
Optimum Condition for the Production of N-acetylglucosamine from
Tiger Shrimp Shells using Semi Pure Extracellular Chitinase Enzyme
Produced by Mucor circinelloides
Yuniwaty Halim
1
, Hardoko
1,2
, Nicholas Candra
1
and Ratna Handayani
1
1
Food Technology Department, Universitas Pelita Harapan, Jl. M.H Thamrin Boulevard, Tangerang, Indonesia
2
Faculty of Fisheries and Marine Sciences, Brawijaya University, Jl. Veteran, Malang, Indonesia
Keywords: Chitin, Chitinase Enzyme, Glucosamine, Mucor circinelloides, Tiger Shrimp Shells.
Abstract: Chitin is a biodegradable polysaccharide, commonly found in shrimp shells and further processed into its
derivatives, such as glucosamine that is extensively used in dietary supplements for the treatment
of osteoarthritis, knee pain and back pain. This research was conducted to determine the optimum pH,
temperature, substrate concentration and fermentation time for semi pure extracellular chitinase enzyme from
Mucor circinelloides to be used in N-acetylglucosamine production. The optimum pH was determined at
different pH of 3, 4, 5, 6, 7, 8 and 9 and optimum temperature was determined at 30, 40, 50, 60, 70 and 80°C
by measuring chitinase activity. Substrate concentration varies from 0.5, 1.0, 1.5 and 2.0% and fermentation
time varies from 2, 4, 6 and 24 hours were used to determine the optimum condition for N-acetylglucosamine
production. Results showed that optimum pH of extracellular chitinase enzyme produced by Mucor
circinelloides with colloidal chitin as a substrate was 8 with chitinase activity of 5.76 ± 0.17 U/ml and
optimum temperature was 50°C with chitinase activity of 6.78 ± 0.13 U/ml. The optimum substrate
concentration of extracellular chitinase enzyme with chitin as substrate was 1.5% chitin with concentration
of N-acetylglucosamine produced of 1285.73 ± 66.19 ppm and the optimum fermentation time was 2 hours
with concentration of N-acetylglucosamine produced of 1322.71 ± 45.43 ppm.
1 INTRODUCTION
Chitin is a non-toxic biodegradable polysaccharide
that is commonly found in shrimp shells. Chitin is
hard to be absorbed by human body because chitin
has low solubility and large molecular size.
Therefore, it is commonly further processed into its
derivatives, such as glucosamine and chitosan (Haliza
and Suhartono, 2012). Glucosamine is a derived
chitin monomer and an important precursor in the
biosynthesis of glycolipids, glycoproteins and
proteoglycans proven to be involved in maintaining
joint health (Kardiman, 2013). Glucosamine can be
found naturally in the human body and is a precursor
for the biochemical synthesis of glycosaminoglycans
found in cartilage. Glucosamine is extensively used
in dietary supplements for the treatment of
osteoarthritis, knee pain and back pain (Benavente et
al., 2015). The conventional production of
glucosamine using chemical treatment has many
disadvantages as it is not environment friendly due to
acidic wastes, the yield is low and hard to control
(Sashiwa et al., 2002). Enzymatic hydrolysis method
as an alternative treatment using chitin directly from
crab or shrimp shells is faster, simpler and more
environmentally friendly compared to chemical
treatment (Krokeide et al., 2007). Glucosamine
produced by enzymatic hydrolysis is in the form of
N-acetylglucosamine.
Mucor circinelloides is one of the filamentous
fungi that produce chitinase enzyme to degrade chitin
into glucosamine (Shubakov and Kucheryavykh,
2004). In this research, chitin degradation into
glucosamine is done using semi pure extracellular
chitinase enzyme produced and collected from
Mucorcircinelloides because of the ability of Mucor
circinelloides to secrete chitinase enzyme
extracellularly (Luong et al., 2010) and higher purity
of chitinase enzyme increases the chitinase enzyme
activity (Suryadi
a
et al., 2013). The aim of this
research was to utilize Tiger shrimp (Penaeus
monodon) shells to produce N-acetylglucosamine
enzymatically using extracellular semi pure chitinase
Halim, Y., Hardoko, ., Candra, N. and Handayani, R.
Optimum Condition for the Production of N-acetylglucosamine from Tiger Shrimp Shells using Semi Pure Extracellular Chitinase Enzyme Produced by Mucor circinelloides.
DOI: 10.5220/0009980500002964
In Proceedings of the 16th ASEAN Food Conference (16th AFC 2019) - Outlook and Opportunities of Food Technology and Culinary for Tourism Industry, pages 185-191
ISBN: 978-989-758-467-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
185

enzyme from Mucor circinelloides. Several
parameters that contribute to enzymatic reaction, such
as pH, temperature, substrate concentration and
fermentation time were also determined.
2 MATERIALS AND METHODS
The materials used in this research were chitin
isolated from Tiger shrimp shells (Penaeus monodon)
obtained from PT. Lola Mina, Muara Baru, Jakarta,
Mucor circinelloides culture isolated from Tiger
shrimp shells, Potato Dextrose Agar (PDA)
“MERCK” and Potato Dextrose Broth (PDB) “DB
Filco”, N-acetylglucosamine standard “SIGMA
ALDRICH”, 3-5 dinitro salicylic acid “MERCK”,
bovine serum albumin (BSA), dipotassium phosphate
(K2HPO4), potassium dihydrogen phosphate
(KH2PO4), magnesium sulphate heptahydrate
(MgSO4.7H2O), ammonium sulphate ((NH4)2SO4),
disodium hydrogen phosphate (Na2HPO4),
potassium sodium tartrate (Na-K-tartrate),
phosphoric acid (H3PO4), distilled water, NaOH
solution (3.5%, 10 N), HCl solution (37%, 1 M),
tartaric acid 10% and ethanol 96%. The equipment
used in this research were incubator shaker
“HEIDOLPH 22UNIMAX 1010”, analytical balance
“OHAUS U-1800 AR 2140”, oven “MEMMERT”,
centrifuge “MPW-223e”, microcentrifuge
“HETTICH ZENTRIFUGE EBA 20”, microscopic
camera “OLYMPUS DP21”, UV-VIS
spectrophotometer “THERMO SCIENTIFIC
GENESYS 10S”, pH meter “METROHM 913”,
quartz cuvette “HELLMA Analytics”, micropipette
and glassware.
2.1 Colloidal Chitin Preparation (Setia
and Suharjono, 2015)
Colloidal chitin was prepared as substrate in optimum
pH and optimum temperature determination and
added in culture media to induce the chitinase
production. Ten grams of isolated chitin was added
with 140 ml of 37% HCl and stirred with magnetic
stirrer for 2 hours to dissolve the chitin. The mixture
was added with 500 ml of absolute ethanol and
filtered with Buchner funnel. The residue obtained
was added with 5 N of NaOH until the pH reached
neutral and centrifuged with speed 4000 rpm for 5
minutes. The precipitate obtained was collected as the
colloidal chitin.
2.2 Production of Semi Pure Chitinase
(Jenifer et al., 2014)
About 5 ml of Mucor circinelloides spore culture was
added into 250 ml Potato Dextrose Broth (PDB)
media containing 0.5% colloidal chitin, 0.5%
Na2HPO4 and 0.5% MgSO4.7H2O. The mixture was
incubated for 2 days in incubator shaker at room
temperature. The grown culture suspension was
centrifuged at 3300 rpm for 10 min at 4°C and the
supernatant obtained was the extracellular crude
chitinase. The extracellular crude chitinase was
precipitated with 90% ammonium sulphate while
stirred at 4°C. This suspension left for 24 hours at 4°C
then centrifuged at 3300 rpm for 10 minutes. The
precipitate was taken and dissolved in 8 ml of 0.05 M
phosphate buffer solution (pH 8) per 150 ml of grown
culture suspension (Lawati, 2013). The precipitate
taken was the extracellular semi pure chitinase
enzyme and was stored at 4°C prior to usage in
fermentation.
2.3 Determination of Optimum pH and
Temperature (Jenifer et al., 2014)
1 ml of 0.5% colloidal chitin in each pH buffers (pH
3-9) were added by 1 ml of semi pure chitinase
enzyme then incubated in room temperature for 1
hour. The optimum pH was determined from highest
chitinase activity. Furthermore, 1 ml of 0.5%
colloidal chitin in the optimum pH buffer were added
by 1 ml of semi pure chitinase enzyme then incubated
in temperature of 30, 40, 50, 60, 70 and 80°C for 1
hour. The optimum fermentation temperature was
determined from highest chitinase activity.
2.4 Determination of Optimum
Substrate Concentration and
Fermentation Time (Herdyastuti et
al., 2009)
Different levels of chitin concentration of 0.5, 1, 1.5
and 2% chitin were added to the optimum pH buffer.
1 ml of different concentration of chitin in the
optimum pH buffer were added by 1 ml of semi pure
chitinase enzyme then incubated in the optimum
temperature for 2, 4, 6 and 24 h. The optimum
substrate concentration and fermentation time were
determined by the highest N-acetylglucosamine
concentration.
16th AFC 2019 - ASEAN Food Conference
186

2.5 Analysis of Chitinase Enzyme
Activity (Rahmansyah and
Sudiana, 2003)
Chitinase activity was measured using DNS
(dinitrosalicylic) colorimetric method. The
determination of chitinase activity used colloidal
chitin as the substrate. 1 gram of 3,5-dinitrosalicylic
acid (DNS) was dissolved in 20 ml distilled water
then added with 1 gram of NaOH, 0.2 gram of phenol
and 0.05 gram of sodium sulphite. The mixture was
then transferred and diluted into 100 ml volumetric
flask. The mixture was then centrifuged at 3300 rpm
for 5 minutes. 1 ml of supernatant was taken and
added with 2 ml of modified DNS and 1 ml of 4%
potassium sodium tartrate. The mixture then heated
for 15 minutes at boiling temperature. The mixture
then was observed using spectrophotometer at 540
nm. Chitinase activity were calculated using the
formula:
Chitinase Activity (U/ml) =
Nace
t
ylglucosamineconcen
t
ra
t
ion
x1000x
enzy
m
e
Nace
t
ylglucosaminemolecularweigh
t
x
incuba
t
ionperiod
h
(1)
2.6 N-acetylglucosamine Concentration
Quantification (Rahmansyah and
Sudiana, 2003)
Glucosamine standard curve was prepared for
quantification of N-acetylglucosamine concentration.
Blank was first prepared by adding 2 ml of modified
DNS and 1 ml of 4% potassium sodium tartrate into
1 ml of distilled water then heated for 15 minutes in
boiling temperature. N-acetylglucosamine standard
was prepared in concentration of 200, 400, 600, 800
and 1000 ppm. 1 ml from each concentration was
added with 2 ml of modified DNS and 1 ml of 4%
potassium sodium tartrate. The mixture heated for 15
minutes in boiling temperature. The heated mixture
of blank and each concentration of glucosamine
standard were observed by spectrophotometer at 540
nm. N-acetylglucosamine content calculated by using
the linear equation of glucosamine standard. 1 ml of
N-acetylglucosamine from the fermentation was
added with 2 ml of modified DNS and 1 ml of 4%
potassium sodium tartrate. The mixture was heated at
boiling temperature for 15 minutes and the
absorbance observed using spectrophotometer at 540
nm.
2.7 Data Analysis
The experimental design used was Completely
Randomized Factorial Design with 1 factor for
optimum pH or temperature determination and 2
factors for optimum substrate concentration and
fermentation time determination. All data obtained
were analyzed using SPSS version 22.
3 RESULT AND DISCUSSION
3.1 Characteristics of Isolated Chitin
Chitin used in this research was isolated from Tiger
shrimp shells through demineralization and
deproteination processes and then analysed for its
yield, moisture content, protein content, ash content
and degree of deacetylation. The results can be
observed on Table 1.
Table 1: Characteristics of isolated chitin.
Parameter Content
Yield (% db) 8.85 ± 0.33
Moisture content (% wb) 4.59 ± 0.32
Ash content (%) 0.46 ± 0.03
Protein content (%) 1.74 ± 0.07
Degree of Deacetylation (%) 28.07
Yield of isolated chitin obtained in this research
was lower compared to the previous study by Hossain
and Iqbal (2014) that showed the yield content of
chitin from shrimp shell is in range of 13.12-17.36%.
The moisture content of isolated chitin is comparable
to the previous studies (Arif et al., 2013; Isa et al.,
2014; Liu et al., 2013; Sanusi, 2004) that showed the
moisture content of chitin is fewer than 10%, i.e.
about 8.70%, 7.64% and 5.22%, respectively. The ash
content of chitin is lower compared to the previous
studies by Arif et al. (2013), Isa et al. (2014) and Liu
et al. (2013) that was about 2%, 5.60% and 1.59%,
respectively. This shows that the demineralization
process in this research was more effective to remove
the minerals from shrimp shells. Furthermore, protein
content of isolated chitin was lower compared to by
Arif et al. (2013) that showed that the protein content
of isolated chitin was 4.16%. This result also shows
that the deproteination process in this research was
more effective. Degree of deacetylation is an
indicator of chitin purity (Sanusi, 2004). The degree
of deacetylation (DD) of isolated chitin obtained was
28.07%, in accordance with previous researches (Arif
et al., 2013; Younes and Rinaudo, 2015) that
mentioned degree of deacetylation of chitin was 15 -
70% and less than 50%, respectively.
Optimum Condition for the Production of N-acetylglucosamine from Tiger Shrimp Shells using Semi Pure Extracellular Chitinase Enzyme
Produced by Mucor circinelloides
187
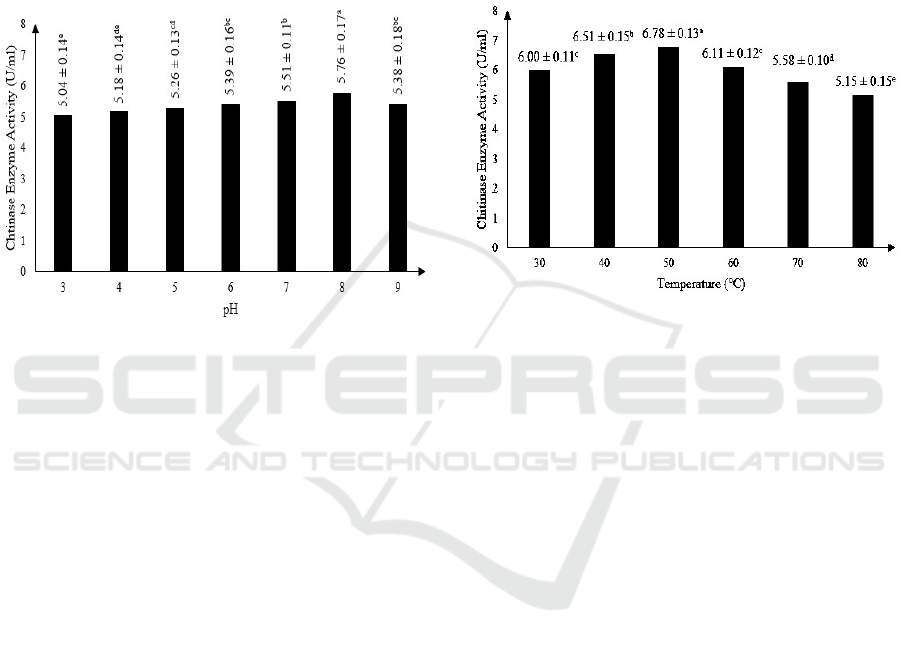
3.2 Effect of pH on Semi Pure
Extracellular Chitinase Activity
Statistical analysis using ANOVA shows that pH
gave significant effect on enzyme activity of chitinase
produced by Mucor circinelloides. Results also show
that the optimum pH for semi pure extracellular
chitinase activity is at pH 8, with enzyme activity of
5.76 ± 0.17 U/ml. The results can be observed on
Figure 1.
Figure 1: The effect of pH on chitinase enzyme activity
(Note: Different letter notations indicates a significant
difference at p≤0.05).
This result is in accordance with a previous study
who stated that the chitinase activity may increase
and decrease because of the difference in pH as a
factor (Purkan et al., 2014). Chitinase enzyme
produced by M. circinelloides that is optimum at pH
8, is similar to chitinase enzyme produced by
Moniliophthora perniciosa (Galante et al., 2012),
Aeromonas sp. (Haliza and Suhartono, 2012) and
Bacillus cereus (Suryadib et al., 2013) that were also
optimum at pH 8.
3.3 Effect of Temperature on Semi
Pure Extracellular Chitinase
Activity
Statistical analysis using ANOVA shows that
temperature of reaction gave significant effect on
enzyme activity of chitinase produced by Mucor
circinelloides. Results also show that the optimum
temperature for semi pure extracellular chitinase
activity is at 50
o
C, with enzyme activity of 6.78 ±
0.13 U/ml. The results can be observed on Figure 2.
Temperature affects the kinetic energy of
molecule which can accelerate enzyme hydrolysis
reaction with substrate when the temperature is raised
(Murray et al., 2005). However, after optimum
temperature is reached, the chitinase enzyme activity
decrease as the temperature is raised because enzyme
can be denatured in high temperature (Lehninger et
al., 2004). The optimum temperature of 50°C
obtained in this research is similar to chitinase
enzyme produced by Aspergillus tereus (Farag et al.,
2016) and Serratia marcescens (Zeki and Muslim,
2010). Chitinase enzyme produced by M.
circinelloides can be considered as thermostable.
Figure 2: The effect of temperature on chitinase enzyme
activity (Note: Different letter notations indicates a
significant difference at p≤0.05).
Temperature affects the kinetic energy of
molecule which can accelerate enzyme hydrolysis
reaction with substrate when the temperature is raised
(Murray et al., 2005). However, after optimum
temperature is reached, the chitinase enzyme activity
decreases as the temperature is raised because enzyme
can be denatured in high temperature (Lehninger et
al., 2004). The optimum temperature of 50°C
obtained in this research is similar to chitinase
enzyme produced by Aspergillus tereus (Farag et al.,
2016) and Serratia marcescens (Zeki and Muslim,
2010). Chitinase enzyme produced by M.
circinelloides can be considered as thermostable.
3.4 Effect of Substrate Concentration
and Fermentation Time on
N- acetylglucosamine Production
Statistical analysis using Univariate shows that
interaction between substrate concentration and
fermentation time did not affect the N-
acetylglucosamine (NAG) concentration produced by
chitinase enzyme. However, substrate concentration
and fermentation time affected the NAG
concentration produced by chitinase enzyme. These
results can be observed on Figure 3 and Figure 4.
16th AFC 2019 - ASEAN Food Conference
188
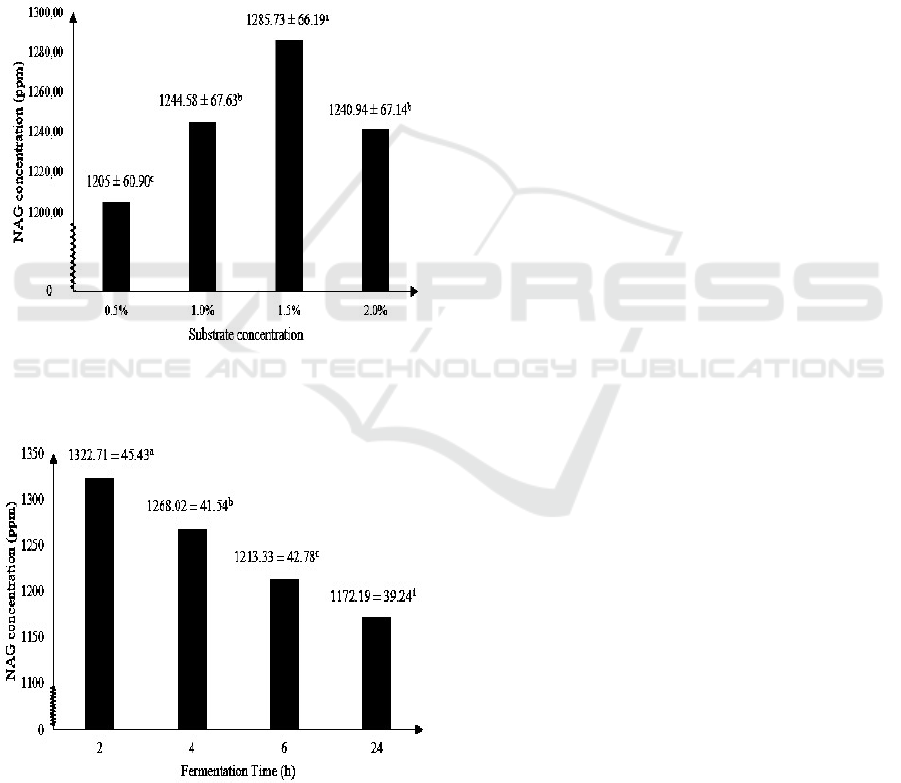
Figure 3 shows the highest NAG produced by
chitinase enzyme of M. circinelloides with isolated
chitin as substrate is at 1.5% substrate concentration,
i.e. about 1285.73 ± 66.19 ppm. The optimum
substrate concentration of 1.5% is according to a
previous study that stated chitinase activity reached
maximum activity at chitin concentration of 1.5%, in
which optimum chitinase activity maximizes the
production of NAG (Karthik et al., 2014). Optimum
substrate concentration for chitinase isolated from M.
circinelloides of 1.5% is also in accordance with
optimum substrate concentration of chitinase isolated
from Streptomyces viridificans (Gupta et al., 1995)
and Aeromonas sp. (Huang et al., 1996).
Figure 3: The effect of substrate concentration on NAG
concentration (Note: Different letter notations indicates a
significant difference at p≤0.05).
Figure 4: The effect of fermentation on NAG concentration
(Note: Different letter notations indicates a significant
difference at p≤0.05).
Figure 4 shows that the highest NAG
concentration produced by chitinase enzyme of M.
circinelloides is at 2 hours of fermentation, i.e. about
1322.71 ± 45.43 ppm. The ability of chitinase to
degrade chitin structure affected the time needed to
produce NAG (Wulandari, 2009). Chitinase activity
isolated from Aeromonas sp. reached maximum
chitinase activity of 8.7 U/ml at 72 hours of
incubation (Younes et al., 2013), Trichoderma
harzianum reached maximum chitinase activity of 5.4
U/ml after incubation of 72 hours (Sandhya et al.,
2005) and Streptomyces rubiginosus reached
maximum chitinase activity of 2.2 U/ml after 72 h of
incubation (Jha et al, 2016). This also means that
chitinase produced by Mucor circinelloides reaches
its maximum activity and NAG production much
faster compared to chitinase produced by Aeromonas
sp., Trichoderma harzianum and Streptomyces
rubiginosu.
4 CONCLUSIONS
This research confirmed the potency of extracellular
semi pure chitinase enzyme produced by Mucor
circinelloides as an alternative method to produce N-
acetylglucosamine. The optimum pH of extracellular
semi pure chitinase from Mucor circinelloides is 8
with chitinase activity of 5.76 ± 0.17 U/ml and the
optimum temperature is 50°C with chitinase activity
of 6.78 ± 0.13 U/ml. The optimum substrate
concentration to produce N-acetylglucosamine is
1.5% of chitin with 1285.73 ± 66.19 ppm of N-
acetylglucosamine produced and the optimum
fermentation time is 2 hours with concentration of N-
acetylglucosamine produced is about 1322.71 ± 45.43
ppm.
ACKNOWLEDGEMENTS
The authors would like to thank Center of Research
and Community Development, Universitas Pelita
Harapan, Tangerang, Indonesia for financially
supporting this research through Project no: P-
0004/FaST/I/2018.
REFERENCES
Arif, A.R., Ischaidar, N., Hasnah, Dali, S. 2013. Isolasi
Kitin dari Limbah Udang Putih (Penaeus merguiensis)
secara Enzimatis. Seminar Nasional Kimia, pp. 10-16.
Benavente, M., Arias, S., Moreno, L., Martinez, J. 2015.
Production of Glucosamine Hydrochloride from
Optimum Condition for the Production of N-acetylglucosamine from Tiger Shrimp Shells using Semi Pure Extracellular Chitinase Enzyme
Produced by Mucor circinelloides
189

Crustacean Shell. Journal of Pharmacy and
Pharmacology, volume 3, pp. 20-26.
Farag, A.M., Hanan, M.A., Hassan, A.H., Moustafa, E.
2016. Purification, Characterization and Antimicrobial
Activity of Chitinase from Marine-derived Aspergillus
terreus. The Egyptian Journal of Aquatic Research,
volume 42(2), pp. 185-192.
Galante, R.S., Taranto, A.G., Koblitz, M.G.B., Góes-Neto,
A., Pirovani, C.P., Cascardo, J.C.M., Cruz, S.H.,
Pereira, G.A.G., De Assis, S.A. 2012. Purification,
Characterization and Structural Determination of
Chitinases Produced by Moniliophthora perniciosa.
Anais Da Academia Brasileira De Ciências, volume
84(2), pp. 469-486.
Gupta, R., Saxena, R.K., Chaturvedi, P.I., Virdi, J.S. 1995.
Chitinase Production by Streptomyces viridificans: Its
Potential in Fungal Cell Wall Lysis. Journal of Applied
Bacteriology, volume 78, pp. 378–383.
Haliza, W., Suhartono, M.T. 2012. Karakteristik Kitinase
dari Mikroba. Balai Teknologi Pascapanen Pertanian,
volume 8(1), pp. 1-14.
Herdyastuti, N., Raharjo, T., Mudasir, M., Matsjeh, S.
2009. Chitinase and Chitinolytic Microorganism:
Isolation, Characterization and Potential. Indo J Chem.,
volume 9(1), pp. 37-47.
Hossain, M.S., Iqbal, A. 2014. Production and
Characterization of Chitosan from Shrimp Waste. J.
Bangladesh Agric. Univ., volume 12(1), pp. 153–160.
Huang, J.H., Chen, C.J., Su, Y.C. 1996. Production of
Chitinolytic Enzymes from a Novel Species of
Aeromonas. J Ind Microbiol., volume 17, pp. 89-95.
Isa, M.T., Ameh, O.A., Danlami, A., Abutu, D. 2014.
Kinetic Modelling of the Demineralization of Shrimp
Exoskeleton using Citric acid. Leonardo Electronic
Journal of Practices and Technologies, volume 13(25),
pp. 99-108.
Jenifer, S., Jesayree, J., Laveena, D.K., Manikandan, K.
2014. Purification and Characterization of Chitinase
from Trichoderma viride N9 and Its Antifungal Activity
Against Phytopathogenic Fungi. World Journal of
Pharmacy and Pharmaceutical Sciences, volume 3(12),
pp. 1604-1611.
Jha, S., Hasmukh, A.M., Jha, C.K. 2016. Characterization
of Extracellular Chitinase Produced from Streptomyces
rubiginosus Isolated from Rhizosphere of Gossypium
sp. Cogent Food and Agriculture, volume 2(1), pp. 1-
12.
Kardiman, C. 2013. Manfaat Glukosamin, Kondroitin, dan
Metilsulfonilmetana pada Osteoartritis. Cermin Dunia
Kedokteran, volume 40(12), pp. 936-939.
Karthik, N., Akanksha, K., Binod, P., Pandey, A. 2014.
Production, Purification and Properties of Fungal
Chitinases. Indian Journal of Experimental Biology,
volume 52, pp. 1025-1035.
Krokeide, I.M., Synstad, B., Gaseidnes, S., Horn, S.J.,
Eijsink, V.G., Sorlie, M. 2007. Natural Substrate Assay
for Chitinases Using High Performance Liquid
Chromatography: a Comparison with Existing Assays.
Anal Biochem. volume 363, pp. 128-134.
Lawati, N. 2013. Pemurnian Parsial dan Karakterisasi
Enzim Kitinase dari Beauveria bassiana. Thesis. Institut
Pertanian Bogor, Bogor.
Lehninger, A.L., Nelson, D.L., Cox, M.M. 2014. Principles
of Biochemistry. Worth Press, New York, 4th edition.
Liu, L., Liu, Y., Shin, H., Chen, R., Li, J., Du, G., Chen, J.
2013. Microbial Production of Glucosamine and N-
acetylglucosamine: Advances and Perspectives. Appl
Microbiol Biotechnol., volume 97, pp. 6149–6158.
Luong, D.T., Tuan, N.A., Van, N.T., Yen, L.T.H., Versali,
M.F.J., Dommes, J., Duong, V.H. 2010. Study in an
Actinomycetes Producing Chitinase and Chitin
Deacetylase. AnnuRep, pp. 439-448.
Murray, R.K., Granner, D.K., Mayes, P. W., Rodwell, V.
W. 2005. Harper’s Illustrated 13 Biochemistry.
McGraw-Hill, New York, Twenty-sixth edition.
Purkan, Badiatul A., Baktir, A., Sumarsih, S. 2014.
Eksplorasi Bakteri Kitinolitik dari Sampah Organik:
Isolasi dan Karakterisasi Enzim Kitinase. Molekul,
volume 9(2), pp. 128-135.
Rahmansyah, M., Sudiana, I.M. 2003. Optimasi Analisis
Amilase dan Glukanase yang Diesktrak dari Miselium
Pleurotus ostreatus dengan Asam 3,5 Dinitrosalisilat.
Ber Penel Hayati. volume 9, pp. 7-12.
Sandhya, C., Binod, P., Nampoothiri, K.M, Szakacs, G.,
Pandey, A. 2005. Microbial Synthesis of Chitinase in
Solid Cultures and Its Potential as a Biocontrol Agent
against Phytopathogenic Fungus Colletotrichum
gloeosporioides. Applied Biochem Biotechnol.,
volume 127, pp. 1-15.
Sanusi, M. 2004. Transformasi Kitin dari Hasil Isolasi
Limbah Industri Udang Beku menjadi Kitosan. Mar
Chim Acta., volume 5(2), pp. 28-32.
Sashiwa, H., Fugishima, S., Yamano, N., Nakayama, A.,
Muraki, E., Hiraga, K., Oda, K., Aiba, S. 2002.
Production of N-acetyl-D-glucosamine from α-chitin
by Crude Enzymes from Aeromonas hydrophila H-
2330. Carbohydrate Research, volume 337, pp. 761-
763.
Setia, I.N., Suharjono. 2015. Chinolytic Assay and
Identification of Bacteria Isolated from Shrimp Waste
Based on 16S rDNA Sequences. Advances in
Microbiology, volume 5, pp. 541-548.
Shubakov, A.A., Kucheryavykh, P.S. 2004. Chitinolytic
Activity of Filamentous Fungi. Applied Biochemistry
and Microbiology, volume 40(5), pp. 445-447.
Suryadi, Y., Priyatno, T.P., Samudra, I.M., Susilowati,
D.N., Lawati, N., Kustaman, E. 2013a. Pemurnian
Parsial dan Karakterisasi Kitinase Asal Jamur
Entomopatogen Beauveria bassiana Isolat BB200109.
Jurnal AgroBiogen, volume 9(2), pp. 77-84.
Suryadi, Y., Priyono, T.P., Susilowati, D.N., Samudra,
I.M., Yudhistira, N., Purwakusumah, E.D. 2013b.
Isolasi dan Karakterisasi Kitinase Asal Bacillus cereus
11 UJ. Jurnal Biologi Indonesia, volume 9, pp. 51-62.
Wulandari, F. 2009. Optimasi Produksi N-asetilglukosamin
dari Kitin melalui Fermentasi oleh Aspergillus
rugulosus 501. Thesis. Institut Pertanian Bogor, Bogor.
Younes, I., Rinaudo, M. 2015. Chitin and Chitosan
Preparation from Marine Sources. Structure, Properties
16th AFC 2019 - ASEAN Food Conference
190

and Applications. Mar Drugs., volume 13(3), pp. 1133-
1174.
Younes, G., Zahra, D., Mohkam, M., Kargar, M. 2013.
Isolation and Optimization of Cultivation Conditions
for Production of Chitinase by Aeromonas sp. ZD_05
from the Persian Gulf. Journal of Pure and Applied
Microbiology, volume 7(2), pp. 913-918.
Zeki, N.H., Muslim, S.N. 2010. Purification,
Characterization and Antifungal Activity of Chitinase
from Serratia marcescens Isolated from Fresh
Vegetables. Ibn Al-Haitham J Pure Appl Sci., volume
23(1), pp. 13-25.
Optimum Condition for the Production of N-acetylglucosamine from Tiger Shrimp Shells using Semi Pure Extracellular Chitinase Enzyme
Produced by Mucor circinelloides
191
