
Effect of Heating Condition and pH on Stability of Total Phenolic
Content and Antioxidant Activities of Samui (Micromelum Minutum)
Extract
Wanrada Krungkri and Varipat Areekul
Faculty of Agroindustry, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, Thailand
Keywords: Temperature, pH, Total Phenolic Content, Antioxidant, Samui (Micromelum Minutum) Extract.
Abstract: Samui (Micromelum minutum) leaf, commonly consumed in southern Thailand has a high potential of
antioxidant. In this study, this plant was extracted with three various solvents; water, ethanol (95%) and
acetone (60%), freeze-dried and then, re-dissolved in water. The factorial design was applied to evaluate the
effect of pH (ranged from 5-7) and heat condition (60-80°C) on the stability of bioactive activity. The total
phenolic content (TPC), DPPH radical scavenging, ABTS radical scavenging, ferric ion reducing antioxidant
power (FRAP) and antioxidation by TBARs method were determined. The result indicated the influence of
the solvent. Acetone extract seemed to be more effective compared to ethanolic and aqueous extracts. In
addition, pH and heat conditions significantly affected the stability of TPC and antioxidant capacities
(p≤0.05). Generally, the TPC and antioxidant capacities were higher at higher pH. On the other hand,
increasing heat conditions significantly deteriorated the TPC and antioxidant capacities. In conclusion, all
studied factors influenced the stability of bioactive compounds and their activities which need to be considered
when applying the extract into food.
1 INTRODUCTION
Plants are rich source of phytochemicals, for instance,
phenolic compounds such as flavonoids, and tannins.
All act as antioxidants which have been linked with
several health benefits due to their medicinal
properties and high nutritional value. Antioxidants
also control, reduce or inhibit lipid oxidation caused
by reactive oxygen species in foods, therefore,
enhancing their shelf-life and quality (Cherkupally, et
al, 2017; Altemimi, et al, 2017). Phytochemicals have
been recognized their antioxidant potential which
prevents oxidation of fat as well as consuming this
type of vegetables may help prevent chronic non-
communicable diseases (Thomas, et al, 2016).
Samui (Micromelum minutum) is a traditional
vegetables commonly found in Southern Thailand.
Apical bud and young leaves are popular in various
main dishes as well as fresh consumption. It consists
of several phytochemicals such as phenol, coumarin,
alkaloids, and beta-sitosterol (Bunyapraphatsara,
Chokchaijareunporn and Herbs, 2000; Areekul, 2552;
van Valkenburg and Bunyapraphatsara, 2001). This
coumarin had been reported it properties for
inhibition of the cancer cells such as A549 (lung),
ACHN (renal), H727 (lung), MCF-7 (breast) and HL-
60 (leukemia) (Sakunpak, A., et al, 2013). This plant
showed high potential sources of phenolic content
and antioxidant compounds (Areekul and
Promkraiwan, 2009; Friedman and Jurgens, 2000). In
the previous study, Samui extract at a concentration
of 500 ppm was effective in inhibiting the oxidation
reaction in the water-based emulsion (Soto, et al,
2019).
The antioxidant efficiency of the extract can be
changed through several factors including
temperature and pH. Generally, heating causes an
acceleration of the initiation reactions and hence
decreases in the antioxidant activity. In addition, the
pH also affects the decomposition of important
substances, the stability of the phenolic compounds,
and the antioxidant activity (Promkraiwan, 2009;
Maisuthisakul, et al, 2007). In food production, it is a
fact that food undergoes to the processing step
including pH adjustment and heating process.
Therefore, the understanding of the stability of plant
extracts is necessary in order to apply them to food.
The objective of this study was to evaluate the
126
Krungkri, W. and Areekul, V.
Effect of Heating Condition and pH on Stability of Total Phenolic Content and Antioxidant Activities of Samui (Micromelum minutum) Extract.
DOI: 10.5220/0009980800002964
In Proceedings of the 16th ASEAN Food Conference (16th AFC 2019) - Outlook and Opportunities of Food Technology and Culinary for Tourism Industry, pages 126-132
ISBN: 978-989-758-467-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

stability of phenolic compound and their antioxidant
activity extracted from various solvents at different
pH and heating conditions.
2 MATERIALS AND METHODS
2.1 Materials
2.1.1 Chemicals
All chemical were purchased;Acetone (CH
3
COCH
3
;MacronFine Chemicals,USA), Methanol (Lab-scan,
Ireland), Ethanol (Lab-scan, Ireland), 2,2-diphenyl-1-
picrylhydrazyl (DPPH, C
18
H
12
N
5
O
6
; Aaldrich, USA),
Folin–ciocalteu reagent (VWR, Prolabo, Ecuador),
Sodium carbonate (Na
2
CO
3
; Ajax Finechem,
Australia), Hydrochloric acid (HCl; J.T. Baker,
USA), Gallic acid (C
7
H
6
O
5
; Sigma-Aldrich,
Germany), 6-hydroxy-2,5,7,8-tetramethylchroman-
2-carboxylic acid (Trolox, C
14
H
18
O
4
; BBL, USA),
Linoleic acid (C
7
H
6
O
5
; Sigma-Aldrich, Germany),
Thiobarbituric acid (Merck, Germany), Trichloro -
acetic acid (Merck, Germany), 2,2’-Azinobis (3-
ethylbenzo-thiazoline-6-sulfonic acid)(Sigma,USA)
and 2,4,6-Tripyridyl-s-triazine (TPTZ) (Sigma,USA)
2.1.2 Plant Materials
Samui (Surat Thani, Thailand) was purchased from
local market in Surat-thani province Apical bud and
young leave was selected, cleaned with water, and
then dried in the hot air dryer at a temperature of 40°C
until the final moisture content below 10% The
sample was ground and sifted through a 40 mesh
sieve. The powdered plant specimens was put into
polyethylene bags (PE), vacuum sealed and stored at
-20°C.
2.2 Preparation of Extracts
Three solvents were used to extract: water, ethanol
(95%) and acetone (60%) The ground plant was
weighed 5 gram and mixed with 100 ml of each
solvent, stirred continuously with a temperature-
controlled shaker (25 ± 2°C), at a speed of 200 rpm
for 12 hours. The extract was then filtered with
Whatman Filter No. 4. The filtrate was evaporated at
a temperature of 35°C. After that, the freeze-drying
was performed. The freeze dried sample was kept at -
18°C.
2.3 Study of Heat Stability and pH
Thirty mg. of freeze-dried samples were re-dissolved
in 30 ml DI water. Extract According to the method
selected from Article 2.2 and then studied the effect
of 3 levels of acidity, 5, 6 and 7 by dissolving 30 mg
powder plant with DI 30 ml of water. And adjust the
pH with a concentration of 0.3-0.4 molar acetate
buffer at pH 6-7. Then divided into 3 parts, bringing
10 ml samples into the test tube Soak into the bath,
After adjusting the pH, each sample was submerged
into water-bath at three different conditions as
following; 60, 70 and 80°C for 30, 15 and 3 min,
respectively. After heat treatment, the sample was
immediately cooled down in the ice-water and
determined for all chemical analysis.
2.4 Chemical Analysis
2.4.1 Total Phenolic Content
Total phenolic contents of samples were determined
by the Folin–Ciocalteu method (Shaghaghi, et al,
2008). Briefly, aliquots of 40 µl of samples and
standards were mixed with 100 µl of deionized water,
20 µl of Folin–Ciocalteau reagent, and 40 µl of
10%sodium carbonate (Na
2
CO
3
). After incubation at
room temperature for 30 min in the dark, the
absorbance of the reaction mixture was measured at
765 nm against a deionized water blank by a
microplate reader (Biochrom, EZ Read 2000). Using
standard curve of Gallic acid solutions, the total
phenolic contents of samples were determined in
triplicates.
2.4.2 DPPH Radical-Scavenging Activity
Assay
The radical-scavenging activity was determined by
the DPPH method (Murakami, 2004). Briefly,
aliquots of 50 µl of sample were mixed with 150 µl
of 0.22M DPPH in ethanol (final concentration of
95%). The mixture was shaken vigorously and left to
stand for 30 min at room temperature in the dark.
Controls or blanks were prepared without the sample
solution. The absorbance at 517 nm by DPPH was
measured with a microplate reader (Biochrom, EZ
Read 2000). Using standard curve of Trolox
solutions, the DPPH in samples were calculated.
2.4.3 ABTS+ Radical Scavenging Activity
The radical-scavenging activity was determined by
the ABTS
+
method (Zhou and Yu, 2004). Briefly,
aliquots of 50 µl of sample solution were mixed with
Effect of Heating Condition and pH on Stability of Total Phenolic Content and Antioxidant Activities of Samui (Micromelum minutum)
Extract
127

100 µl of 5 M ABTS
+
. The mixture was shaken
vigorously and left to stand for 5 min at room
temperature in the dark. Controls or blanks were
prepared without the sample solution. The absorbance
at 734 nm was measured with a microplate reader
(Biochrom, EZ Read 2000). The ABTS
+
of samples
were calculated using a standard curve of Trolox
solutions, the ABTS
+
in samples were calculated.
2.4.4 Ferric Reducing/Antioxidant Power
The Ferric reducing/antioxidant power was deter-
mined (Benzie and Strain, 1996). Briefly, aliquots of
10 µl of sample solution were mixed with 300 µl of
FRAP. The mixture was shaken vigorously and left to
stand for 8 min at room temperature in the dark. The
absorbance at 593 nm was measured with a
microplate reader (Biochrom, EZ Read 2000). The
FRAP of samples were calculated using a standard
curve of Trolox solutions, the FRAP in samples were
calculated.
2.4.5 Ant-Thiobarbituric Acid Reactive
Substances
Anti-Thiobarbituric acid reactive substances was
determined by the Anti-TBARs method (McDonald
and Hultin, 1987). Briefly, aliquots of 0.2 mL of
sample and standards were mixed with 0.8 ml of 1%
linolenic acid, Leave in a water bath at a temperature
of 50 ± 1°C for 18 hours. 2 mL of TCA-TBA-HCl
solution to boil for 15 minutes, Rest to cool before
spinning, at a speed of 5,500 rpm for 5 min, the
absorbance at 520 nm by Anti-TBARs was measured
with a microplate reader (Biochrom, EZ Read 2000).
Using standard curve Butylated hydroxyanisole
(BHA) solutions, the Anti-TBARs in samples were
calculated.
2.5 Statistical Analysis
The results were expressed as the mean ± standard
deviation (SD) calculated using Microsoft Excel.
Data were analyzed for analysis of variance using
SPSS program using a variance (ANOVA) followed
by the two-tailed Duncan’s multiple range test
(DMRT). P-values less than 0.05 were considered
significant (p<0.05).
3 RESULTS AND DISCUSSION
3.1 Total Phenolic Content
This experiment studied the stability of bioactive
compounds extracted from three different solvents
under the various pH and heating condition. The
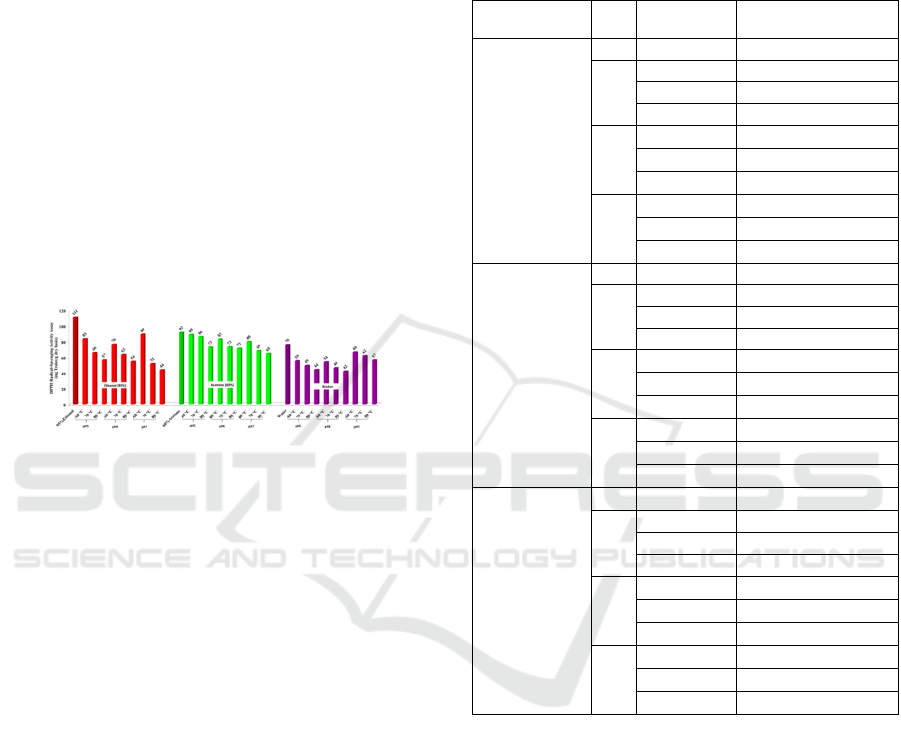
result is shown in Figure 1. TPCs in treated samples
ranged between 36.22–78.82 mg GAE/g dry basis
which significant lower compared with control (88.39
mg GAE/g dry basis). The was found that using
different solvents affected the stability of extracted
compounds. The higher TPC indicated the higher
stability of phenolic compounds. For this experiment,
the acetone extract provided the highest stability of
phenolic compounds, followed by ethanol and water,
respectively (p≤0.05). Sweet potato leaf polyphenol
had high retention under pH 5-7 and mind heat
condition while increasing heating temperature
and/or increasing acidity or alkalinity had a great
impact on its stability (Sun, et al, 2017).
The effects of pH and heating condition also
pronounced on the stability of phenolic content
(p≤0.05). However, the heating condition had higher
effect. As increasing heating temperature, TPC
retention is lower. Generally, heating causes an
acceleration of the initiation reactions and hence
decreases in the phenolic. High temperature and long-
term heat treatment should be avoided (Evans, et al,
1996). The result showed that control had highest
TPC indicating the presence of H+ and heat treatment
may make the phenolic unstable. The hydrolyzation
of phenolic acid under alkaline and strong acidic
condition will induce the decrease of the number of
phenolic (Medina, et al, 2007). From the result, the
highest remaining TPC was found in the acetone
extract at pH 7.0 with lowest heat condition.
Figure 1: Effect of heating condition and pH on stability of
total phenolic content of Samui extract.
3.2 DPPH Radical-Scavenging Activity
Assay
The stability of Samui extracts from three various
solvents on DPPH radical-scavenging activity is
shown in Figure 2. DPPH values significantly
decreased in all samples after treated with pH and
16th AFC 2019 - ASEAN Food Conference
128
heating condition from 111.02 mg Trolox/g dry basis
(control) to 42.12-91.56 mg Trolox/g dry basis (p
<0.05). This result was similar to TPC result where
acetone extract had the highest stability followed by
ethanol and water (p<0.05), respectively.
The effect of pH and heating condition also
pronounced on the stability of plant extract (p≤0.05).
However, the heating condition had higher impact
compared with pH. As increasing heating
temperature, DPPH retention was lower. This could
be due to the decomposition of the antioxidant
compound associated with the phenolic compounds.
The lowest total phenolic content was attained under
high heat (Sulaiman, 2017). The result showed that
the control sample had highest DPPH indicating the
presence of the antioxidant activity related to the
number of phenolic hydroxyl and the electron-
donating ability of molecules (Ruenroeng- klin, et al,
2008).
Figure 2: Effect of heating condition and pH on stability of
DPPH radical-scavenging activity assay of Samui extract.
3.3 ABTS
+
Radical Scavenging Activity
Same as the result of TPC and DPPH, the control
sample had the highest ABTS
+
and after treatment, all
samples had significantly lower ABTS
+
(Table 1)
except the ethanolic extract. At pH 7, the ABTS
+
of
ethanolic extract (65.38-79.54 mg Trolox/g dry basis)
was higher than that of control 61.77 mg Trolox/g dry
basis. This may occur when some polyphenol from
reaction of ABTS
+
radicals and the antioxidants
added to the medium. Therefore, effective in the
ABTS
+
radical scavenging activity (Huyut, et al,
2017).
In addition, the phytochemical stability of
aqueous extract was the lowest. pH and heating
condition also affected the stability of ABTS
+
(p≤0.05). However, as increasing heating
temperature, ABTS
+
retention is lower resulting from
high temperature influencing on the inhibition the
process of hydrogen or electron donation (Sulaiman,
2017). The ABTS
+
radical is soluble in both aqueous
and organic solvents. Thus, ABTS
+
method evaluates
the antioxidant activity of both hydrophilic and
lipophilic compounds from the process of hydrogen
or electron donation (Aliakbarlu, et al, 2018). From
the result, the highest remaining ABTS
+
was found in
the ethanolic extract at pH 7.0 with lowest heat
condition.
Table 1: Effect of heating condition and pH on stability of
ABTS
+
radical scavenging activity of Samui (Micromelum
minutum) extract.
Solvent pH Temp
ABTS
mg Trolox/g dry basis
95%Ethanol
28.55±0.17
d
5
60 28.17±1.28
de
70 26.13±0.11
fg
80 23.63±1.20
ij
k
6
60
25.78±0.09
fgh
70
24.14±0.09
hij
80
22.86±0.90
jkl
7
60
41.63±0.50
a
70
37.23±1.43
b
80
33.99±0.31
c
60%Acetone
26.74±0.44
e
f
5
60 24.19±0.31
hij
70 22.24±0.12
kl
80 20.46±0.31
m
6
60
25.47±0.77
fgh
70
23.65±0.42
ijk
80
21.68±0.18
lm
7
60
28.11±1.64
de
70
24.75±2.02
ghi
80
23.39±0.41
ijk
Water
24.58±0.29
ghij
5
60 13.73±1.09
op
70 13.18±0.73
op
q
80 10.93±1.51
q
6
60
14.82±1.17
no
70
13.81±0.60
op
80
11.90±0.08
qr
7
60
15.93±0.64
n
70
14.60±0.51
nop
80
12.95±0.34
pq
Means with different superscript letters (a–z) in the
same column differ significantly (p< 0.05).
3.4 Ferric Reducing/Antioxidant Power
The value of FRAP (Table 2) from ethanolic extract
significantly decreased from 277.62 mg Trolox/g dry
basis (control) to 103.32-233.78 mg Trolox/g dry
basis (p <0.05). The result from acetone and aqueous
extract were similar. In addition, higher pH and
higher heating condition affected their stabilities. The
antioxidant potential of the extract was ascertained
from FRAP assay based on their ability to reduce
TPTZ-Fe
3+
complex to TPTZ-Fe
2+
. TPTZ-Fe
2+
is an
intensive blue color and can be monitored at 593 nm.
Reducing power is associated with antioxidant
Effect of Heating Condition and pH on Stability of Total Phenolic Content and Antioxidant Activities of Samui (Micromelum minutum)
Extract
129
activity and may serve as a significant reflection of
the antioxidant activity (Tinrat, 2016). From the
result, the highest remaining FRAP was found in the
water extract at pH 5.0 with the lowest heat condition.
Table 2: Effect of heating condition and pH on stability of
Ferric reducing/antioxidant power of Samui (Micromelum
minutum) extract.
Solvent pH Temp
FRAP
mg Trolox/g dry basis
95%Ethanol
256.87±0.36
c
5
60 241.88±0.83
d
70 227.42±2.30
e
80 205.66±1.51
g
6
60 201.37±1.55
gh
70 179.68±1.32
jk
80 154.22±1.03
m
7
60 179.58±1.17
jk
70 129.62±1.48
o
80 117.85±1.41
p
60%Acetone
274.43±2.28
b
5
60 217.69±0.62
f
70 178.25±1.82
k
80 142.97±1.46
n
6
60 198.35±1.55
ghi
70 191.79±0.24
hi
80 167.32±1.25
l
7
60 222.70±0.87
ef
70 199.54±1.19
gh
80 153.42±0.87
m
Water
299.47±0.86
a
5
60 272.58±1.02
b
70 230.76±1.02
e
80 199.13±1.30
gh
6
60 205.80±1.02
gh
70 188.94±1.24
ij
80
152.78±0.81
m
7
60 173.82±0.84
kl
70 127.26±0.84
o
80
104.16±0.55
q
Means with different superscript letters (a–z) in the
same column differ significantly (p< 0.05).
3.5 Ant-Thiobarbituric Acid Reactive
Substances
The values of ant-TBARs radical scavenging activity
are shown in Table 3. Ant-TBARs decreased in all
samples after treated with pH and heating condition.
However, it was noted that acetone extract had less
stability when exposed to pH and heat treatment
which is not similar to the result of TPC and other
antioxidant capacities. Ant-TBARs follow lipid
oxidation by product analysis as produce from the
propagation of lipid oxidation such as
hydrogenperoxide compound and radical of
hydrocarbon that reacts with thiobarbituric acid and
form to complex compound (pink to red of color). If
extraction leads to high of Ant-TBARs, it is well
inhibit lipid oxidation (Maisuthisakul, et al, 2007).
The highest remaining Ant-TBARs was found in the
water extract at pH 7.0 with lowest heat condition.
Table 3: Effect of heating condition and pH on stability of
Ant-Thiobarbituric acid reactive substances of Samui
(Micromelum minutum) extract.
Sovent pH Temp
Ant-TBARs
mg BHT/g dry basis
95%Ethanol
1127.61±0.77
b
5
60 838.91±1.55
de
f
70 759.62±0.44
fgh
80 719.31±1.99
h
6
60 873.02±1.55
de
70 812.97±1.17
efg
80 744.29±1.33
gh
7
60 1252.00±2.63
a
70 1216.50±0.92
a
80 1054.08±2.63
bc
60%Acetone
1001.72±0.39
c
5
60 469.10±1.11
lmn
70 417.79±1.69
mn
80 409.42±0.87
n
6
60 500.85±1.37
klm
70 477.32±1.61
lmn
80 521.14±8.99
jkl
7
60 627.16±0.65
i
70 598.17±2.83
ij
80 555.29±0.74
ijkl
Water
1008.22±0.39
c
5
60 556.54±0.64
ijkl
70 546.19±1.06
ijkl
80 528.23±0.87
jkl
6
60 592.01±1.06
ij
70 577.95±0.73
ijk
80 555.85±1.75
ijkl
7
60 915.58±1.43
d
70 832.36±1.64
ef
80 803.48±1.14
efg
Means with different superscript letters (a–z) in the
same column differ significantly (p< 0.05).
4 CONCLUSIONS
In conclusion, the extraction solvent affected on the
stability of Samui (Micromelum minutum) extracts.
Acetone extract seem to be more stability in TPC and
antioxidant capacities except of TBARs when
16th AFC 2019 - ASEAN Food Conference
130

compared to ethanolic and aqueous extracts.
Additionally, pH and heat conditions significantly
affected the stability of TPC and antioxidant
capacities (p≤0.05). Generally, TPC and antioxidant
capacities were generally higher at higher pH. On the
other hand, the increasing heat condition significantly
deteriorated the TPC and antioxidant capacities. All
factors influenced the stability of bioactive
compounds and their activities which need to be
considered when applying the extract into food.
REFERENCES
Aliakbarlu, J., Ghiasi, S., & Gilani, B.B. 2018. Effect of
extraction conditions on antioxidant activity of barberry
(Berberis vulgaris L.) fruit extracts. Veterinary
Research Forum. Volume 9(4), pp. 361–365.
Altemimi, A., Lakhssassi, N., Baharlouei, A., & Watson,
DG. 2017. Lightfoot DA. Phytochemi cals: Extraction,
Isolation, and Identification of Bioactive Compounds
from PlantExtract. Plants (Basel). Volume 6(4), pp. 42.
Areekul, S. 2552. Knowledge of wild plant use in the north
of Thailand 2. Chiang Mai: Royal Project Foundation.
Areekul, V., & Promkraiwan, N. 2009. Anti-rancidity effect
of plant extracts in emulsion. Bangkok: Master of
Science Thesis. Faculty of Agro-Industry, King
Mongkut's Institute of Technology Ladkrabang. pp.
103.
Benzie, I.F.F., & Strain, J.J. 1996. The ferric reducing
ability of plasma (FRAP) as a measure of ‘‘antioxidant
power’’: the FRAP assay. Analytical Biochemistry.
Volume 239(1), pp. 70–76.
Bunyapraphatsara, N. & Chokchaijareunporn, O.H. 2000.
Local plants. Search PHARM Database: Faculty of
Pharmacy, Mahidol University. Bangkok.Thailand.
Cherkupally, R., Kota, S. R., Amballa, H., & Reddy, B.N.
2017. In vitro antifungal potential of plant extracts
against Fusarium oxysporum, Rhizoctonia solani and
Macrophomina phaseolina. Annals of Plant Sciences.
Volume 6(9), pp. 1676-1680.
Evans, R., C.A., Miller, N.J., & Paganga, G. 1996.
Structure-Antioxidant Activity Relationships of
Flavonoids and Phenolic Acids. Free Radical Biology
and Medicine. Volume 20(7), pp. 933–956.
Friedman, M., & Jurgens, H.S. 2000. Effect of pH on the
Stability of Plant Phenolic Compounds. Journal of
Agricultural and Food Chemistry. Volume 48(6), pp.
2101-2110.
Huyut, Z., Beydemir, F., & Gulcin, I. 2017. Antioxidant and
Antiradical Properties of Selected Flavonoids and
Phenolic Compounds. Biochemistry Research Inter-
national. 10. Article ID 7616791,https://doi.org/10.11 5
5/2017/7616791.
Maisuthisakul, P., Suttajit, M., & Pongsawatmanit, R. 2007.
Characterization of the phytochemicals and antioxidant
properties of extracts from Teaw (Cratoxylum
formosum Dyer). Food Chemistry. Volume 100, pp.
1620-1629.
Maisuthisakul, P., Suttajit, M., & Pongsawatmanit, R. 2007.
Characterization of the phytochemicals and antioxidant
properties of extracts from Teaw (Cratoxylum
formosum Dyer). Food Chemistry. Volume 100, pp.
1620-1629.
McDonald, R.E., & Hultin H.O. 1987. Some characteristics
of the enzymic lipid oeroxidation system in the
microsomal fraction of flounder skeletal muscle.
Journal of Food Scienc.
Volume 52, pp. 15-21.
Medina, I., Gallardo, J.M., & González, M.J. 2007. Effect
of Molecular Structure of Phenolic Families as
Hydroxycinnamic Acids and Catechins on Their
Antioxidant Effectiveness in Minced Fish Muscle.
Journal of Agricultural and Food Chemistry. Volume
55(10), pp. 3889–3895.
Murakami, M., Shukla, Y.N., Jain, S.P., & Kumar, S. 2004.
Effect of thermal treatment on radical-scavenging
activity of single and mixed polyphenolic compounds.
Journal of Food Science. pp. 69.
Promkraiwan, N. 2009. Antioxidant capacities and active
constituents of some wild plant extracts. King
Mongkut's Institute of Technology Ladkrabang:
Faculty of Agro-Industry; Bangkok: Master of Science
Thesis. Faculty of Agro-Industry, King Mongkut's
Institute of Technology Ladkrabang. pp. 158.
Ruenroengklin, N., Zhong, J., Duan, X., Yang, B., Li, J., &
Jiang, Y. 2008. Effects of Various Temperatures and
pH Values on the Extraction Yield of Phenolics from
Litchi Fruit Pericarp Tissue and the Antioxidant
Activity of the Extracted Anthocyanins. International
Journal of Molecular Sciences. 9; 1333-1341. DOI:
10.3390/ijms 9071333.
Sakunpak, A., Matsunami, K., Otsuka, H., &
Panichayupakaranant, P. 2013. Isolation of new
monoterpene coumarins from Micromelum minutum
leaves and their cytotoxic activity against Leishmania
major and cancer cells. Food Chemistry. Volume 139,
pp. 458-463.
Shaghaghi, M., Manzoori, J.L., & Jouyban, A. 2008.
Determination of total phenols in tea infusions, tomato
and apple juice by terbium sensitized fluorescence
method as an alternative approach to the Folin-
Ciocalteu spectrophotometric method. Food Chemistry.
Volume 108, pp. 695-701.
Soto, E.R., Quijal, F.J.M., Cilla, A., Munekata, P.E.S.,
Lorenzo, J.M., Remize, F. & Barba, F.J. 2019.
Influence of Temperature, Solvent and pH on the
Selective Extraction of Phenolic Compounds from
Tiger Nuts by-Products: Triple-TOF-LC-MS-MS
Characterization. Molecules. Volume 24(4), pp. 797.
Sulaiman, I.S.C., Basri, M., Masoumi, H.R.F., Chee, W.J.,
Ashari, S.E., & Ismai, M. 2017. Effects of temperature,
time, and solvent ratio on the extraction of phenolic
compounds and the anti-radical activity of
Clinacanthus nutans Lindau leaves by response surface
methodology. Chemistry Central Journal. Volume 11,
pp. 54. DOI: 10.1186/s13065-017-0285-1.
Effect of Heating Condition and pH on Stability of Total Phenolic Content and Antioxidant Activities of Samui (Micromelum minutum)
Extract
131

Sun, H.N, Mu, T.M., Xi, & L.S. 2017. Effect of pH, heat,
and light treatments on the antioxidant activity of sweet
potato leaf polyphenols. International Journal of Food
Properties. Volume 20(2), pp. 318-332. DOI: 10
.1080/10942912.2016.1160410.
Thomas, R., Jebin, N., Saha, R., & Sarma, D.K. 2016.
Antioxidant and antimicrobial effects of kordoi
(Averrhoa carambola) fruit juice and bamboo
(Bambusa polymorpha) shoot extract in pork nuggets.
Food Chemistry. Volume 190, pp. 41-49.
Tinrat, S. 2016. Antioxidant Activities and Total Phenolic
Content of Multi-colored Fruits and Vegetables in
Thailand. KKU Research Journal. Volume 21(1), pp.
11.
van Valkenburg, J.L.C.H., & Bunyapraphatsara, N. 2001.
Plant Resources of South-East Asia. Journal of Natural
Products. Volume 12(2), pp. 350.
Zhou, K., & Yu, L. 2004. Antioxidant properties of bran
extracts from Trego wheat grown at different location.
Journal of Agricultural and Food Chemistry. Volume
52, pp. 1112-1117.
16th AFC 2019 - ASEAN Food Conference
132
