
The Role of Single Layer Immobilized Cells in Mead Fermentation
Rate
Monika Rahardjo
1
, Lindayani
2
and Laksmi Hartayanie
2
1
Food Technology Study Program, Satya Wacana Christian University, Salatiga, Indonesia
2
Food Technology Study Program, Soegijapranata Catholic University, Semarang, Indonesia
Keywords: Fermentation, Food Innovation, Honey, Immobilized Cells, Mead.
Abstract: The research consisted of the making of honey-must, the making of single layer immobilized cells, and
mead fermentation. Mead fermentation carried out with variation of treatments which were control (C) and
single layer immobilized cells (SL). The analysis performed were analysis of alcohol content, sugar content,
and yeast assimilable nitrogen (YAN) concentration which determine fermentation rate. The result of this
research explained that during mead fermentation, sugar content decreased with latest sugar content varied
namely 4,30-4,70
o
Brix, alcohol content with the latest value namely 8,5-9,5%, and fermentation rate for SL
was faster than C namely 0,105 g.ml
-1
.hr
-1
.
1 INTRODUCTION
Honey is one of bee’s derivative product with
various health benefits and also contributes to
enhance community’s economical state. Mead (as
known as honey wine) is one way to innovate honey
by fermenting honey solution (Pereira et al., 2013).
Mead is probably the oldest fermented beverage in
the world that was produced, but its production has
declined in recent years partly due to a lack of
scientific progress in this field.
Based on studies of wine, wine is considered as a
safe and healthy beverage in the moderate amount of
consumption because it had a positive effect on the
cardiovascular system (German & Walzem, 2000;
Snopek et al., 2018; Wurz, 2019). Alcohol that
contained in wine included as macro nutrition and
worked as an energy source, able to provide calories
for biological activities for humans, energy for
physical work, and thermogenesis (Joshi et al.,
2012). It is also studied before that antioxidant
content, especially honey phenolic compounds,
increased from mead fermentation process
(Wintersteen et al., 2005). In addition, the fermented
ethanol compounds in mead can enhance the
solubility and extraction strength for beneficial
compounds contain in mead (Roldán et al., 2011).
Over past few decades, the use of immobilized
cells methods had received attention of researchers
and had been successfully applied in the production
of alcohol (ethanol), organic acids, enzymes, and
fermented food products (beer and wine) (Liouni et
al., 2008; Reddy et al., 2008) and proved can
overcome some common problems that occur in the
fermentation process. During mead fermentation
process, some common problems were the inability
to obtain the desired alcohol content, a long
fermentation process, heterogeneity of the final
product (Pereira et al., 2014), and the re-
fermentation from the yeast that increase volatile
acids thus produced unwanted aroma (Ramalhosa et
al., 2011). Immobilized cells also allow easier
handling of yeast cells, clear end products, and
continuity of use (Kourkoutas et al., 2004; Park &
Chang, 2000). The purpose of this research was to
study the role of single layer immobilized cells in
mead fermentation rate including its alcohol and
sugar content.
2 MATERIALS AND METHODS
The main and chemical ingredients that used in this
research were honey from Ceiba pentandra,
Saccharomyces cerevisiae var. bayanus (dry yeast),
malic acid (C
4
H
6
O
5
), formaldehyde (CH
2
O),
distilled water, sodium alginate, natrium hydroxide
(NaOH), and calcium chloride (CaCl
2
). The
192
Rahardjo, M., Lindayani, . and Hartayanie, L.
The Role of Single Layer Immobilized Cells in Mead Fermentation Rate.
DOI: 10.5220/0009981800002964
In Proceedings of the 16th ASEAN Food Conference (16th AFC 2019) - Outlook and Opportunities of Food Technology and Culinary for Tourism Industry, pages 192-196
ISBN: 978-989-758-467-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

equipment used in this research were refractometer
(Hanna Instrument 96801), pH meter (Hanna
Instrument pH 213), and autoclave.
The research steps were the making of honey-
must, the making of single layer immobilized cells,
and mead fermentation. Honey-must is a solution
resulting from dilution of honey in distilled water.
At the stage of making honey-must, dilution of
honey in distilled water done until the concentration
of sugar reach 15
o
Brix (Srimeena et al., 2014).
Insoluble materials then filtered so the clear honey-
must solution was obtained. The honey-must then
adjusted to pH around 3,7 using malic acid
(C
4
H
6
O
5
). The parameters measured before and after
honey-must adjustment were sugar content (
o
Brix),
pH, and yeast assimilable nitrogen concentration
(YAN). YAN was determined by the formaldehyde
method (Pereira et al., 2014). The next step was
honey-must pasteurization by heating at temperature
of 65
o
C for 10 minutes (Mendes-Ferreira et al.,
2010) using a water bath and then cooled it to room
temperature.
The making of single layer immobilized cells
began with preparing starter yeast culture. The
starter yeast culture was prepared by rehydrating two
grams of dry yeast in 20 ml of distilled water at
38oC to obtain a suspension of yeast ca. 108 CFUs
per ml (Pereira et al., 2014). Sodium alginate was
dissolved in distilled water at a concentration of 4%
(w/v) and sterilized by autoclaving at temperature of
121
o
C for 20 minutes. To inoculate honey-must
solution with 106 CFU per ml, the appropriate
amount of the yeast suspension was added to sodium
alginate solution. This polymer cells mixture was
added drop wise to a 180 mM calcium chloride
sterile solution and allowed to harden for 30 minutes
at 4
o
C.
For mead fermentation step, firstly honey-must was
divided into 6 polyethylene terephthalate (PET)
gallons. Honey-must in the first three gallons were
used as control (C) where only 106 CFU per ml
yeast added, and the rest three gallons were added
with single layer immobilized cells (SL). Each
mixture was then incubated at room temperature
(Roldán et al., 2011) and batch fermented under
anaerobic condition. Each gallons were closed and
clamped, the remaining air was removed using a
pump. Fermentation was considered complete when
the YAN concentration, alcohol content, and sugar
content were constant. Measurement of YAN
concentration, alcohol content, and sugar content
carried out every two days and with thrice
replications.
3 RESULTS
The free cells yeast in control (C) and wet beads
yeast in single layer immobilized cells (SL) were
weighted before being put into honey-must. In
addition, the pH value, sugar content (
o
Brix), and
YAN concentration after adjustment were measured.
The initial physicochemical characteristics of honey-
must can be seen in Table 1.
Table 1: Initial characteristics of honey-must after
adjustment.
Control
Single
Layer
Total yeast weight (g)
26.49±0.02
31.61±0.01
pH
3.72±0.00
3.72±0.00
Sugar content (
o
Brix)
15.30±0.00
15.20±0.00
Yeast Assimilable
Nitrogen (mg/L)
248.00±0.00
249.10±0.01
The YAN value after honey-must adjustment
could be included in high levels (>225 mg/L) so it
didn’t require additional nutrients. Changes in sugar
content (
o
Brix), alcohol content (%), and YAN
concentration during mead fermentation can be seen
in Table 2 and the ANOVA result for each
parameters can be seen in Table 3.
Table 2: Sugar content, alcohol content, and yeast
assimilable nitrogen concentration during mead
fermentation.
D
a
y
Sugar content
(
o
Brix)
Alcohol content
(%)
Yeast
assimilable
nitrogen
concentration
(mg/L)
Control
Single
layer
Control
Single
layer
Control
Single
layer
1
14.47±
0.00
13.4±
0.00
0±0.00
0±0.00
256.67±
0.00
270.67
±0.00
3
14.13±
0.00
11.77±
0.00
0±0.00
1.83±
0.08
275.33±
0.00
254.33
±0.00
6
12.50±
0.00
10.73±
0.00
3.17±
0.08
4.67±
0.08
270.67±
0.00
228.67
±0.00
8
11.13±
0.00
9.73±
0.00
4.17±
0.08
6.67±
0.08
263.67±
0.00
212.33
±0.00
10
10.57±
0.00
7.30±
0.01
5.00±
0.00
8.60±
0.03
254.33±
0.00
189.00
±0.00
The Role of Single Layer Immobilized Cells in Mead Fermentation Rate
193
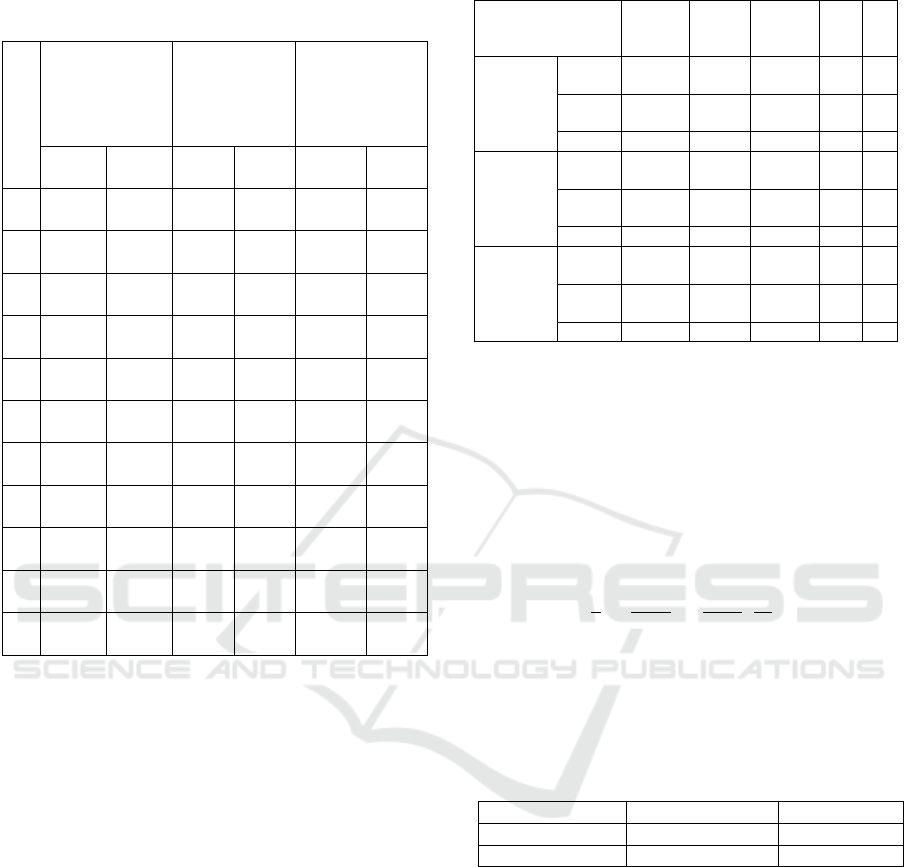
Table 2: Sugar content, alcohol content, and yeast
assimilable nitrogen concentration during mead
fermentation (Cont.)
D
a
y
Sugar content
(
o
Brix)
Alcohol
content (%)
Yeast
assimilable
nitrogen
concentration
(mg/L)
Control
Single
layer
Control
Single
layer
Control
Single
layer
13
9.70±
0.00
5.53±
0.00
6.00±
0.00
9.17±
0.00
249.67±
0.00
168.00
±0.00
15
7.57±
0.00
4.37±
0.00
7.00±
0.00
9.43±
0.00
238.00±
0.00
165.67
±0.00
17
5.53±
0.00
4.30±
0.00
7.70±
0.03
9.50±
0.00
235.67±
0.00
163.33
±0.00
20
4.97±
0.00
4.30±
0.00
8.00±
0.00
9.50±
0.00
224.00±
0.00
161.00
±0.00
22
4.90±
0.00
4.30±
0.00
8.27±
0.00
9.50±
0.00
219.33±
0.00
163.33
±0.00
24
4.87±
0.00
4.27±
0.00
8.47±
0.00
9.50±
0.00
217.00±
0.00
161.00
±0.00
27
4.73±0.
00
4.30±
0.00
8.50±
0.00
9.50±
0.00
214.67±
0.00
161.00
±0.00
29
4.7±
0.00
4.30±
0.00
8.50±
0.00
9.50±
0.00
214.67±
0.00
158.67
±0.00
30
4.7±
0.00
4.30±
0.00
8.50±
0.00
9.50±
0.00
212.33±
0.00
158.67
±0.00
31
4.7±
0.00
4.30±
0.00
8.50±
0.00
9.50±
0.00
212.33±
0.00
158.67
±0.00
32
4.7±
0.00
4.30±
0.00
8.50±
0.00
9.50±
0.00
212.33±
0.00
158.67
±0.00
Control (C) hadn’t significant difference with
single layer immobilized cells (SL). During mead
fermentation, the value of sugar content (
o
Brix) will
decrease over time until it finally stabilized. The
final value of the sugar content was almost the same,
ranging from 4,3 to 4,7. SL had lower latest sugar
content compared to C.
Changes in the value of alcohol content hadn’t
significant difference from C and SL. During mead
fermentation, the value of alcohol content (%)
increase over time until finally stabilized. The value
of alcohol content varied from 8,5 to 9,5%. SL
produced higher alcohol content compared to C.
From table 2, the relationship between sugar content
and alcohol content can be addressed, the higher the
alcohol content along with the decrease in sugar
content of mead.
Table 3: Analysis of variance (ANOVA) result.
Sum of
squares
Degree
of
freedom
Mean
square
F
Sig.
Sugar
content
(
o
Brix)
Between
groups
15.60
1
15.60
1.30
0.26
Within
groups
360.04
30
12.00
Total
375.64
31
Alcohol
content (%)
Between
groups
20.46
1
20.46
2.26
0.14
Within
groups
272.27
30
9.08
Total
292.74
31
Yeast
assimilable
nitrogen
(mg/L)
Between
groups
22171.97
1
22171.97
23.00
0.00
Within
groups
28920.53
30
964.02
Total
51092.50
31
Fermentation rate of wine was associated with
yeast assimilable nitrogen (YAN) value, where an
increase in fermentation rate is associated with a
decrease in the number of YAN (Pereira et al.,
2014). There was a significant difference in the
value of YAN between C and SL. The value of mead
fermentation rate is calculated using Michaelis
Menten equation (Levenspiel, 1999) which was
approached by the linear equation as follows:
1
𝑉
=
1
𝑉𝑚𝑎𝑥
+ (
𝐾𝑚
𝑉𝑚𝑎𝑥
)
1
[𝑆]
(1)
Vmax values (maximum reaction speed) and Km
(Michaelis Menten constant) can be obtained from
the equation and can be seen in Table 4. When
compared, SL had faster fermentation rate than C.
Table 4: Vmax and Km value from mead fermentation.
Vmax (g.ml
-1
.hr
-1
)
Km (g.ml
-1
)
Control
0.035
6.641
Single layer
0.105
23.627
4 DISCUSSION
4.1 Effect of Immobilized Cells on
Alcohol Content (%) and Sugar
Content (Obrix) of Mead
Conversion of sugar into alcohol was an important
process in the fermentation of wine. During mead
fermentation process, alcohol content formed was
related to sugar content. The higher the alcohol
content formed, the lower the sugar content value in
the mead. Total sugar content (
o
Brix) during
16th AFC 2019 - ASEAN Food Conference
194

fermentation was an indicator of yeast’s activity in
fermenting solution (Kaur et al., 2014). When the
value of sugar content decreased, it was due to the
formation of alcohol.
The alcohol content achieved in this research
varied between 8,5-9,5%, where fermentation using
immobilized cells achieved higher alcohol content
and faster fermentation rate than control. This
proved that fermentation using immobilized cells
caused yeast cells to have higher alcohol tolerance
that control. Besides, this difference was due in
immobilized cells, yeast cells were protected from
inhibitors and other unfavourable fermentation
conditions that caused faster development of yeast
cells (Park & Chang, 2000). This result was in
accordance with the research conducted before (Yu
et al., 2007), where the productivity of alcohol in
fermentation was higher in immobilized system
compared to the free cells system because yeast cells
in the immobilized system consumed sugar faster
and efficiently.
4.2 Effect of Immobilized Cells on
Mead Fermentation Rates
Mead fermentation rate is associated with the value
of yeast assimilable nitrogen (YAN), where there
was an increase of fermentation rate associated with
a decrease in the amount of YAN (Pereira et al.,
2014). Fermentation rate depends in the
concentration of the inhibitors such as fatty acids
(hexanoic acid, octanoic acid, decanoic acid),
protein (enzymes), furfural, and
hydroxymethilfurfural (Sroka et al., 2013). High
concentration of inhibitors will inhibit the
fermentation rate. Inhibitors interact synergistically
with high osmotic pressure and an increase in
alcohol content during fermentation.
Thus in this research, mead fermentation using
immobilized cells resulted in a faster fermentation
rate and fermentation time than free cells system
because of the yeast cells were protected from
inhibitors and other unfavourable fermentation
conditions. This result was in accordance with the
research before (Navrátil et al., 2001), where the use
of immobilized cells can increase the rate of
fermentation because yeast cells were protected in
the matrix from adverse environmental conditions
such as pH, temperature, and inhibitors so their
growth were faster. In addition, the reduced
intracellular pH value in immobilized cells increases
the permeability of cytoplasmic membranes with
protons thereby increasing ATP consumption which
cause an increase in glucolytic activity and an
increase in glucose consumption in the medium
(Kourkoutas et al., 2004) thereby increasing the rate
of mead fermentation.
5 CONCLUSIONS
During mead fermentation, there were a decrease in
sugar content and YAN concentration, as well as an
increase in alcohol content. Fermentation using
immobilized cells achieved higher alcohol content
and faster fermentation rate and fermentation time
than control (free cells system).
REFERENCES
German, J. B., & Walzem, R. L. (2000). The Health
Benefits of Wine. Annual Review of Nutrition, 20(1),
561–593.
https://doi.org/10.1146/annurev.nutr.20.1.561
Joshi, V. K., Kumar, V., & Kumar, A. (2012). Physico-
chemical and sensory evaluation of wines from
different citrus fruits of Himachal Pradesh, 2(2), 145–
148.
Kaur, B., Kumar, B., Kaur, N., Sirhindi, G., Silakari, O.,
Garg, N., & Kaur, P. (2014). Role of lactobacillus
fermentum as a starter culture for malolactic
fermentation to improve quality of white wines. World
Journal of Pharmacy and Pharmaceutical Sciences,
3(3), 1687–1712.
Kourkoutas, Y., Bekatorou, A., Banat, I. M., Marchant, R.,
& Koutinas, A. A. (2004). Immobilization
technologies and support materials suitable in alcohol
beverages production: a review. Food Microbiology,
21, 377–397. https://doi.org/10.1016/j.fm.2003.10.005
Levenspiel, O. (1999). Chemical reaction engineering.
Wiley. Retrieved from https://www.wiley.com/en-
us/Chemical+Reaction+Engineering%2C+3rd+Edition
-p-9780471254249
Liouni, M., Drichoutis, P., & Nerantzis, E. T. (2008).
Studies of the mechanical properties and the
fermentation behavior of double layer alginate–
chitosan beads, using Saccharomyces cerevisiae
entrapped cells. World Journal of Microbiology and
Biotechnology, 24(2), 281–288.
https://doi.org/10.1007/s11274-007-9467-7
Mendes-Ferreira, A., Cosme, F., Barbosa, C., Falco, V.,
Inês, A., & Mendes-Faia, A. (2010). Optimization of
honey-must preparation and alcoholic fermentation by
Saccharomyces cerevisiae for mead production.
International Journal of Food Microbiology, 144(1),
193–198.
https://doi.org/10.1016/j.ijfoodmicro.2010.09.016
Navrátil, M., Šturdík, E., & Gemeiner, P. (2001). Batch
and continuous mead production with pectate
immobilised, ethanol-tolerant yeast. Biotechnology
The Role of Single Layer Immobilized Cells in Mead Fermentation Rate
195

Letters, 23(12), 977–982.
https://doi.org/10.1023/A:1010571208324
Park, J. K., & Chang, H. N. (2000). Microencapsulation of
microbial cells. Biotechnology Advances, 18(4), 303–
319. Retrieved from
http://www.ncbi.nlm.nih.gov/pubmed/14538106
Pereira, A. P., Mendes-Ferreira, A., Oliveira, J. M.,
Estevinho, L. M., & Mendes-Faia, A. (2013). High-
cell-density fermentation of Saccharomyces cerevisiae
for the optimisation of mead production. Food
Microbiology, 33(1), 114–123.
https://doi.org/10.1016/j.fm.2012.09.006
Pereira, A. P., Mendes-Ferreira, A., Oliveira, J. M.,
Estevinho, L. M., & Mendes-Faia, A. (2014). Effect of
Saccharomyces cerevisiae cells immobilisation on
mead production. LWT - Food Science and
Technology, 56, 21–30.
https://doi.org/10.1016/j.lwt.2013.11.005
Ramalhosa, E., Gomes, T., Pereira, A. P., Dias, T., &
Estevinho, L. M. (2011). Mead Production. In
Advances in food and nutrition research (Vol. 63, pp.
101–118). https://doi.org/10.1016/B978-0-12-384927-
4.00004-X
Reddy, L. V., Reddy, Y. H. K., Reddy, L. P. A., & Reddy,
O. V. S. (2008). Wine production by novel yeast
biocatalyst prepared by immobilization on watermelon
(Citrullus vulgaris) rind pieces and characterization of
volatile compounds. Process Biochemistry, 43(7),
748–752.
https://doi.org/10.1016/J.PROCBIO.2008.02.020
Roldán, A., van Muiswinkel, G. C. J., Lasanta, C.,
Palacios, V., & Caro, I. (2011). Influence of pollen
addition on mead elaboration: Physicochemical and
sensory characteristics. Food Chemistry, 126(2), 574–
582. https://doi.org/10.1016/j.foodchem.2010.11.045
Snopek, L., Mlcek, J., Sochorova, L., Baron, M.,
Hlavacova, I., Jurikova, T., … Sochor, J. (2018).
Contribution of Red Wine Consumption to Human
Health Protection. Molecules (Basel, Switzerland),
23(7). https://doi.org/10.3390/molecules23071684
Srimeena, N., Gunasekaran, S., & Murugesan, R. (2014).
Optimization of fermentation conditions for producing
Indian rock bee (Apis dorsata) mead using response
surface methodology. Journal of Applied and Natural
Science (Vol. 6). Retrieved from
www.ansfoundation.org
Sroka, P., Satora, P., Tarko, T., Duda-Chodak, A., &
Kepska, K. (2013). Immobilization Of Yeast on
Grapes for Mead Production. Retrieved from
https://www.semanticscholar.org/paper/IMMOBILIZ
ATION-OF-YEAST-ON-GRAPES-FOR-MEAD-
Sroka-
Satora/7d7a5ead42c344a72eac3ff1f70fff6a1bcddc57
Wintersteen, C. L., Andrae, L. M., & Engeseth, N. J.
(2005). Effect of Heat Treatment on Antioxidant
Capacity and Flavor Volatiles of Mead. Journal of
Food Science, 70(2), C119–C126.
https://doi.org/10.1111/j.1365-2621.2005.tb07071.x
Wurz, D. A. (2019). Wine and health: A review of its
benefits to human health. BIO Web of Conferences,
12, 04001. https://doi.org/10.1051/bioconf/
20191204001
Yu, J., Zhang, X., & Tan, T. (2007). An novel
immobilization method of Saccharomyces cerevisiae
to sorghum bagasse for ethanol production. Journal of
Biotechnology, 129(3), 415–420.
https://doi.org/10.1016/j.jbiotec.2007.01.039
16th AFC 2019 - ASEAN Food Conference
196
