
Effect of Soaking Formalin Solution on the Quality in Bean Sprout
Harirat Nimprasert and Varipat Areekul
Faculty of Agro-Industry, King Mongkut’s Institute of Technology Ladkrabang, Ladkrabang, Bangkok, Thailand
Keywords: Bean Sprout, Formaldehyde, Formalin.
Abstract: Formaldehyde is believed to preserve several agriculture produces not only seafood but also fruits and
vegetables. There are news spreading on the illegal adding this compound in bean sprout and other
vegetables but no scientific document has been investigated the effect of formaldehyde on it. Therefore, this
study was focused on the effect of formaldehyde on quality changes of bean sprout during chilled storage.
Bean sprout were soaked in three levels (10, 100 and 500 ppm) of formaldehyde solution for 15 min, rinsed
and stored at 8
o
C for 18 days, the samples were taken every 3 days to evaluate free and total formaldehyde
content, firmness, browning index (BI) and weight loss. It was found that the higher concentration of
soaking, the higher residues were significantly remained in free and total formaldehyde content (p≤0.05).
However, both residuals rapidly decreased and mostly was statistically indifference within 9 days (p>0.05).
After 9 days storage, the BI and weight loss were higher as increasing soaking concentration especially
soaking with 500 ppm formaldehyde solution (p≤0.05). For firmness, there were insignificantly among
untreated and treated samples (p>0.05). In conclusion, soaking with formaldehyde solution could not help
for preserving bean sprout but speedily deteriorate of this produces.
1 INTRODUCTION
Thailand cultivates vast variety of fruits and
vegetables. These nutrition produces contain high
amount in fiber, vitamins and minerals which are
benefits for health especially lower the risk of
intestinal cancer and other disease (Thai Health,
2017). In Southeast Asia, bean sprout is commonly
well-known in many varieties of soup, salad, stirred
fried vegetables and side dishes (Liu, 2008). It is
made either from soybean or mung bean by
germinating in the dark. It is rich in vitamins,
minerals, and phytochemicals (Guo et al., 2012).
Unfortunately, this sprout has a short period of shelf-
life which is about two weeks in a refrigerator
(Fresher Pantry, 2017). This produce is easily
infected by mold and bacteria and rapidly become
rotten because it is cultivated under conditions of
wetness and darkness (Hur & Koh, 2002).
Therefore, the agriculturists may use some
chemicals for extending shelf-life.
The rumor of using illegal chemicals in foods is
widely spread in the country. One of them is
formaldehyde (or methanol) or generally known as
formalin. This chemical is a precursor to produce the
resin for particle bonding or coating in several
industrial applications for example, textile and
furniture. Formaldehyde is a hazardous chemical for
human. At low volume of formaldehyde in food, it is
metabolized to formate and CO
2
and then excreted
from body but the metabolite may be toxic to liver,
kidneys, heart and nervous system. However, at high
volume of formaldehyde, it is collected in formic
acid form which can be toxic with cells, tissues,
digestive system and may result in death (Silpakorn
University, 2017; Changsap, 2015). The minimum
risk level (MRL) for oral exposure to formaldehyde
is 0.3 mg/kg/day which is derived for intermediate-
duration exposure and an MRL of 0.2 mg/kg/day is
derived for chronic-duration exposure (ATSDR,
1999). The United States Environmental Protection
Agency (US EPA) advises the recommended daily
intakes (RDI) for formaldehyde is 0.2 mg/kg/day
(Yeh et al., 2013). In addition, this chemical is also
recognized as biocide to fix animal organs and
bodies. Therefore, the agriculturists who lack of
knowledge may misuse to preserve meats, fruits and
vegetables. This chemical does not be legally
allowed to add into any agricultural produces and
foods. However, formaldehyde is naturally presented
in fruits and vegetables when plant cell wall
undergoes to de-methyl-esterified process. It produces
Nimprasert, H. and Areekul, V.
Effect of Soaking Formalin Solution on The Quality in Bean Sprout.
DOI: 10.5220/0009981700002964
In Proceedings of the 16th ASEAN Food Conference (16th AFC 2019) - Outlook and Opportunities of Food Technology and Culinary for Tourism Industry, pages 227-231
ISBN: 978-989-758-467-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
227

methanol which convert to formaldehyde by the
catalase-H
2
O
2
system, cytochrome P450 (CYP2E1)-
mediated oxidation and the alcohol dehydrogenase 1
(ADH1) class of enzymes (Dorokhov et al., 2015).
The previous studies revealed various amounts of
formaldehyde in a variety of fruits and vegetables.
Formaldehyde content in cauliflower was reported at
5.94 ppm using HPLC procedure (Wahed et al.,
2016) and 26.9 ppm using colorimetric method
(CFS, 2009). Some fruits and vegetables were also
reported high formaldehyde content for example,
apple (17.3 ppm) (Tsuchiya et al., 1975). However,
the high amount of formaldehyde may contain in the
fresh produces and cause adverse effect for human
(Yuyong, 2016). In addition, there is controversial
on the effect of formaldehyde in fruits and
vegetables. The previous study revealed the
formaldehyde could extend shelf-life of mushrooms
while it presented negative effect on fruits and
vegetables (Antora et al., 2018). The objective of
this study was to investigate the effect of soaking
formaldehyde solution on the quality in bean sprout
during chilled storage.
2 MATERIALS AND METHODS
2.1 Chemicals
Formaldehyde solution (36-38%) was purchased
from GPO (Thailand). Trichloroacetic acid (TCA)
was bought from Fisher Scientific (England).
Phosphoric acid was obtained from MERCK
(Germany). Ammonium acetate was purchased from
LOBA Chemie (India). Acetic acid and Potassium
hydroxide (KOH) was bought from SIGMA-
ALDRICH (United States). Acetylacetone was
purchased from CARLO ERBA (New Zealand).
2.2 Sample Preparation
First, the Bean sprout was purchased from the
Amornphan village market, Bangkok, Thailand.
Next, four different concentrations of formaldehyde
solution (0, 10, 100 and 500 ppm) were prepared by
dilution with distilled water. Each sample about 200
g was soaked in 1L formaldehyde solution for 15
min. After that, the sample was rinsed with tap
water, drained and put in to HDPE bag. All samples
were kept in refrigerator at 8 °C and were sampling
every 3 days for analysis.
2.3 Methods
2.3.1 Determination of Weight Loss
Every sample was weighed before and after storage
by 2 digits analytical balance. The weight loss
percentage was calculated by using the following
formula:
%ℎ=
−
× 100
(1)
2.3.2 Determination of Browning Index
The sample of each treatment was tightly arranged
in area of 4 x 6 cm. After that, it was determined L
*
,
a
*
and b
*
by Hunter Lab (Color Quest XE, USA)
which, the reflectance specular excluded (RSEX)
mode, D65 of illuminant and 10° of observer was set
for this operation (Palou et al., 1999).
The browning index was calculated by using the
following formula:
=
[100 ×
(
− 0.31
)
]
0
.172
(2)
=
[
∗
+
(
1.75 ×
∗
)
]
[
(
5.645 ×
∗
)
+
[
∗
−(3.012 ×
∗
)
(3)
2.3.3 Determination of Firmness
The samples were investigated with a Texture
Analyzer (TA-XT Plus, UK) couple with needle
probe which, the compression mode, 10 g of contact
force and 2 mm/sec of test speed was set for this
operation (Paciulli et al., 2015).
2.3.4 Determination of Free and Total
Formaldehyde Contents
For free formaldehyde extraction, 5 g of minced
sample was mixed with 30 ml of 5% trichloroacetic
acid and homogenized at a speed of 11,000 rpm for
2 min. Next, the homogenate was filtered with the
Whatman No. 4 filter paper. Then, the filtrate was
adjusted to pH of 6.0-6.5 using 1 N KOH and was
made up to a final volume of 50 ml with distilled
water. This made-up volume filtrate was used for the
determination.
For total formaldehyde extraction, 20 g of
minced sample was mixed with 10 ml of 10%
phosphoric acid and 200 ml of distilled water. Next,
the mixture was homogenized with a homogenizer at
a speed of 11,000 rpm for 2 min. Then, the
homogenate was transferred to distillation flask and
the distillation was conducted for approximately 1
16th AFC 2019 - ASEAN Food Conference
228
hour or until the distillate of around 15 ml was
obtained. The distillate was used for the
determination.
For formaldehyde determination, 3 ml of filtrate
or distillate was mixed with 3 ml of Nash reagent
(0.2% Acetylacetone; 0.1 M Acetic acid; 3.89 M
Ammonium acetate). After that, the mixture was
reacted at 60 °C in the water bath for 15 min and
cooled with running water. Finally, the sample was
measured by Spectrophotometer (Evolution 201,
USA) at 412 nm of absorbance and the
formaldehyde content was calculated from the
standard curve prepared using standard
formaldehyde solution ranging 0-10 ppm (Sochaya
& Soottawat, 2013).
3 RESULTS AND DISCUSSIONS
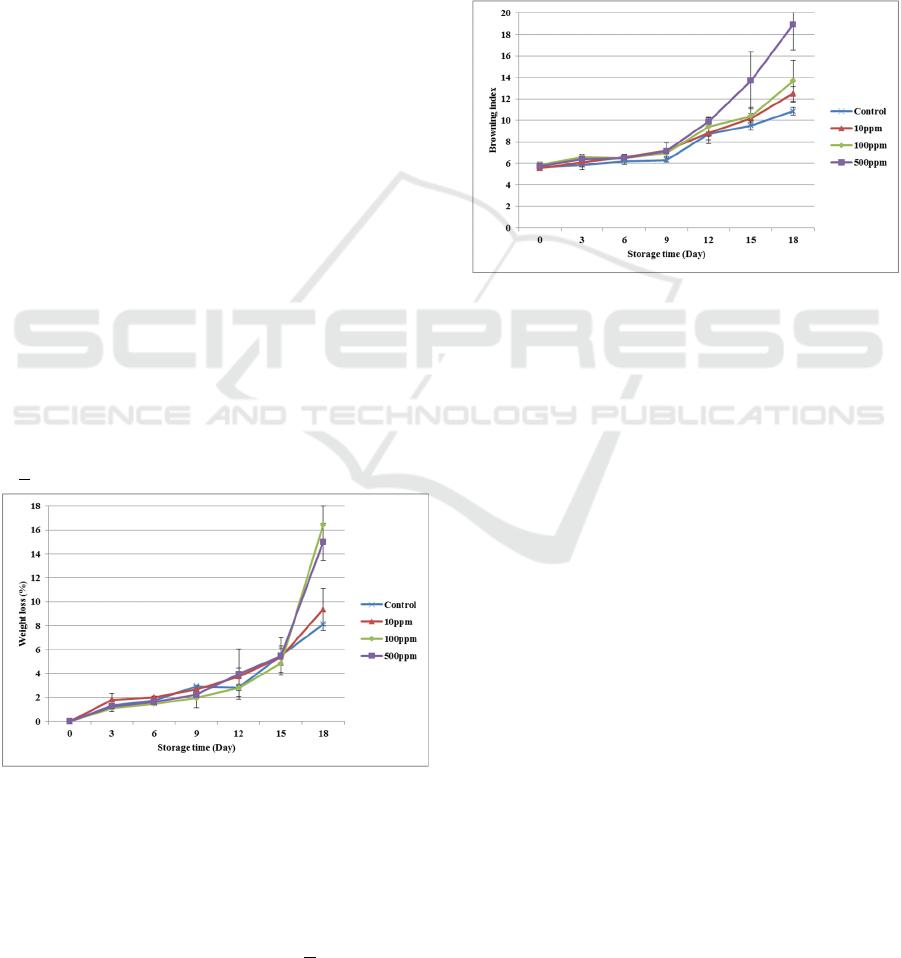
The weight loss of treated sample during chilled
storage is shown in Figure 1. During first 15 days of
storage, the weight loss in samples continuously
increased and were insignificantly among untreated
and treated samples (p>0.05). After that, during 15
to 18 days, the weight loss in sample soaked with
100 and 500 ppm formaldehyde solution was rapidly
increased and much higher than other samples
especially control. Weight loss occur when plant
transpire for heat transferring from respiration
(Siripanich, 2006). This result indicated that soaking
with formaldehyde could not extend the shelf-life
but it accelerated the deterioration of bean sprout
(p<0.05).
Figure 1: Change of weight loss percentage for
formaldehyde treated bean sprout graph.
The result of change in BI is presented in Figure
2. For the first 9 days of storage, BI slightly
increased after that, rapidly increased. The treated
sample at higher concentration were significantly
higher BI compared with control (p<0.05). During
storage, plant slightly produce ethylene which is
speedily respiration rate and browning reaction
(Siripanich, 2006). Soaking with formaldehyde
solution, the formaldehyde residues may be corroded
and oxidized to formic acid (Val Tech, 2014)
causing cell lysis. The enzyme such as polyphenol
oxidase will react with the substrate resulting in
formation of browning compounds and,
subsequently, increasing BI value. The result also
confirmed that soaking with formaldehyde is
speeding the deterioration of bean sprout. The final
sentence of a caption must end with a period.
Figure 2: Change of browning index for formaldehyde
treated bean sprout graph.
The firmness of all treated samples and control
were insignificantly different (p>0.05). However, all
samples were slowly decreased when the storage
time increased (p≤0.05). This phenomena is a
natural deterioration of plant caused by the change
of enzymatic pathways activation (Swieca & Dziki,
2015) and mechanism of cell wall will destroy
pectin that is structure of cell wall when plant cell
develop and grow (Dorokhov et al., 2015).
The free formaldehyde content in sample as
shown in Table 1. The soaked with water (Control)
was 0.58 ppm. After soaking in the formaldehyde
solution, the treated samples contained higher free
formaldehyde contents compared with control (0
ppm). As soaking the samples in higher
concentrations, the formaldehyde contents in the
samples were significantly higher (p≤0.05). This
result indicated that the compound can diffuse in the
plant tissue. It was noted that the intense of
formaldehyde smell was so strong after treating the
bean sprout at the concentration of 100 and 500 ppm
and could be noticed at the first sight. During the
chilled storage, the free formaldehyde content in
control slightly increased at the first 3 days of
storage from 0.58 to 0.79 ppm and after that, it
remained constant until the end of storage. For
Effect of Soaking Formalin Solution on The Quality in Bean Sprout
229
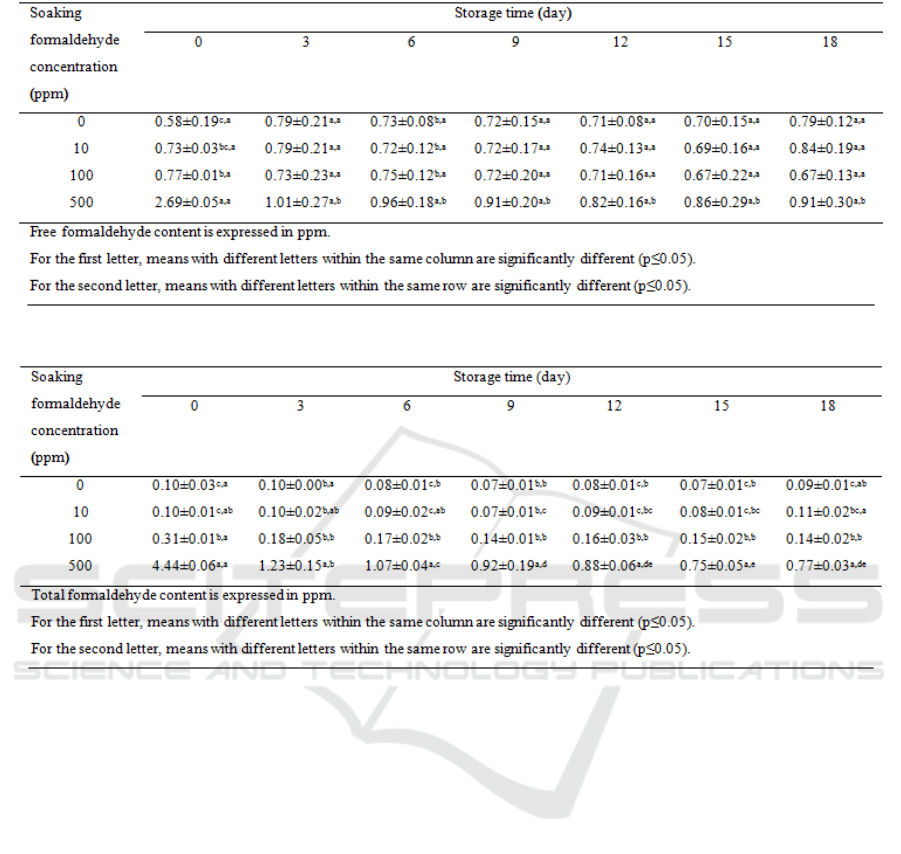
Table 1: Change of free formaldehyde content in treated bean sprout.
Table 2: Change of total formaldehyde content in treated bean sprout.
sample soaked with 10 and 100 ppm, the free
formaldehyde contents tended to be constant along
with the storage period. However, for the sample
soaked with 500 ppm, the free formaldehyde content
significantly decreased from 2.69 to 1.01 ppm at the
first 3 days storage which indicated that the
formaldehyde could be volatized at the chilled
temperature. However, the free formaldehyde
content seemed to remain constant (0.81-0.95 ppm)
for the rest of storage period same as other samples.
The final sentence of a caption must end with a
period.
For total formaldehyde content as shown in
Table 2. In control, we found that formaldehyde
contents were insignificantly changed during storage
ranged from 0.07 - 0.10 ppm. Surprisingly, the
amounts of total formaldehyde contents were lower
than those of free formaldehyde content. It indicated
that the condition of steam distillation may cause
loss of formaldehyde during extraction. After
soaking with formaldehyde solution, all samples
except soaked at 10 ppm had significantly higher
amount of total formaldehyde contents (p≤0.05).
These results agreed with the result obtained from
those of free formaldehyde content.
4 CONCLUSION
In conclusion, bean sprout soaked with
formaldehyde solution prior to storage could not
help for preservation. Moreover, it was speedily the
deterioration especially soaking at high
concentration. Therefore, this result was scientific
proof that the belief in its preservation benefit in
bean sprout was wrong.
ACKNOWLEDGEMENTS
The author would like to thank to faculty of Agro-
industry, KMITL for financial support this study and
Dr.Rachit Suwapanich for valuable guidance.
16th AFC 2019 - ASEAN Food Conference
230

REFERENCES
Agency for Toxic Substances and Disease Registry
(ATSDR). 1999. Toxicological Profile for
Formaldehyde. United States Department of Health
and Human Services.
Antora, R. A., Hossain, M. P., Monira, S. S. U., Aziz, M.
G. 2018. Effect of Formaldehyde on Some Post-
Harvest Quality and Shelf-Life of Selected Fruits and
Vegetables. Journal of Bangladesh Agricultural
University, Volume 16 (1), pp. 151-157.
Centre for Food Safety (CFS). 2009. Formaldehyde in
Food. [online]. Available at: http://www.cfs.gov.hk/
english/programme/programme_rafs/programme_rafs_
fa_02_09.html. [Accessed 27 August 2017].
Changsap, B. 2015. Formaldehyde/Formalin Nearly
Dangerous. Journal of Science and Technology of
Huachiew Chalermprakiet University, Volume 1 (1),
pp. 97-109.
Dorokhov, Y. L., Shindyapina, A. V., Sheshukova, E. V.,
Komarova, T. V. 2015. Metabolic Methanol:
Molecular Pathways and Physiological Roles.
Physiological Reviews, Volume 95 (2), pp. 603-644.
Fresher Pantry. 2017. Shelf-life of Bean Sprouts. [online].
Available at: https://fresherpantry.com/vegetables/
how-long-do-bean-sprouts-last/. [Accessed 28 August
2019].
Guo, X., Li, T., Tang, K., Liu, R. H. 2012. Effect of
Germination on Phytochemical Profiles and
Antioxidant Activity of Mung Bean Sprouts (Vigna
radiata). Journal of Agricultural and Food Chemistry,
Volume 60 (44), pp. 11050-11055.
Hur, J. S. & Koh, Y. 2002. Bactericidal Activity and
Water Purification of Immobilized TiO
2
Photocatalyst
in Bean sprout Cultivation. Biotechnology Letters,
Volume 24, pp. 23-25.
Liu, K. S. 2008. Food Use of Whole Soybeans. [online].
Available at: https://www.sciencedirect.com/topics/
agricultural-and-biological-sciences/bean-sprouts.
[Accessed 28 August 2019].
Paciulli, M., Ganino, T., Pellegrini, N., Rinaldi, M.,
Zaupa, M., Fabbri, A., Chiavaro, E. 2015. Impact of
the Industrial Freezing Process on Selected
Vegetables-Part I. Structure, Texture and Antioxidant
Capacity. Food Research International, Volume 74,
pp. 329-337.
Palou, E., Malo, A. L., Canovas, G. V. B., Chanes, J. W.,
Swanson, B. G. 1999. Polyphenoloxidase Activity and
Color of Blanched and High Hydrostatic Pressure
Treated Banana Puree. Journal of Food Science,
Volume 64 (1), pp. 42-45.
Silpakorn University. 2017. Formalin: Chemical
Contamination in Food. [online]. Available at:
http://oldweb.pharm.su.ac.th/chemistry-in-
life/d023.html. [Accessed 1 December 2017].
Siripanich, J. 2006. Physiology and Postharvest
Technology of Vegetables and Fruits. Nakhon Pathom:
Delectable Publications.
Sochaya, C. & Soottawat, B. 2013. Effect of
Formaldehyde on Protein Cross-Linking and Gel
Forming Ability of Surimi from Lizardfish Induced by
Microbial Transglutaminase. Food Hydrocolloids,
Volume 30, pp. 704-711.
Swieca, M. & Dziki, U. G. 2015. Effects of Sprouting and
Postharvest Storage Under Cool Temperature
Conditions on Starch Content and Antioxidant
Capacity of Green Pea, Lentil and Young Mung Bean
Sprouts. Food Chemistry, Volume 185, pp. 99-105.
Thai Health. 2017. 6 management of eat fruits and
vegetables for safety. [online]. Available at:
http://www.thaihealth.or.th/Content/38872-รุก%206%
20มาตรการขับเคลื่อนก
ิ
น%20"ผักผักผล%"20ปลอดภัย%20400%20ก
รัม.html. [Accessed 20 December 2017].
Tsuchiya, K., Hayashi, Y., Onodera, M., Hasegawa, T.
1975. Toxicity of Formaldehyde in Experimental
Animals-Concentrations of the Chemical in the
Elution from Dishes of Formaldehyde Resin in Some
Vegetables. Keio Journal of Medicine, Volume 24 (1),
pp. 19-37.
Val Tech. 2014. Formaldehyde, 37% w/w Safety Data
Sheet. Federal Register, Volume 77 (58).
Wahed, P., Razzaq, M. A., Dharmapuri, S., Corrales, M.
2016. Determination of Formaldehyde in Food and
Feed by an In-House Validated HPLC Method. Food
Chemistry, Volume 202, pp. 476-483.
Yeh, T. S., Lin, T. C., Chen, C. C., Wen, H. M. 2013.
Analysis of Free and Bound Formaldehyde in Squid
and Squid Products by Gas Chromatography-Mass
Spectrometry. Journal of Food and Drug Analysis,
Volume 21, pp. 190-197.
Yuyong, S. 2016. Formalin-Formaldehyde. [online].
Available at: http://www.pharmacy.mahidol.ac.th/th/
knowledge/article/322/ฟอร์มาลิน-ฟอร์มัลดีไฮด์/. [Accessed 1
December 2017].
Effect of Soaking Formalin Solution on The Quality in Bean Sprout
231
