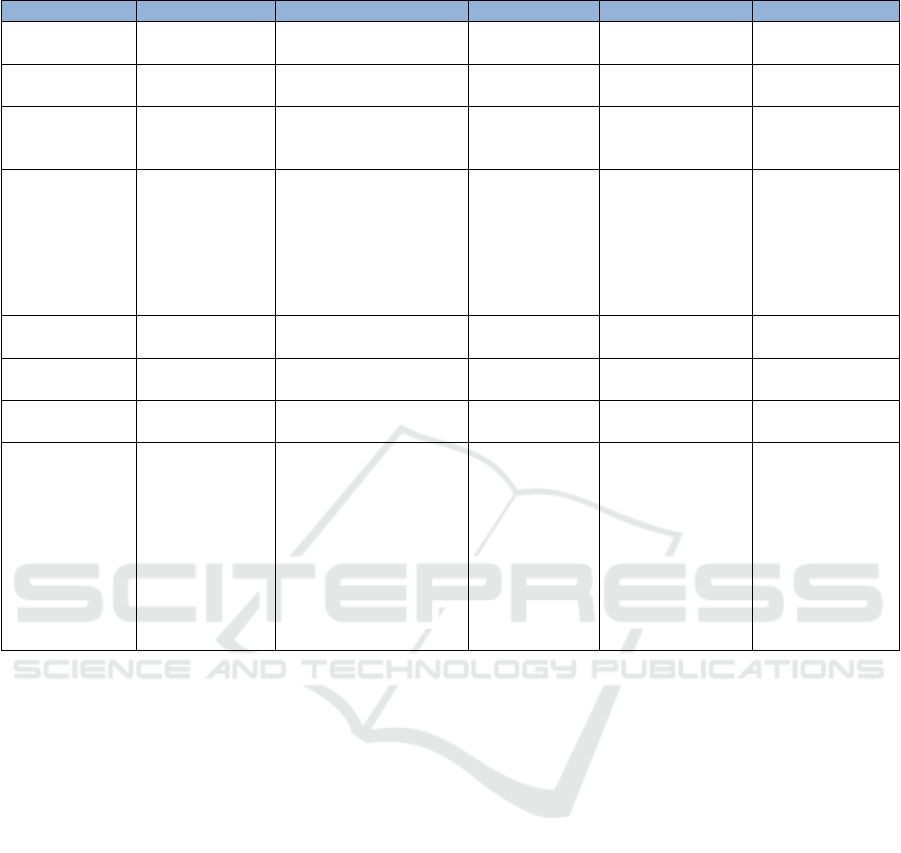
Table 3: Comparison of regulation to other countries base on content of regulation.
Information
Indonesia
Canada
Thailand
Malaysia
India
Specific
Regulation
Yes
No
No
Yes
Yes
Year of
Implementation
2005
1998
1998
2010
2006
Clear definition
about
functional food
Yes before
2006/ No after
2006
Yes
Yes
No
Yes
Regulatory
Agencies
NADCF/BPOM
Health Canada and the
Canadian Food
Inspection Agency
(CFIA)
Food and Drug
Administration
Office
Ministry of
Health and
Expert
Committee on
Nutrition, Health
Claims and
Advertisement
the Food Safety
and Standards
Authority of
India
(FSSAI)
Nutrition
function claim
Yes
Yes
Yes
Yes
Yes
Other function
claim
Yes
Yes
Yes
Yes
Yes
Disease Risk
Reduction
Yes
Yes
Yes
No
Yes
Clinical Trials
For disease risk
reduction claim
need Random
Clinical Trials
(RCT), and for
Probiotic need
clinical trial in
Indonesia
For disease risk
reduction, must
be based on adequate
scientific evidence and
it should be reasonable
and feasible for an
individual to consume
an effective amount of
the food in the context
of a healthy diet
Need scientific
evidence that
publish on
journal and
other evidence
like clinical
trial.
Prohibited
Health Claims
supported with
strong scientific
evidence
has established a specific regulation on claim label of
food such as nutrient claim and health claims. Canada
and Thailand regulate functional food on the Food
and Drugs regulation. That two countries do not have
specific regulation, but they have established of
functional food regulation in 1998 before Indonesia,
India and Malaysia. Each country has specific
regulatory body under Ministry of Health who
supervised functional food. Based on Approval
Claim, all countries permitted for nutrient claims and
other function claims. But in Malaysia, this country
has not allowed for disease risk reduction claims.
Based on regulation, Indonesia has implemented
specific regulations related to functional food,
although it later turned into regulation related to food
with claims. However, the growth of functional food
products in Indonesia is still not growing as shown in
figure 2. This is because functional food industries,
especially new industries, have not fully understood
the regulations regarding food with this claim which
they consider to be very complicated.
5 CONCLUSIONS
The development of functional food product in
Indonesia based on its regulation. Clarity of
regulation will be followed and obeyed by food
industry. Registration of processed food with claim
will be increase. Revised regulations regarding
claimed food specifically related to the list of
permitted claims and clarity in the procedure for
submitting food with claims, especially on claims for
disease risk reduction, will increase the number of
functional food products in Indonesia.
If we compared to other countries, Indonesia has
established the complete regulation for registration of
food with claims. The content of regulation no much
differs with Indonesia, Malaysia and India. In
Indonesia, there are nutrition function claim, other
function claim and disease risk reduction, same as
with Canada, Thailand and India. Except for
Malaysia, they do not permitted claims for disease
risk reduction.
The Development of Functional Food in Indonesia: Based on Regulation Compared to Other Countries
163

ACKNOWLEDGEMENTS
We acknowledge the support from the Ministry of
Research, Technology and Higher Education to fund
this research.
REFERENCES
Arshad, F., 2003. Functional foods from the dietetic
perspective in Malaysia. NUTRITION AND
DIETETICS, 60(2), pp.119-121.
Farid, M., Kodama, K., Arato, T., Okazaki, T., Oda, T.,
Ikeda, H. and Sengoku, S., 2019. Comparative Study of
Functional Food Regulations in Japan and Globally.
Global Journal of Health Science, 11(6).
FDA, MPOH, 2016, Public Manual of Requesting for
assessment of health claim, Thailand,
http://www.fda.moph.go.th/sites/fda_en/Shared%20D
ocuments/Manual/%E0%B8%9B%E0%B8%A3%E0
%B8%B0%E0%B9%80%E0%B8%A1%E0%B8%B4
%E0%B8%99%E0%B8%84%E0%B8%A7%E0%B8
%B2%E0%B8%A1%E0%B8%9B%E0%B8%A5%E0
%B8%AD%E0%B8%94%E0%B8%A0%E0%B8%B1
%E0%B8%A2/02%20PM%20Requesting%20for%20
assessment%20of%20health%20claim.pdf (August 20,
2019)
Ginting, E., Utomo, J.S., Yulifianti, R. and Jusuf, M., 2015.
Potensi ubijalar ungu sebagai pangan fungsional. Iptek
Tanaman Pangan, 6(1).
Hapsari, R.T., 2014. Prospek uwi sebagai pangan
fungsional dan bahan diversifikasi pangan. Buletin
Palawija, (27), pp.26-38.
Harrison, J.R. and Nestmann, E.R., 2014. Current Canadian
Regulatory Initiatives and Policies for Natural Health
Products (Dietary Supplements). In Nutraceutical and
Functional Food Regulations in the United States and
Around the World (pp. 183-200). Academic Press.
Health Canada. 1998. “Nutraceuticals/Functional Foods
and Health Claims on Foods.” Policy Paper
http://www.hc-sc.gc.ca/fn-an/alt_formats/hpfb
dgpsa/pdf/label-etiquet/nutrafunct_foods-nutra-
fonct_aliment-eng.pdf (July 20, 2019).
Health Canada, 2019, Health Claims,
https://www.canada.ca/en/health-
canada/services/food-nutrition/food-labelling/health-
claims.html (accessed July 20, 2019)
Iwatani, S. and Yamamoto, N., 2019. Functional food
products in Japan: A review. Food Science and Human
Wellness.
Kelley C Fitzpatrick, 2004. Regulatory issues related to
functional foods and natural health products in Canada:
possible implications for manufacturers of conjugated
linoleic acid, The American Journal of Clinical
Nutrition, Volume 79, Issue 6, June 2004, Pages
1217S–1220S, https://doi.org/10.1093/ajcn/79.6.1217S
Keservani, R.K., Sharma, A.K., Ahmad, F. and Baig, M.E.,
2014. Nutraceutical and functional food regulations in
India. In Nutraceutical and Functional Food
Regulations in the United States and Around the World
(pp. 327-342). Academic Press.
Lau, T.C., 2014. Overview of Regulations and
Development Trends of Functional Foods in Malaysia.
In Nutraceutical and Functional Food Regulations in
the United States and Around the World (pp. 465-478).
Academic Press.
Malla, S., Hobbs, J.E. and Sogah, E.K., 2013. Functional
foods and natural health products regulations in Canada
and around the world: nutrition labels and health
claims. Saskatoon, Saskatchewan, Canada: Report
prepared for the Canadian Agricultural Innovation and
Regulation Network, pp.447-454.
McMahon, E., & Reguly, T. 2010. Canada’s approach to
functional foods. Update, 2, 26-29.
National Institute of Health Research and Development
2018. Basic Health Research (Riset Kesehatan Dasar
Riskesdas). Jakarta: Badan Penelitian dan
Pengembangan Kesehatan; 2018 (downloaded
February, 2019).
Nor, N.A.A.M., Masdek, N.R.N.M. and Sulaiman, N.H.,
2016. Functional food business potential analysis in
Malaysia, Thailand, Indonesia and the Philippines.
Economic and Technology Management Review,
pp.99-110.
Nur, M.M.A., 2014. Potensi Mikroalga sebagai Sumber
Pangan Fungsional di Indonesia. Eksergi, 11(2), pp.1-
6.
Ratanakorn, S., 2016. Food Regulations and Enforcement
in Thailand. Elsevier.
Shamal, S. and Mohan, B.C., 2015. Functional Food
Acceptance in India: Socio-Demographic and Lifestyle
Determinants.
Sharma, A., Kumar, P., Sharma, P. and Shrivastav, B.,
2013. A Comparative Study of Regulatory Registration
Procedure of Nutraceuticals in India, Canada and
Australia. Int J Pharm Qual Assur, 4(4), pp.61-66.
Siong, D.T.E., 2010. Guide to Nutrition Labelling and
Claims. Ministry of Health, Malaysia.
https://extranet.who.int/nutrition/gina/sites/default/file
s/MYS%202010%20Guide%20to%20Nutrition%20La
belling%20and%20Claims.pdf (July 20, 2019)
Siong, T.E., 2014. Regulatory framework of functional
foods in Southeast Asia. In Clinical Aspects of
Functional Foods and Nutraceuticals (pp. 218-237).
CRC Press.
Supachaturat, S., Pichyangkura, R., Chandrachai, A. and
Pentrakoon, D., 2017. Perspective on functional food
commercialization in Thailand. International Food
Research Journal, 24(4).
Tee, E.S., Chen, J. and Ong, C.N., 2004. Functional foods
in Asia: Current status and issues. International Life
Sciences Institute (ILSI), Southeast Asia Region.
Tee, E.S., Wong, J. and Chan, P., 2018. Functional foods
Monographs. ILSI Southeast Asia Region Monograph
Series. International Life Sciences Institute (ILSI),
Southeast Asia Region.
16th AFC 2019 - ASEAN Food Conference
164

Verma, B. and Popli, H., 2018. Regulations of
nutraceuticals in India & US. The Pharma Innovation
Journal 2018; 7(7): 811-816.
Verschuren, P.M., 2002. Functional foods: scientific and
global perspectives. British Journal of Nutrition,
88(S2), pp. S126-S130.
Walji, Rishma and Heather Boon. 2008. “Natural Health
Products Regulations: Perceptions and Impacts.” Food
Science and Technology 19: 494-497.
Weststrate, J.A., Van Poppel, G. and Verschuren, P.M.,
2002. Functional foods, trends and future. British
Journal of Nutrition, 88(S2), pp. S233-S235.
Zawistowski, J., 2014. Regulation of functional foods in
selected Asian countries in the pacific rim. In
Nutraceutical and Functional Food Regulations in the
United States and Around the World (pp. 419-463).
Academic Press.
The Development of Functional Food in Indonesia: Based on Regulation Compared to Other Countries
165
