Difference in Characteristic of Beetroot (Beta Vulgaris L.) Biomass
through Microfiltration Technique to Prevent Natural Infection
Agustine Susilowati, Aspiyanto, Hani Mulyani, Puspa D. Lotulung and Yati Maryati
Research Center for Chemistry, Indonesian Institute of Sciences (LIPI), 452 Building Kawasan PUSPIPTEK, Serpong,
South Tangerang, 15314, Banten, Indonesia
Keywords: Beetroot (Beta Vulgaris L.), Betacyanin, Polyphenol, Microfiltration (MF), Antibacteria.
Abstract: Fermentation of beetroot (Beta vulgaris L.) by using Kombucha culture produces biomass as a source of
polyphenol, particularly betacyanins having potential use as prevention of natural infection. This experiment
activity aims to find out separation optimization of beetroot biomass through microfiltration (MF) technique
and characteristic of retentate and permeate as a result of MF on composition, monomer domination of
polyphenol and betacyanin compounds, particle size distribution, and ability of anti-bacteria to prevent natural
infection on Staphylococcus aureus Ina CC-B4 and Escherichia coli Ina CC-B5. Separation was conducted
at room temperature, strirrer rotation speed (SRS) 200, 300 and 400 rpm, and fixed transmembrane pressure
(TMP) 40 psia. Result of experiment work showed that based on betacyanin, the best treatment is achieved at
SRS 300 rpm yielding retentate and permeate with composition of betacyanin of 0.31 and 0.16 µg/mL, total
polyphenol of 0.55 and 0.37%, total acids 1.00 and 0.72%, reducing sugars 16.33 and 28.97 mg/mL, total
solids 10.05 and 9.38%, dissolved protein 18.50 and 24.35 mg/mL, particle size 3002.4 and 1962.0 nm,
particle index 0.468 and 0.370, respectively. Identificaion on betacyanin and gallic acid monomers as total
polyphenol at retentate is dominated by monomer with molecular weight (MW) 551.13, 551.53, and 171.02,
171.24 and 171.76 Dalton (Da.), meanwhile permeate is dominated by monomer with MW 551.16 and 171.23,
171.72 Da. and relative intensities 100%, respectively. Ability to inhibit the growth of bacteria of
Staphylococcus aureus Ina CC-B4 and Escherichia coli Ina CC-B5 is achieved by retentate with zone area of
inhibiting 11 and 10 mm, respectively. In this optimum condition, MF membrane technique was able to retain
betacyanin and total polyphenol as anti-bacteria compounds in retentate with 416% (4.16-folds) and 83.33%
and pass them in permeate 166% (1.67-folds) and 23.33% compared to prior to process (feed).
1 INTRODUCTION
Fermentation of beetroot (Beta vulgaris L.) by
Kombucha cultures generates biomass with
beneficial as source of polyphenol and natural organic
acids as functional food. Beetroot is the deep violet
tubers by betacyanin pigment (Sarkar et al, 2015).
being having potential uses as antioxidant, anti-
cholesterol, natural detoxification, and prevention of
hypertension. Betacyanin including in betalain
pigment can be categorized as phenolic compound
(Coultate, 2009). Betalain pigment is synthesized
from amino acid of tirosyne containing nitrogen
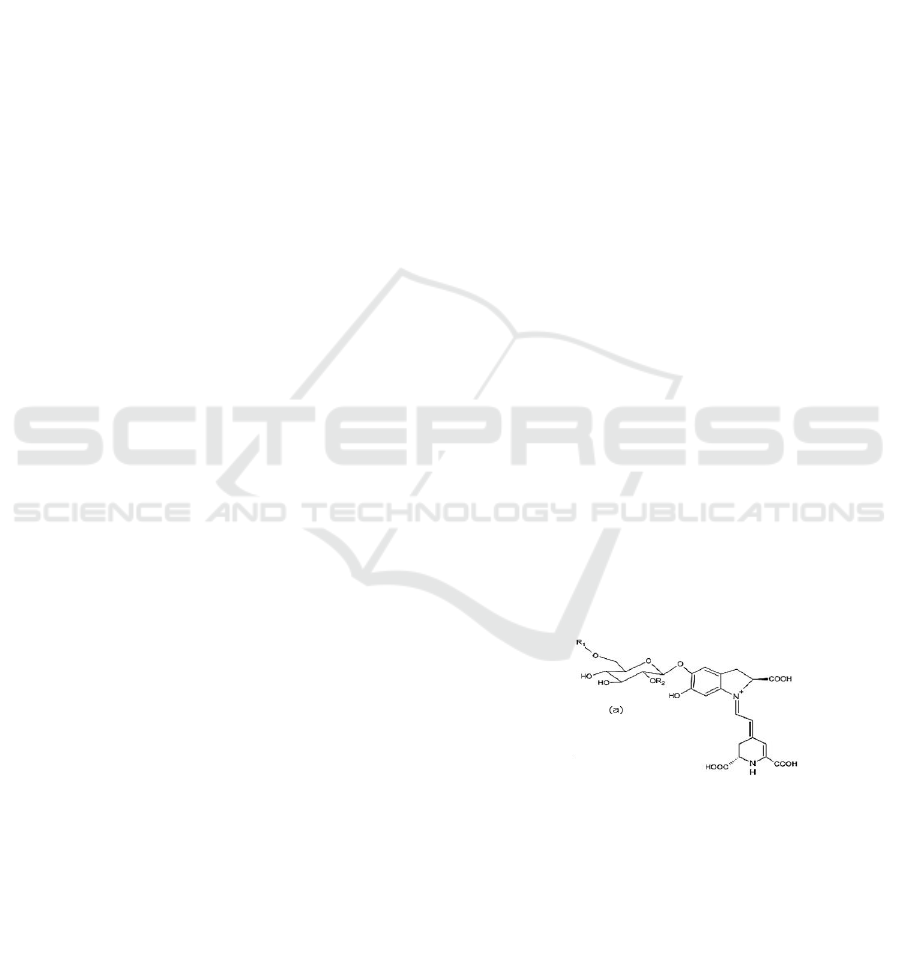
element (Pavoković & Krsnik-Rasol, 2011) as shown
in Fig. 1. Betacyanin has a property of exclusive-
mutual on anthocyanin pigment. In other words, both
these pigments are not found together with
anthocyanin (Cai et al, 2005; Grotewold, 2009).
Figure 1: Chemical structure of betacyanin.
Recovery of betacyanin as biomass of fermentation
by Kombucha culture is related to microbe activity in
degrading sucrose into glucuronic acid, acetic acid,
malic acid (Malbaša et al, 2008) and other
compounds, such as betacyanin being possibility has
beneficial to prevent infection of Stapylococcus
aureus Ina CC-B4 and Escherichia coli Ina CC-B5
through chemical reactions assimilative and
dissimulative during fermentation (0 –12 days). In its
function as natural detoxification, they have the
Susilowati, A., Aspiyanto, ., Mulyani, H., Lotulung, P. and Maryati, Y.
Difference in Characteristic of Beetroot (Beta Vulgaris L.) Biomass through Microfiltration Technique to Prevent Natural Infection.
DOI: 10.5220/0009981000002964
In Proceedings of the 16th ASEAN Food Conference (16th AFC 2019) - Outlook and Opportunities of Food Technology and Culinary for Tourism Industry, pages 301-311
ISBN: 978-989-758-467-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
301

ability to prevent infection-related to the role of
betacyanin as phenolic compounds.
Betacyanin in retentate (concentrate) and
permeate as a result of MF on fermented beetroot
suspensions have potential use as prevention of
natural infection being generated via separation by
means of MF membrane. MF membrane is able to
sieve particles with particles in the ranges larger than
0.1 to 10 μm at trans membrane pressure (TMP) 5 –
50 psia (0.3 – 3.3 bar), so that it retains fat particle (1
– 10 μm), protein (0.04 – 2 μm), polysaccharides (8 –
20 μm) on the membrane surface. Particles with size
in the ranges of smaller than 0.1 to 10 μm will pass
freely as permeate, particularly colour pigment, such
as anthocyanin, chlorophyll, β-carotene (0,001 – 0.1
μm), organic acids, amino acids, vitamin and mineral
(0.001 – 0.1 μm) (Raja et al 2003; Field et al 1993
and Mulder, 2012). The presence of fouling becomes
a possibility when compounds retained on the
membrane surface are affected by type of membrane,
characteristic of biomass, operation condition
(temperature, flow rate or stirrer rotation speed, trans
membrane pressure, TMP) (Field, 1993; Mulder
2012). MF membrane being made of fluoropolymer
has specification with pores size in the ranges of 0.15
µm to 0.65 µm and is able to operate at TMP in the
range of 1 bar to 10 bar (module scale) or TMP in the
ranges of 20 psia to 40 psia (dead-end stirred
ultrafiltration cell, DESUFC), flow rate in the ranges
of 3.5 L/minute to 15 L/minute (module scale) or
stirrer rotation speed (SRS) in the ranges of 200 rpm
to 400 rpm (DESUFC), temperature in the ranges of
0 to 60 °C and pH ranges of 1 to 11 (Millipore, 2008),
so that assessment on the effect of SRS at fixed TMP
enables to get optimization of betacyanin.
Characterization of betacyanin compounds in
fermented beetroot biomass is performed by
identifying betacyanin compounds using Liquid
Chromatography coupled with Mass Spectrometry
(LC-MS). By using LC-MS, MWs range of purified
polyphenol compound could be known and estimated
so that the functional property of betacyanin could be
declared. Chromatography separates mixtures of
molecular based on difference in migration speed and
molecules distribution in stagnant phase (adsorbent)
and moved phase (eluent), while mass spectrometry
ionizes analytes based on the principle of electrospray
ionization (ESI) to the gas phase (fine aerosol)
(Onggo et al, 2009). LC-MS will separate betacyanin
monomer and identify MW (Eichhorn, 2001),
whereas particle size and particle size distribution can
be known by means of Particle Size Analyzer (PSA)
(Dapkunas et al, 2001); Retsch-Technology GmbH,
2019). In progress, the ability of anti-bacteria activity
of Staphylococcus aureus Ina CC-B4 and Escherichia
coli Ina CC-B5 in permeate and concentrate as a
result of separation of target and desired compounds
from fermented beetroot biomass through MF
membrane was performed via analysis of
microbiology covering inhibition power to the growth
of both types of microbes. Staphylococcus aureus is
a Gram-positive, a facultative anaerobe, round-
shaped bacterium with diameter size (Ø) ranging 0.8
– 1.0 µm, and the optimum growth at 37 ºC. S. aureus
can become an opportunistic pathogen, being a
common cause of skin infections including abscesses,
respiratory infections associated with various
pathologies condition, such as sinusitis, pneumonia,
meningitis, arthritis, and food poisoning (Madigan et
al, 2008). Meanwhile, Escherichia coli known E. coli
is a non-spore-forming, Gram-negative, facultative
anaerobic, rod-shaped, and coliform bacterium of the
genus Escherichia which is harmless, but some
serotypes can cause serious food poisoning and
contamination in their hosts because it produces
exotoxin, which can stop synthesis of protein
(Levinson, 2008). Characteristic of biomass,
concentrate, and permeate on the domination of
betacyanin monomer, particle size, and particle size
distribution are enabled to affect on ability of their
materials in inhibiting the growth of Staphylococcus
aureus Ina CC-B4 and Escherichia coli Ina CC-B5.
This matter enables to get the functional property as
anti-bacteria being can prevent infections of both
microbes.
The aim of this experiment work was to find out
process optimization of MF based on difference in
SRS on composition, particularly total polyphenol
and betacyanin, monomer domination of polyphenol
and betacyanin, particle size, particle size
distribution, and ability to inhibit the growth of
bacteria of Staphylococcos aureus Ina CC-B4 and
Escherichia coli Ina CC-B5.
2 MATERIALS AND METHODS
2.1 Materials and Equipments
The materials used for this experiment activity were
fresh beetroot tuber procured from local market,
Kombucha culture (Research Center for Chemistry –
LIPI), sucrose, Staphylococcus aureus Ina CC–B4
and Eschericiae coli Ina CC-B5 bacteria (Research
Center for Biology – LIPI), commercial composite
fluoropolymer MF membrane with pore size of 0.15
µm (FSM-0.15-PP, Alfa Laval, Nakskov, Denmark)
(according to the manufacturer), and chemicals with
16th AFC 2019 - ASEAN Food Conference
302

analytical grade quality used for preparation and
analysis purposes, such as gallic acid.
Meanwhile, main equipments applied in this
experimental activity were digital balance (Fujitsu,
Japan), peeler (local product), autoclave (Cheng Yi,
LS-50 L, China), blender (National, local), sieve of
60 mesh (Retsch, Germany), incubator (local), series
of fermentation system in laboratory scale (local),
stopwatch (Hanhart Profil2, Germany), magnetic
stirrer (HI 303 N, HANNA Instrument, Japan),
pressure gauge of technical nitrogen (Fisher
Scientific Company, England), cylinder tank for
technical nitrogen (local), Dead-End Stirred
Ultrafiltration Cell (DESFC) (MILLIPORE Model
8200, U.S.A.), UV-vis Spectrophotometer (Model
RF-550, Shimadzu, Japan), Liquid Chromatography-
tandem Mass Spectrometry (Mariner
Biospectrometry) equipped with LC (Hitachi L 6200)
[2] and Particle Size Analyzer (PSA) with SZ 100-
nano Partica Dynamic Light Scattering (DLS) system
(Beckman Coulter LS 100 Q, U. S. A) (Dapkunas et
al, 2001).
2.2 Experimental Design
Experiment activity was conducted using beetroot
biomass fermented by Kombucha culture. Beetroot
biomass was concentrated through a MF membrane
fitted in DESUFC at room temperature, SRS 200, 300
and 400 rpm, and TMP 40 psia for 30 minutes.
Analysis was conducted on initial material of biomass
(feed), retentate, and permeate covers total solids
(Gravimetric method) (AOAC, 2019), total acids
(titratable acids method), total polyphenol (Folin-
Denis or Folin-Ciocalteu method) (Liu, 2006),
dissolved protein (Lowry method)(Lowry, 1951), and
betacyanin (Wong et al, 2015). Aliquot from the best
condition treatment was performed by identifying
betacyanin by means of LC-MS (Eichhorn, 2001)
and anti bacteria activity of Staphylococcos aureus
Ina CC-B4 and Escherichia coli Ina CC-B5 through
investigation of inhibition zone (Bell et al, 1984),
particle size distribution through PSA (Dapkunas et
al, 2001). Process and analysis were performed in
duplicate. Data were processed in this description
based on result of average analysis.
2.3 Procedure
A series of the process initiated by preparing
inoculum of fermented beetroot covers sorting,
cutting of beetroot, blanching at 80 ºC for 5 minutes,
pulverizing by adding clean water at ratio ranged
from 1 to 4, and filtering via 60 mesh in order to get
filtrate. Further, filtrate was autoclaved at 90 – 95 °C,
added sucrose 10% (w/v, filtrate), cooled, inoculated
with Kombucha culture 10% (v/v, filtrate), and
fermented in closed container in darkroom so that it
is produced inoculum of fermented beetroot.
Fermentation process was conducted with similar
initial step, however process of pulverizing beetroot
was carried out by adding clean water at ratio of
beetroot/water (1 : 8, w/w), sucrose 10% (w/v filtrate
of beetroot) and inoculum of fermented beetroot 10%
(v/v filtrate of beetroot), and fermentation of mixture
of pulverized beetroot, sucrose and inoculum of
fermented beetroot was performed in closed container
in darkroom at room temperature for 12 hours so that
it is generated biomass of fermented beetroot. This
recovery of biomass of fermented beetroot is used as
feed in purifying by means of MF membrane.
Separating and/or concentrating biomass of
fermented beetroot was conducted through fitted in
DESMFC mode with a capacity of 180 mL
(laboratory scale). Empty DESMFC was filled by
biomass of fermented beetroot, flown by nitrogen gas
at a pressure of 40 psia, and stirred at room
temperature, SRS of 200 rpm and TMP of 40 psia for
± 0 (initial process) and 30 minutes
(Millipore. 2008).
Both permeate passing across MF membrane and
remained retentate were collected in small beaker
glasses and analysed. After used, the membrane was
washed with distilled water. This procedure is
conducted according to experimental design.
3 RESULTS AND DISCUSSION
3.1 Characteristic of Materials
Composition of beetroot before and after
fermentation process was tabulated in Table 1, in
which it had been appeared that fermentation process
generates biomass with composition of dominant on
polyphenol in its function as prevention of natural
infections. The fermentation process increases
organic acids and reducing sugars, but decreases
almost the whole components. Increasing both these
components are caused by activity of Kombucha
culture degrading sucrose and produce ethanol
followed by oxidizing ethanol to acetaldehyde and
then generate acetic acid. Accumulation from each
metabolite will form organic acids, such as
glucorunic acid, acetic acid, malic acid, butyric acid,
and other components as antioxidant. Betacyanin is
able to bind sugars so that total acids is yielded higher
compared to prior to fermentation. Betacyanins are
able to bind several molecules of sugars (glucose,
Difference in Characteristic of Beetroot (Beta Vulgaris L.) Biomass through Microfiltration Technique to Prevent Natural Infection
303
fructose, galactose, arabinose and several other
molecules of sugar, such as disaccharides and
polysaccharides, and flavonoid compound present in
polyphenol group (Welch et al, 2008) which have
possibility activity of anti bacteria. Declining total
solids, polyphenol, betacyanin, dissolved protein, and
pH are occurred by increasing total acids causing its
occurrence of dilution of the whole components by
chemical reaction on biomass.
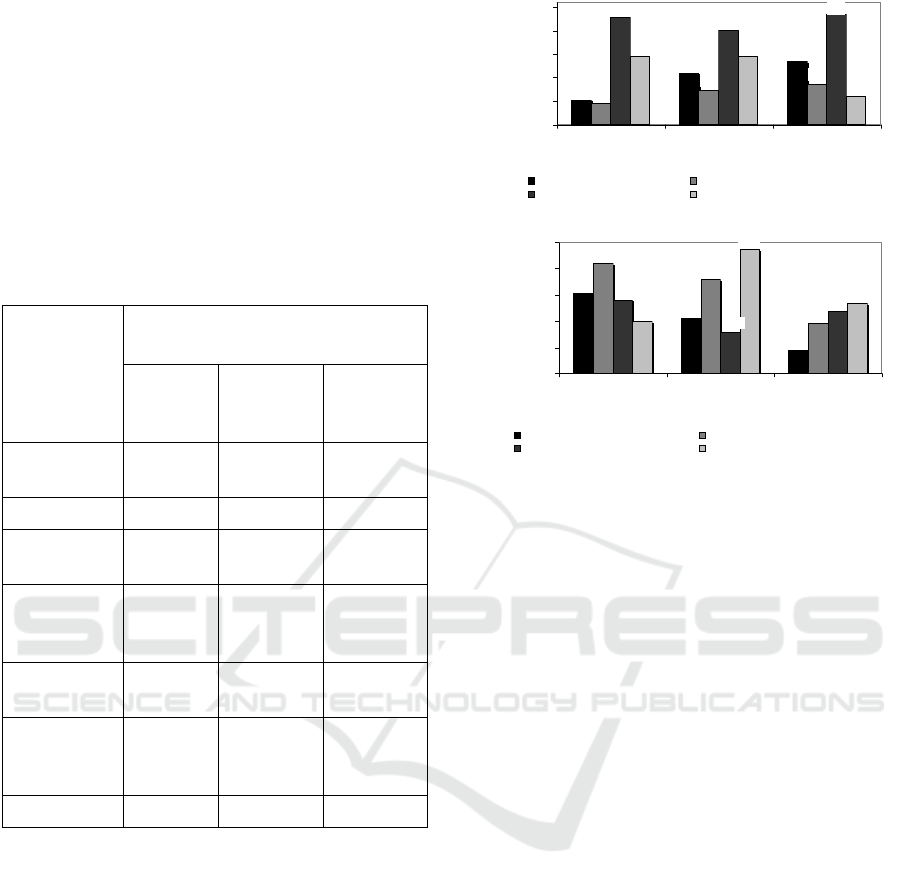
Table 1: Materials composition as a result of purification of
biomass of fermented beetroot through MF membrane for
natural anti-bacteria.
Components
Kind of materials
Pulp of
beetroot*
Fermented
beetroot 0
day*
Fermented
beetroot
12 day*
Betacyanin
(µg/mL)
0.43 0.45 0.060
Polyphenol 0.45 0.43 0.30
Total acids
(%)
0.06 0.16 0. 50
Reducing
sugars
(mg/mL)
9.45 14.66 17.33
Total solids
(%)
10.07 10.22 10.02
Dissolved
protein
(mg/mL)
21.41 21.20 15.36
pH 6.38 4.32 3.64
Legend: *ratio of beetroot/water (1: 8, w/w),
**fermentation for 0 day, ***fermentation for 12 days.
3.2 Influence on Process Condition of
MF Membrane on Composition
3.2.1 Total Polyphenols (%), Total Acids
(%), Dissolved Protein (mg/mL) and
Reducing Sugars (mg/mL)
Separation process of biomass of beetroot at SRS 200,
300 and 400 rpm, and TMP 40 psia for 30 minutes
generates retentate (concentrate) and permeate
(extract) as clear liquid by degrading color from
magenta to clear red. Increasing SRS is able to retain
total polyphenols and total acids more much on the
membrane surface compared to them passing in
permeate, as shown in Fig 2a.
(a)
(b)
Figure 2: Effect of SRS at TMP 40 psia on (a) total
polyphenols and total acids, and (b) dissolved protein and
reducing sugars contents in retentate and permeate as a
result of MF of fermented beetroot suspensions.
SRS becoming more and higher will increase total
polyphenols in concentrate. This reason is possibily
caused by its formation of ‘cake’ due to decreasing
water mass passing through membrane so that it
inhibits total polyphenol to pass through membrane
(Mulder, 2012). Particle size of polyphenol
compounds (MW between 200 and 600 Da.) (Liu,
2006), has smaller particle size than that pores size of
MF membrane (0.15 µm), however, there is an
interaction between SRS (200, 300 and 400 rpm) and
fixed TMP 40 psia so that fouling phenomenon has
occurred. Fouling is defined as the process in which
microparticles, colloidal particles, solute molecules
or bacteria trapped or accumulated on the membrane
surface or into the membrane pores such that the
membrane pores are blocked or become smaller, and
formation of cake layer, in turn depletion of water
mass so that particles of polyphenol and organic acids
will be inhibited to pass across membrane.
Polyphenol is chemical component having activity of
anti-bacteria because of ability to increase membrane
permeability from microbial cell so that membrane
becomes unstabil causing cell hemolysis (Cushnie et
al, 2005) Similar pattern is occurred at total acids, in
which fouling is occurred in spite of particle size of
organic acids compound (MW 200 – 250 Da.) are
small (Liu, 2006). Organic acids is possibility have
ability due to hydrophilic property from membrane so
0.26
0.55
0.67
0.22
0.37
0.43
1.00
0.72 0.72
0.30
1.17
1.14
0.00
0.25
0.50
0.75
1.00
1.25
200 300 400
Stirrer rotary speed (rpm)
Total Polyphenol &
Total Acid (%)
Retentate-Total Polyphenol (%) Permeate-Total Polyphenol (%)
Retentate-Total Acid (%) Permeate-Total Acid (%)
26.75
24.35
28.98
13.54
18.50
22.17
17.70
19.53
16.33
21.22
20.68
17.94
10.0
14.0
18.0
22.0
26.0
30.0
200 300 400
Stirrer rotary speed (rpm)
Dissolved protein &
Reducing sugar (mg/mL)
Retentate-Dissolved Protein (mg/mL) Permeate-Dissolved Protein (mg/mL)
Retentate-Reducing sugar (mg/mL) Permeate-Reducing sugar (mg/mL)
16th AFC 2019 - ASEAN Food Conference
304
that it functionates as transport media of positive
charge ions. In other word, H+ diffuse to microbial
cell wall (Trivedi et al, 2010) so that microbial cell
wall will be more polar so that polyphenol
compounds, flavonoid, etc. ease to permeate. On
treatment combination at SRS 400 rpm and TMP 40
psia, optimization of total polyphenols and total
organic acids in concentrate were achieved at 0.67%
and 1.17% higher compared to passing in permeate
0.43% and 0.3%. In this condition, MF membrane
system is able to retain total polyphenols and total
acids in retentate 123.33% (1.23-folds) and 134%
(1.34-folds), however, it passes both them 43.3% and
40% compared to total polyphenols (0.30%) and total
acids (0.50%) in feed. SRS becoming higher, passes
dissolved protein and reducing sugars to get
optimization of MF membrane system followed by
decreasing its concentration. This matter showed that
MF membrane gives ability of separation
unsuccessfully for both types of components, as
indicated in Fig. 2b. Passing level of dissolved protein
and reducing sugars are related with particle size
calculated as amino acids and monosaccharides (0.01
– 0.1 µm) (PCI Membrane, 2005); Michael 1989)
being smaller compared to MF membrane (0.15 µm),
TMP (40 psia), and type of biomass. Interactions
amongs these factors, it is not possibility occurred
fouling so that they pass more much in permeate
compared with being retained in retentate. Dissolved
protein is the whole proteins from raw material of
beetroot tuber (1.61%), in which beetroot tuber is
degraded by microbe activity in Kombucha culture to
dissolved protein derivatives accoding to Lowry
(Lowry, 1951), whereas, reducing sugar is molecule
of sugar having property of reducing because reactive
hydroxyl ion (OH) according to Nelson-Somogyi
method (AOAC 2019). Reducing sugars and
dissolved protein become parameter in converting
carbohydrate and protein by activity of microbes in
Kombucha culture (Wong, 2015). Optimization of
dissolved protein (22.17 mg/mL) and reducing sugars
(21.22 mL) in concentrate is obtained at SRS 200
rpm, and passes dissolved protein (26.75 mg/mL) and
reducing sugars (17.94 mg/mL) in permeate. In this
optimum condition, MF membrane system is able to
retain dissolved protein (44.33%) and reducing sugars
(22.45%) in retentate, however, it passes dissolved
protein 74.15%) and reducing sugars (3.40%) in
permeate compared to dissolved protein (15.36
mg/mL) and reducing sugar (17.33 mg/mL) in feed.
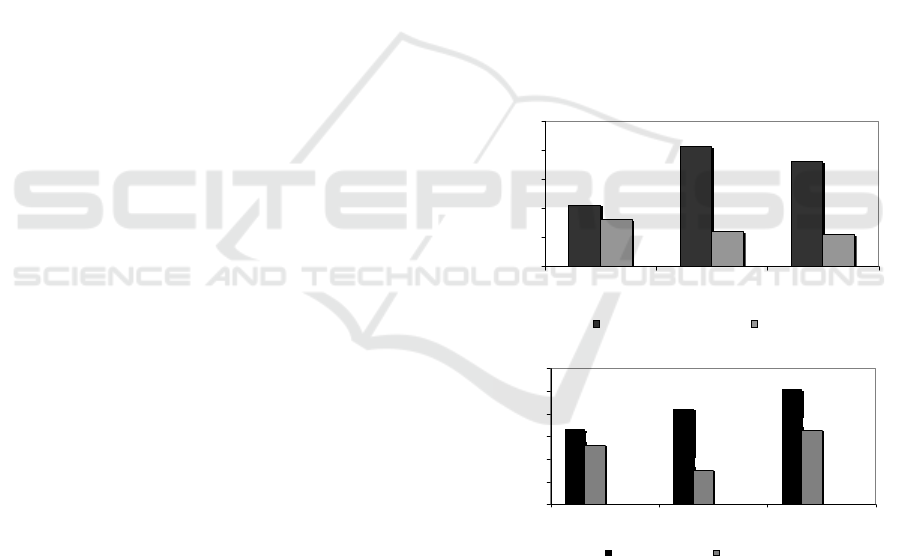
3.2.2 Betacyanin and Total Solid
Betacyanin in biomass of beetroot, retentate and
permeate as a result of MF process are possibility as
compound being have activity of anti bacteria in
phenolic compound group (Coultate, 2009).
Separation process of betacyanin and total solids from
biomass of beetroot showed that increasing SRS
generates increasing betacyanin until it is get the best
SRS followed by dropping betacyanin, however, on
total solids becomes more and more high to optimum
SRS. The best SRS on betacyanin was achieved at
300 rpm, which is able to separate betacyanin in
retentate 0.31 µg/mL and passes in permeate 0.16
µg/mL, as shown in Fig. 3a. In the optimum
condition, MF membrane separates betacyanin in
retentate 416.67% (4.17-folds) compared to
betacyanin in feed (0.06 µg/mL). It had been known
that fermentation process declines betacyanin by
effect of glucosidase enzyme of Kombucha culture
(Havlíková et al, 1983). With particle size ranging
from 0.001 – 0.01 µm, like flavonid compounds
passes freely in permeate, however, due to presence
of fouling causes betacyanin trapped in ‘cake’ layer
on the membrane surface. On total solids, MF
membrane showed increasing total solids in line with
incresing SRS, as showed in Fig. 3b.
(a)
(b)
Figure 3: Effect of SRS at TMP 40 psia on (a) betactanin
and (b) total solids contents in retentate and permeate as a
result of MF of fermented beetroot suspensions.
MF membrane process generates a separation
successfully, in which total solids retained on the
membrane surface in retentate much more than that
passing in permeate for the whole process treatment.
Increasing total solids is caused by deficit of water
mass passing across membrane as a consequence
0.21
0.31
0.28
0.18
0.16
0.16
0.10
0.15
0.20
0.25
0.30
0.3
5
200 300 400
Stirrer rotary speed (ug/mL)
Betacyanin (ug/mL)
Retentate Permeate
9.83
10.05
10.27
9.66
9.38
9.82
9.00
9.25
9.50
9.75
10.00
10.25
10.50
200 300 400
Stirrer rotary spee d (rpm )
Total solid (%) CH+)
Retentate Permeate
Difference in Characteristic of Beetroot (Beta Vulgaris L.) Biomass through Microfiltration Technique to Prevent Natural Infection
305

from interaction between driven force and SRS to
solidify components on the membrane surface.
Optimization of total solids is achieved by retentate
(10.27%) higher compared to pass in permeate
(9.82%) at SRS 400 rpm. In this optimum condition,
MF membrane process is able to retain total solids in
retentate 2.49%, however, pass in permeate 1.99%
compared to total solids in feed (10.02%). Total
solids is an accumulation of the whole components of
beetroot biomass both soluble and insoluble in water
according to Gavimetric method (AOAC 2019) [17].
3.3 Optimum Condition Process of
Microfiltration
Based on the highest betacyanin concentration as
prevention of the best natural infection, separation
process of betacyanin from fermented beetroot was
achieved at SRS 300 rpm and TMP 40 psia. In this
condition is yielded concentrate and permeate of
fermented beetroot with composition of betacyanin
0.31 and 0.16 µg/mL, total polyphenols 0.55 and
0.37%, total acids 1.00 and 0.72%, reducing sugars
16.33 and 28.97 mg/mL, total solids 10.05 and
9.38%, and dissolved protein 18.50 and 24.35
mg/mL. In this condition, MF membrane process is
able to retain components in concentrate and pass
components in permeate on betacyanin 416% (4.16-
folds) and 166% (1.67-folds), total polyphenols
83.33% and 23.33%, total acids 100% (1-fold) and
44%, reducing sugars 6.31% and 88.61%, total solids
0.3% and 6.84%, and dissolved protein 20.44 and
58.53% compared to concentration of components in
feed. Fig. 4 shows feed (biomass of beetroot
fermented for 12 days), concentrate and permeate
yielded from MF membrane process at room
temperature, SRS 300 rpm and TMP 40 psia for 30
minutes.
(a)
(b) (c)
Figure 4: (a) feed, (b) retentate and (c) permeate as a result
of MF of fermented beetroot suspension at optimum
condition (300 rpm and 40 psia).
3.4 Activity of Anti Bacteria from
Fermented Beetroot
Fermented beetroot has possibility activity of natural
anti bacteria relating with presence of polyphenol
compounds, particularly betacyanin. Retentate from
MF membrane process on fermented beetroot at
optimum condition (SRS 300 rpm, TMP 40 psia)
indicates an ability in inhibiting the growth of
Staphylococcos aureus and Escherichia coli on Agar
Sodium with concentration 10 µmL incubated at 37
°C for 18 hours. Fig. 5a and 5b displays ability to
inhibit the growth of Staphylococcus aureus and
Escherichia coli in retentate of fermented beetroot
with diameter of the inhibition zone of 11 mm and 10
mm, respectively.
(a)
(b)
Legend: *Number 20 and 21 are code of samples.
Figure 5: The growth of inhibition ability of (a)
Staphylococcus aureus and (b) Escherichia coli from
retentate of fermented beetroot suspension at optimum
condition.
Feed and permeate does not demonstrate presence
of clear zone arounds colony of Staphylococcus aureus
and Escherichia coli or in other words it does not
display activity of anti bacteria. This matter showed
that MF membrane process tends to affect on ability to
inhibit the growth of Staphylococcus aureus and
Escherichia coli according to concentrations of total
polyphenol (0.55%) and betacyanin (0.31 µg/mL) at
higher concentrate compared to biomass without
through MF membrane process with total polyphenol
(0.30 µg/mL) and betacyanin (0.06 µg/mL). Similar
case is also occurred in permeate according to both
lower concentration of total polyphenol (0.37%) and
betacyanin (0.16 µg/mL) compared to retentate.
Bioactive compound concentration becoming more
and more high will increase its activity as anti- bacteria
(Brooks et al 2010). In general, S. aureus as a gram-
positive bacterium has cell wall composed from
peptidoglycan, in which polyphenol has property of
toxis so that it is able to inhibit bacterial adhesin,
Enzymes function and protein transport on cell cover
(Cowan, 1999). that has deleterious ability on bacteria
function causing lysis.
3.5 Identification of Monomer on
Polyphenol and Betacyanin
Compounds
Identification on polyphenol and betacyanin
monomers is performed on an aliquot of retentate and
16th AFC 2019 - ASEAN Food Conference
306
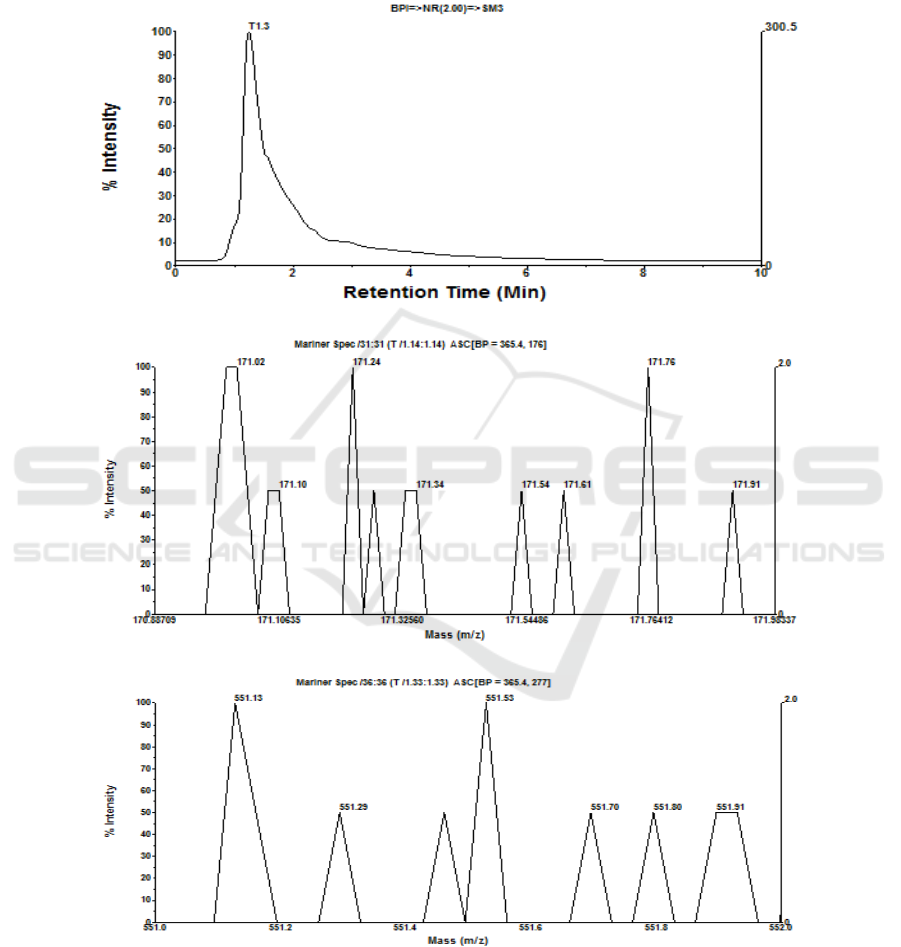
permeate from optimum condition treatment at SRS
300 rpm and TMP 40 psia by means of LC-MS based
on molecular weight (MW) of gallic acid (MW 170
Da.) and betacyanin (MW 550 Da.). By means of LC-
MS method had been known that a compound
indicated difference in MW, in which its possibility is
as M+, M+ Na+, 2M++ or 2M+, Na+ [12]. Operation
condition of LC-MS was adjusted in column of C-8
(15 mm x 2 mm), in which mobile phase (eluent) is
methanol solution at flow rate of 0.1 mL/minute and
injection volume of 5 μL, as displayed in Fig 6a-6f.
(a)
(b)
(c)
Figure 6: (a) Chromatogram of retentate, (b) mass spectra of retentate monomer as gallic acid, (c) mass spectra of retentate
monomer as betacyanin, (d) chromatogram of permeate, (e) mass spectra of permeate monomer as gallic acid, (f) mass spectra
of permeate monomer as betacyanin as a result of MF of fermented beetroot suspensions at room temperature, SRS 300 rpm,
and TMP 40 psia for 30 minutes.
Difference in Characteristic of Beetroot (Beta Vulgaris L.) Biomass through Microfiltration Technique to Prevent Natural Infection
307
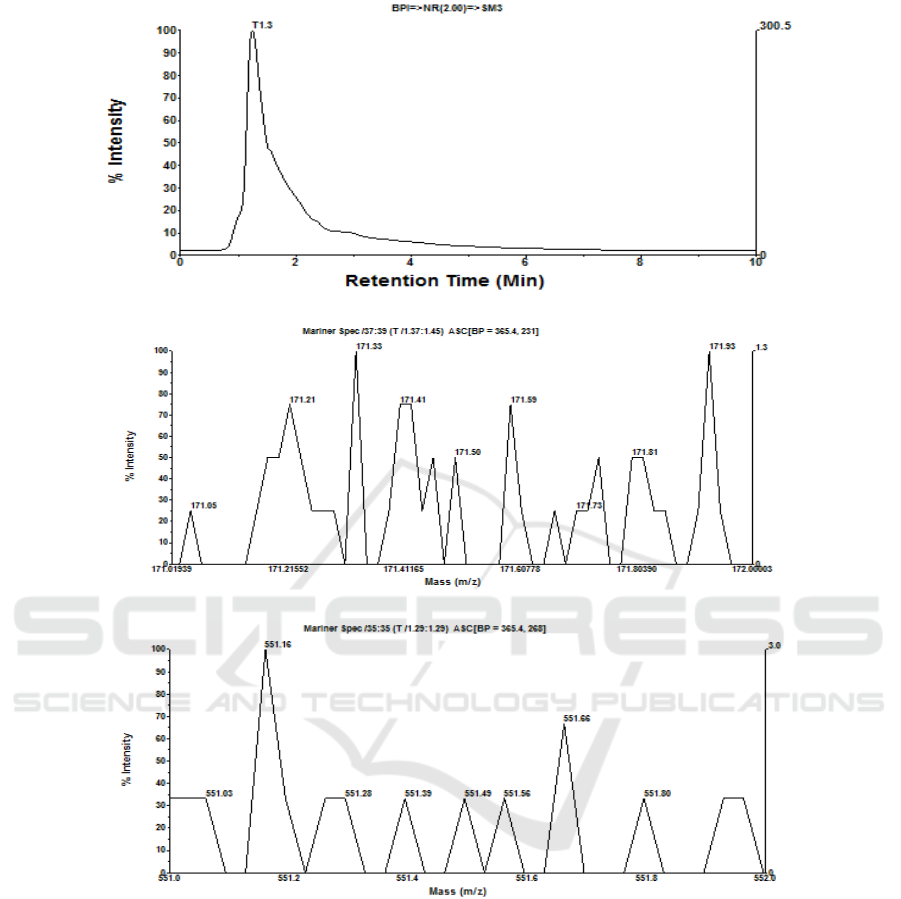
(d)
(e)
(f)
Figure 6: (a) Chromatogram of retentate, (b) mass spectra of retentate monomer as gallic acid, (c) mass spectra of retentate
monomer as betacyanin, (d) chromatogram of permeate, (e) mass spectra of permeate monomer as gallic acid, (f) mass spectra
of permeate monomer as betacyanin as a result of MF of fermented beetroot suspensions at room temperature, SRS 300 rpm,
and TMP 40 psia for 30 minutes (cont.).
On polyphenol monomer as gallic acid,
chromatogram on fermented beetroot concentrate
shows one (1) peak (T1.3), in which at mass spectra
T1.3 is get 8 monomer of gallic acid with MW
between 171.02 and 171.92 Da. dominated by 3
monomers of gallic acid with MW 171.0231, 171.24,
and 171.76 Da. (2M+) and relative intensity of 100%.
Meanwhile, on monomer of betacyanin is get 6
monomers of betacyanin with MW between 551.13
and 551.91 Da. dominated by 2 monomers of
betacyanin with MW 551.13 and 551.53 Da. and
relative intensity 100%, as shown in Fig 6a, 6b, and
6c. Permeate of fermented beetroot is get
chromatogram with one (1) peak with mass spectra
10 monomers of gallic acid dominated by monomer
with MW 171.23 and 171.72 Da. with relative
intensity 100%, whereas monomer of betacyanin is
get 9 monomers of betacyanin with MW between
16th AFC 2019 - ASEAN Food Conference
308
551.03 and 551.95 Da. dominated by monomer of
betacyanin with MW 551.16 Da. and relative
intensity 100%, as showed in Fig 6d, 6e and 6f.
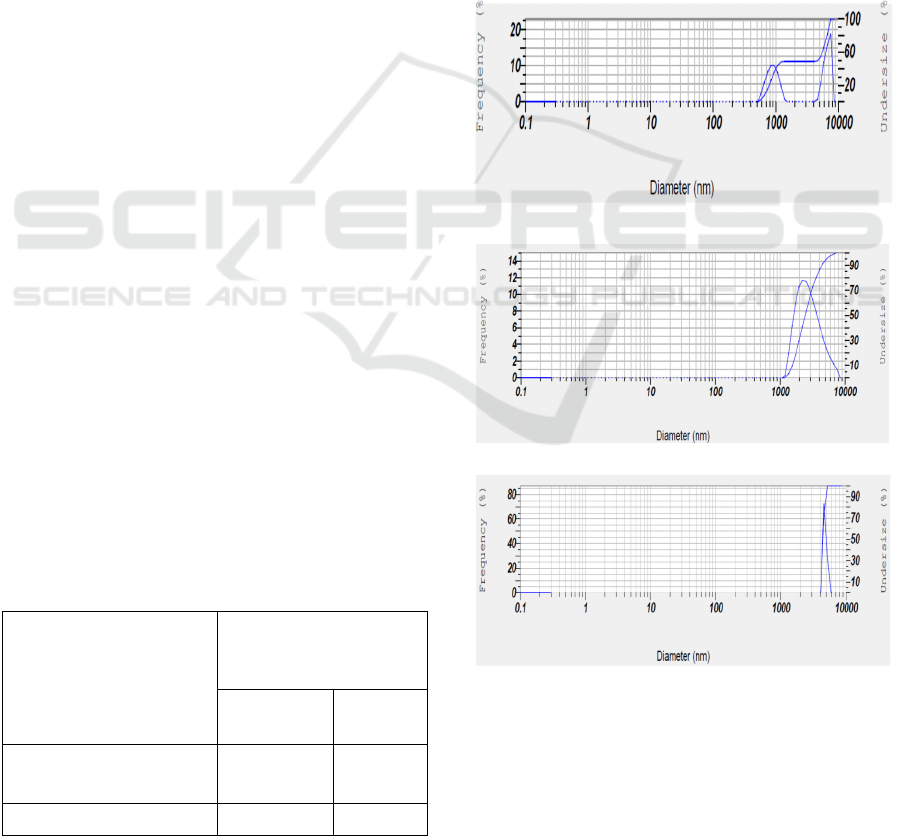
3.6 Distribution of Particle Size
Based on betacyanins, MF membrane process on
fermented beetroot with the best process condition is
resulted from concentrate with thick suspension and
permeate with clear liquid by degrading colour from
red to dark red. By using stirrer at SRS 300 rpm and
TMP 40 psia for 30 minutes affects possibility on
particle size and particle size distribution. Particle
size and particle size distribution are conducted to
know the characteristic of suspension relating with
adsorption aspect in the digestive system. Table 2.
demonstrates larger particle size of feed (5656.4 nm)
than particle size of retentate (3002.4 nm) and
permeate (1962.0 nm) with particle index of 0.370,
1.912 and 0.468, respectively. The difference in this
particle size is possibily caused by MF system being
separating suspension so that biomass feed without
MF system has the highest particle size. Meanwhile,
the concentrate is an accumulation from all
components with particle size larger than 0.15µm due
to fouling phenomenon and permeate has the smallest
particle size because components with particle size
smaller than 0.15µm passes freely in permeate.
Dispersion of particles are displayed as dispersed
particle index (PI), in which feed has the highest PI
(1.912) compared to PI of retentate (0.468) and PI of
permeate (0.370). Particle size becoming more and
more small will be small in PI or in other words
dispersion of particle is more uniform and homogen.
It had been appeared that retentate and permeate have
PI smaller than 1 expressed that particle size
distribution is more homogenous compared to feed
indicating particle size distribution is ununiform (PI
> 1) (Eichhorn, 2001).
Table 2: Characteristic of particles in feed, retentate and
permeate by MF membrane fitted in DESMFC at room
temperature, SRS of 300 rpm and TMP 40 psia for 30
minutes.
Kind of of fermented
beetroot*
Distribution of nano-
polyphenol particles
(nm)
Z-Average
(nm)**
PI***
Feed*
Retentate (concentrate)
5656.4
3002.4
1.912
0.468
Extract (permeate) 1962.0 0.370
Particle size distribution on feed showed that particles
have diameter size (Ø) of 500 – 1200 nm and 700 –
9000 nm (> 10000) at frequency between 0 and 10%,
and between 0 and 15% or the whole particles with Ø
between 500 and 10000 nm (> 10000 nm) at
frequency between 0 and 25%, respectively, as
indicated in Fig 7a. It had been appeared that particles
in retentate have Ø between 1000 and 9500 nm (>
10000 nm) at frequency between 0 and 13% or the
whole particles with Ø between 1000 and 9500 nm (>
10000 nm) at frequency between 0 and 15%, as
demonstrated in Fig. 7b. Meanwhile, on permeate
appears particles with Ø between 6000 and 8000 nm
at frequency between 0 and 65%, as shown in Fig. 7c.
Particle size distribution indicates a difference in the
whole materials, in which feed has particle size with
more variation compared to retentate and permeate.
(a)
(b)
(c)
Figure 7: Particle size distribution of (a) feed, (b) retentate,
and (c) permeate as a result of separation of fermented
beetroot by MF membrane fitted DESUFC at room
temperature, SRS of 300 rpm and TMP 40 psia for 30
minutes.
Difference in Characteristic of Beetroot (Beta Vulgaris L.) Biomass through Microfiltration Technique to Prevent Natural Infection
309

4 CONCLUSIONS
Separation of total polyphenol and betacyanin
compounds from fermented beetroot through MF
membrane generates a separation successfully for
betacyanin, total solids, total acids, and
unsuccessfully for dissolved protein and reducing
sugars. SRS becoming more and higher will retain
total polyphenol, betacyanin, total acids, total solids
in retentate, however SRS pass freely dissolved
protein and reducing sugars in permeate. Based on
recovery of betacyanin, optimization on process
condition by means of MF technique was achieved at
SRS 300 rpm being resulting retentate and permeate
with composition betacyanin of 0.31 and 0.16 µg/mL,
total polyphenols of 0.55 and 0.37%, total acids of
1.00 and 0.72%, reducing sugars of 16.33 and 28.97
mg/mL, total solids of 10.05 and 9.38%, dissolved
protein of 18.50 and 24.35 mg/mL, particle size
3002.4 and 1962.0 nm, particle index 0.468 and
0.370, respectively. Identification on betacyanin and
galic acid monomers as total polyphenol at retentate
is dominated by monomer with molecular weight
(MW) 551.13, 551.53, and 171.02, 171.24 and 171.76
Dalton (Da.), meanwhile permeate is dominated by
monomer with MW 551.16 and 171.23, 171.72 Da.
and relative intensities 100%, respectively. Ability to
inhibit the growth of bacteria of Staphylococcus
aureus Ina CC-B4 and Escherichia coli Ina CC-B5 is
obtained by retentate with zone area of inhibiting 11
and 10 mm, respectively. In this optimum condition,
MF membrane technique was able to retain
betacyanins 416% (4.16-folds), total polyphenol
83.33%, total acids 100% (1-fold), reducing sugars
6.31%, total solids 0.3% and dissolved protein
20.44% in retentate, whereas it passes betacyanin
166% (1.67-folds), total polyphenol 23.33%, total
acids 44%, reducing sugars 88.61%, total solids
6.84% and dissolved protein 58.53% in permeate
compared to components prior to process (feed).
ACKNOWLEDGEMENT
The authors wish to thank the Kemenristekdikti
throughout Program Insentif Riset Sistem Inovasi
Nasional (INSINAS) Fiscal Year 2019 supporting
this research in Program INSINAS Riset Pratama
Individu on Research Field for developing functional
food–based local natural resources.
REFERENCES
Sarkar, T., Sen, M. K., and Nihar, S. 2015. Extraction of
natural pigment from beet root & proper packaging of
that red dye: a review. Journal of Agricultural
Engineering and Food Technology, (2): 116 – 118.
Coultate, Tom P. 2009. Food: the chemistry of its
components. Royal Society of Chemistry,
Pavoković, Dubravko; Krsnik-Rasol, Marijana. 2011.
Complex biochemistry and biotechnological
production of betalains. Food technology and
Biotechnology, 49.2: 145-155.
Cai, Yizhong; Sun, Mei; Corke, Harold, 2005. HPLC
characterization of betalains from plants in the
Amaranthaceae. Journal of chromatographic science, ,
43.9: 454-460.
Grotewold, Erich. 2006, The genetics and biochemistry of
floral pigments. Annu. Rev. Plant Biol., 57: 761-780.
Malbaša, R.; Lončar, E.; Djurić, M. 2008, Comparison of
the products of Kombucha fermentation on sucrose and
molasses. Food Chemistry, 106.3: 1039-1045.
Raja, Ghosh. 2003. Protein Bioseparation Using
Ultrafiltration: Theory, Applications And New
Developments. World Scientific.
Field, R. W. 1993. Transport processes in membrane
systems. In: Membranes in bioprocessing: theory and
applications. Springer, Dordrecht, p. 55-112.
Mulder, J. 2012) Basic principles of membrane technology.
Springer Science & Business Media.
MILLIPORE. 2008. Catalogue and Product Information of
Stirred Ultrafiltration Cell, Amicon Bioseparation,
MILLIPORE, Bedford, U. S. A. www.millipore.com.
Onggo, Djulia, 2009. General Principles in Electrospray
Mass Spectrometry: A New Technique in Mass
Spectral Analysis. Jurnal Matematika dan Sains, , 3.2:
115-131.
Eichhorn, Peter; Knepper, Thomas P. 2001. Electrospray
ionization mass spectrometric studies on the
amphoteric surfactant cocamidopropylbetaine. Journal
of mass spectrometry, 36.6: 677-684.
Dapkunas, Stanley J.; Jillavenkatesa, A.; Lum, L. H. 2001.
Particle Size Characterization. NIST, Gaithersburg,
MD.
Retsch-Technology GmbH. 2019. Nano Particle Analyzer
Horiba SZ-100.https://www.retsch-technology.com.
Accessed at February 3, 2019.
Madigan, M. T., Martinko, J. M., Dunlap, P. V., & Clark,
D. P. 2008. Brock biology of microorganisms 12th
edn. Int. Microbiol, 11, 65-73.
Levinson, W. 2008, Mycology. Review of medical
microbiology and immunology. 10th ed. USA, The
McGraw-Hill Company, 336-52.
AOAC. 2019. Official Methods of Analysis. Association of
Official Analytical Chemists International, 21st
Edition, 2275 Research Blvd, Ste 300, Rockville, MD
20850. http://www.aoac.org. Accessed at 3 September
2019.
Liu, Z. 2006. New techniques for tea catechins extraction.
In: International Training Workshop of Tea Science.
China: Hunan Agricultural University,.
16th AFC 2019 - ASEAN Food Conference
310

Lowry, Oliver H., 1951. Protein measurement with the
Folin phenol reagent. Journal of biological chemistry,
1951, 193: 265-275.
Wong, Yen-Ming; SIOW, Lee-Fong. 2015. Effects of heat,
pH, antioxidant, agitation and light on betacyanin
stability using red-fleshed dragon fruit (Hylocereus
polyrhizus) juice and concentrate as models. Journal of
food science and technology, 52.5: 3086-3092.
Bell, S. M. 1984. Antibiotic sensitivity testing by the CDS
Methods. Dalam Clinical Microbiology Up date
Program. Editor: Hartwig, N. The Prince of Wales
Hospital. New South Wales.
WELCH, Cara R.; WU, Qingli; SIMON, James E. 2008.
Recent advances in anthocyanin analysis and
characterization. Current analytical chemistry, 2008,
4.2: 75-101.
PCI Membrane and Filtration Group. Membrane
Technology For Process Industry, 2005. http.www.
pcims.com./images/ TP105.5us.pdf; PCI Membrane
System Inc., Milford, U.S.A. Accessed 1 September
2019.
Michael, A. S. 1989 Handbook of Industrial Membrane
Technology. Noyes Publications, Park Ridge, USA.
Cushnie, TP Tim; LAMB, Andrew J. 2005. Antimicrobial
activity of flavonoids. International journal of
antimicrobial agents, 26.5: 343-356.
Trivedi, P. C., Pandy, S., and Bhadauri, S.. Text Book of
Microbiology, 1st ed., Aavishakar Publisher, India,
2010 :82 – 83.
USDA, U. 2018. National nutrient database for standard
reference, Legacy Release.
Havlíková, Ludmila; Miková, Kamila; Kyzlink, Vladimír.
1983. Heat stability of betacyanins. Zeitschrift für
Lebensmittel-Untersuchung und Forschung, 177.4:
247-250.
Brooks, George F. Jawetz, Melnick, & Adelberg's 2010.
medical microbiology/Geo. F. Brooks...[et al.]. New
York; Chicago: McGraw Hill Medical.
COWAN, Marjorie Murphy. Plant products as
antimicrobial agents. Clinical microbiology reviews,
1999, 12.4: 564-582.
Difference in Characteristic of Beetroot (Beta Vulgaris L.) Biomass through Microfiltration Technique to Prevent Natural Infection
311
