
Effect of Heat Process for Prebiotic Properties of Taro Starch
(Colocasia Esculenta L. Schott)
R. Haryo Bimo Setiarto
1,2
, Harsi Dewantari Kusumaningrum
2
, Betty Sri Laksmi Jenie
2
,
Tatik Khusniati
1
, Nunuk Widhyastuti
1
and Sulistiani
1
1
Microbiology Division, Research Center for Biology, Indonesian Institute of Sciences (LIPI) Jalan Raya Jakarta,
Bogor Km 46, Cibinong Science Center, Cibinong, Bogor, 16911 West Java, Indonesia
2
Department of Food Science and Technology, Faculty of Agricultural Technology, Bogor Agricultural University,
Bogor 16680, West Java, Indonesia
Keywords: Heat Process, Prebiotic Properties, Taro Starch.
Abstract: This study investigates the effects of a variety of heat processes on the prebiotic properties of taro starch. The
taro starch is treated by the annealing process (24 hours, 50
o
C), the heat moisture treatment (HMT, moisture
25%, 3 hours, 110
o
C), and the autoclaving (15 minutes, 121
o
C) - cooling (24 hours, 4
o
C) cycles with 1, 2, and
3 cycles. The results show that all treatments improve the prebiotic properties of taro starch. The modified
taro starches (MTS) significantly increases their slow digestibility starch (SDS) and resistant starch (RS)
content, while the in-vitro digestibility, very rapid digestible starch (VRDS), rapid digestible starch (RDS)
are relatively decreased. However, the autoclaving-cooling two cycles (AC-2C) results in the MTS with the
best prebiotic properties as shown by its high RS content, low digestibility, high prebiotic effect, high prebiotic
index as well as prebiotics activity towards pathogenic bacteria. The AC-2C modified taro starch is very
prospective to be used as a prebiotic candidate.
1 INTRODUCTION
Colocasia esculenta L. schott known as taro was one
of the tubers from the Araceae family that is rich in
consumable starch (Aboubakar et al., 2009; Kaushal
et al., 2012; Zhu et al., 2015). The most common
consumed parts of taro were corm and cormel, the
thickening root which grows in the soil (Deka & Sit,
2016; Yu et al., 2018a). Taro was one of the most
cultivated tubers in the tropics and the subtropics,
including Southeast Asia, the Caribbean and the
North Atlantic Ocean, South and West Africa, Pacific
Islands and Polynesia (Aboubakar et al., 2009). The
utilization of Taro in Southeast Asia was still minimal
(Kaushal et al., 2012; Zhu et al., 2015; Deka & Sit,
2016). In the last few years, however, taro cultivation
had been increased due to its potential as the
functional food which contains up to 70 – 80 gram /
100 gram of starch content, 2 – 6 gram / 100 gram of
protein, 0.6 – 0.8 gram / 100 gram of fiber, vitamin,
phosphorus, magnesium, and calcium (Kaushal et al.,
2012; Zhu et al., 2015, Li et al., 2018a). Taro could
be widely applied in the food industry and processed
into consumable products such as pasta, starch, flour,
cereal bar, canned product, chips and beverage
powder (Li et al., 2018a; Muñoz-Cuervo et al., 2016).
The digestibility rate of taro starch was very high and
it had been applied to various food products because
of its unique structure and small particle of taro starch
granules (Kaushal et al., 2012; Muñoz-Cuervo et al.,
2016). The utilization of native taro starch was still
limited as it is high in retrogradation, thermal
decomposition, poor in process tolerance, narrow
peak viscosity range and resistant towards low shear
stress (Demirkesen-Bicak et al., 2018; Yu et al.,
2018b). These weaknesses increase the interest of
many researchers to modify starch so its functional
properties could be improved (Sullivan et al., 2017;
Sharlina et al., 2017; Oyeyinka et al., 2018). Starch
modification techniques could be carried out by its
physical, chemical or enzymatic properties (Hazarika
& Sit, 2016). Some physical modification techniques
could improve the functional properties of taro starch
including the cycle of cooling, autoclaving, HMT and
annealing.
Generally, the modification of taro starch was
conducted to enhance its functional properties as well
Setiarto, R., Kusumaningrum, H., Jenie, B., Khusniati, T., Widhyastuti, N. and Sulistiani, .
Effect of Heat Process for Prebiotic Properties of Taro Starch (Colocasia Esculenta L. Schott).
DOI: 10.5220/0009978900002833
In Proceedings of the 2nd SEAFAST International Seminar (2nd SIS 2019) - Facing Future Challenges: Sustainable Food Safety, Quality and Nutrition, pages 77-83
ISBN: 978-989-758-466-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
77

as its physicochemical characteristics. Setiarto et al.
(2018) modified the taro flour to improve its prebiotic
properties, using the fermentation technique and the
autoclaving-cooling cycling. Two cycles of the
autoclaving-cooling technique successfully increased
the RS content by 2.7-fold (from 4.13% to 11.15%)
compared to the control treatment. The modified taro
flour resulted shows a better prebiotic effect, index,
and activity than the control (the one without
fermentation and autoclaving-cooling). This study
aims to determine the physicochemical
characteristics and prebiotic properties of modified
taro starch by implementing annealing, HMT and
autoclaving-cooling treatments. The prebiotic
properties were assessed during this study to provide
the additional information on functional properties
for food industry application.
2 MATERIALS AND METHODS
2.1 Materials
The main raw material used in this study was the
Bogor Taro of Pandan (Colocasia esculenta) with
eight months harvest age, from Cijeruk Bogor West
Java, Indonesia. Lactobacillus plantarum SU-LS 36
and EPEC (Entero Pathogenic Escherichia coli) was
provided from The Laboratory of Food Microbiology,
Research Center for Biology, Indonesian Institute of
Science (LIPI).
2.2 Taro (Colocasia Esculanta) Starch
Extraction Process
Taro starch extraction was performed by applying the
technique from Airul et al. (2014) with a few
modifications. Taro tuber (Colocasia esculanta) was
peeled, washed, and soaked in the mixture of 1%
NaCl (3: 4) for an hour to remove oxalate crystals.
It was then shredded and mixed with distilled water
(3: 1) for one minute using a blender (Phillips,
Netherland). Double fold cotton cloth was utilized to
filter the taro pulp. The obtained taro pulp filtrate was
settled overnight to let the starch sink at the base of
the beaker glass. Taro pulp was centrifuged with High
Speed Centrifuge (Kubota, Japan) at 5000 rpm for 10
minutes to obtain taro starch. Distilled water was used
to clean the taro starch three times to remove the
supernatant. After that, it was oven dried in at 50oC
up to the constant weight. Finally, the dry taro starch
was ground using the disk mill (China).
2.3 Modification of Taro Starch
2.3.1 Annealing Treatment
The annealing treatment of taro starch was conducted
by the technique from Wang et al. (2018). Twelve
grams of taro starch was added to 60 ml of distilled
water with the ratio of taro starch: water (1: 5) (b / v)
was placed in a polyethylene bag. The annealing
treatment was carried out at 50
o
C for 24 hours by
inserting a polyethylene bag which had been tightly
closed into a water bath (Hitachi, Japan). Afterward,
the taro starch was freeze dried, crushed and filtered
using the 100-mesh sieve. The resulting taro starch
from the annealing process was then chilled at 4
o
C
prior to further analysis.
2.3.2 Heat-Moisture Treatment
The taro starch modification using HMT was
obtained following Deka & Sit (2016). Forty-five
grams of taro starch (dry-based) was placed into a
glass container, and distilled water was added to it
while stirring until the water content reached 25%.
Then, the glass container was sealed, balanced for 48
hours at room temperature then heated at 120°C in an
electric oven (Shimizu, Japan) for three hours. The
heated modified taro starch was then dried at 40°C for
overnight, milled and sieved with a 100-mesh sieve.
2.3.3 Autoclaving-Cooling Treatment
The autoclaving-cooling method of taro starch
followed the procedure by Setiarto et al. (2018). The
taro starch was added with aquadest at the ratio of 1:
2, heated in an autoclave (Hitachi, Japan) at 121°C for
15 minutes, then chilled in refrigerator at 4°C for 24
hours. Thereafter, the treated taro starch was dried
(70°C, 16 hours) in an oven (Shimidzu, Japan) until
the moisture content reached 12%, and milled using a
pin disk mill (Shimidzu, Japan). The starch was
sieved to obtain the 100-mesh taro starch. The
autoclaving-cooling treatment was also completed
with two cycles and three cycles.
2.4 In-vitro Digestibility and Digestible
Starch Composition Analysis
In-vitro starch digestibility was analyzed by
measuring the level of maltose as the product of
hydrolysis taro starch by using α-amylase enzyme
(Sigma) compared to starch solution. This analysis
was performed by referring to a method from
Anderson et al., (2002). The absorbance of sample
2nd SIS 2019 - SEAFAST International Seminar
78

and blank solutions was determined by
Spectrophotometer UV-Vis (Shimadzu UV-1800,
Japan) at 520 nm. In this study, the calculation of the
starch digestibility (%) is shown in the following
formula:
Starch digestibility (%) =
x
100%
(1)
The digestible starch composition analysis was
conducted in this study by following Englyst et al.,
(1992) method. There are four types of starch
compositions based on their digestibility times. The
first type is called very rapid digestible starch
(VRDS), which is expressed as the amount of
digested starch in the first minute by porcine
pancreatin and amyloglucosidase 210 U as explained
in the Sigma Cat. No. P7545 and No. A7095,
respectively. The second type is called the rapid
digestible starch (RDS) which is the amount of
digested starch expelled between 1 minute and 20
minutes. The third type is the slow digestible starch
(SDS) which is expressed as the amount of digested
starch between 20 and 120 minutes. Finally, the
resistant starch (RS) is described as non-digestible
starch after 120 minutes of analysis.
2.4.1 Analysis of Prebiotic Effect and
Prebiotic Index of MTS
The analysis of prebiotic effect and index was
conducted by observing the change in the number of
L. plantarum SU-LS 36 colonies on m-MSRB
medium and m-MSRB medium with 2.5% taro starch
(native, AC-1C, AC-2C, AC-3C, annealing and
HMT). They were determined using the methods by
Roberfroid (2007). After incubation process for 24
hours at 37°C, the probiotic cell cultures were
enumerated in the MRSA medium. The same
procedures were conducted using a commercial
prebiotic FOS (fructooligosaccharide) as positive
control. The calculations were finished using these
following equations:
Prebiotic Effect = Log (cfu/mL) 2.5% taro starch
–
Log (cfu/mL) m-MRSB
(2)
Prebiotic Index =
2.5%ℎ−
ℎ ℎ
(3)
2.4.2 Prebiotic Activity Examination of
MTS to Diarrhea-Causal-Bacteria
The examination of prebiotic activity was conducted
by adding 2% (v/v) of L. plantarum SU-LS 36 culture
into m-MSRB with 2.5% (w/v) of glucose or 2.5%
(w/v) of taro starch (native, AC-1C, AC-2C, AC-3C,
annealing and HMT). It was analyzed by referring the
method from Huebner et al. (2007). After 0 hour and
24 hours of incubation times, the samples were
enumerated in the MRSA medium. The examination
was also conducted towards diarrhea-causal-bacteria,
Entero Pathogenic Escherichia coli (EPEC). The
EPEC culture of 2% (v/v) was added into different
Erlenmeyer containing m-TSB 2.5% (w/v) of glucose
or 2.5% (w/v) taro starch (native, AC-1C, AC-2C,
AC-3C, annealing and HMT). The culture was
incubated at 37°C, and enumerated in the TSA
medium after 0 hour and 24 hours of incubation
times. Prebiotic activity value was calculated using
this equation:
Prebiotic Activity Value =
{
(/)
}-
{
(/)
}
(4)
Note
N = number of L. plantarum SU-LS 36 (log cfu/mL)
t
0
= start of incubation time (0 hour)
E = number of Entero Pathogenic Escherichia coli
(log cfu/mL)
t
1
= end of incubation time (24 hour)
2.5 Statistical Data Analysis
There were three replications in this experiment,
where the statistical analyses were implemented
to process the research data. The Duncan statistical
test was implied to examine the considerable
differences at the level of p <0.05 utilizing the SPSS
18.0 statistical software (SPSS, Inc., Chicago,
IL, USA).
Effect of Heat Process for Prebiotic Properties of Taro Starch (Colocasia Esculenta L. Schott)
79
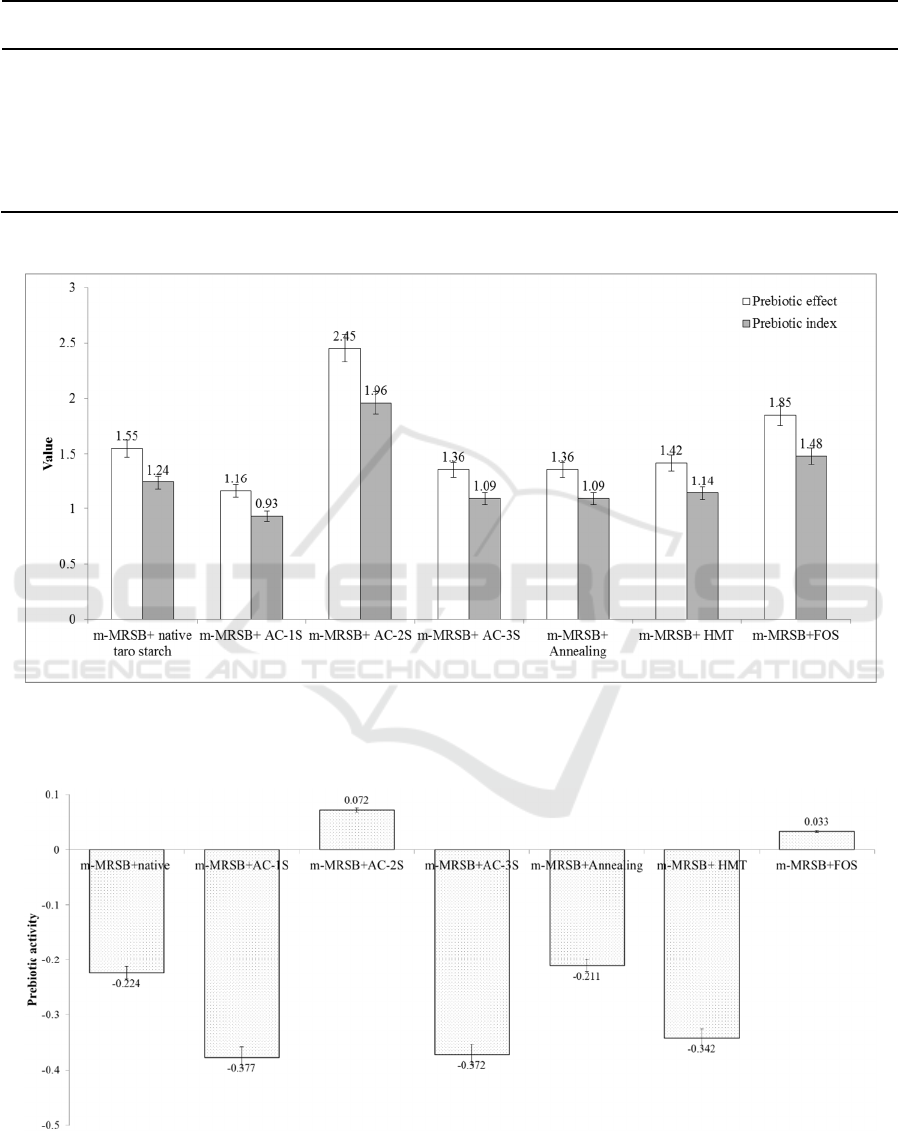
Table 1: In vitro digestibility and starch digestibility profile.
Treatment
In-vitro
digestibility (%)
VRDS
(% dry weight)
RDS
(% dry weight)
SDS
(% dry weight)
RS
(% dry weight)
Native taro
starch
80.17±0.63
c
37.30±0.42
d
32.07±0.25
d
23.15±0.63
a
7.48±0.94
a
AC-1C
70.77±0.52
b
31.95±0.81
c
27.13±0.48
c
25.60±0.74
b
15.32±0.86
b
AC-2C
64.41±0.76
a
29.29±0.46
b
22.20±0.59
b
27.17±0.38
c
21.34±078
c
AC-3C
62.83±0.82
a
27.42±0.35
a
21.20±0.72
b
28.67±0.55
c
22.71±0.21
c
Annealing
67.24±0.26
b
33.27 ±0.87
c
24.02±0.29
b
25.27±0.88
b
17.44±0.69
b
HMT
65.66±0.31
a
30.58±0.93
b
18.54±0.46
a
27.26±0.61
c
23.62±0.49
c
Note: In-vitro digestibility, VRDS, RDS, SDS and RS content with the different superscript letters within a row were
significantly different at p<0.05 level
Figure 1: Prebiotic effect and index of native taro starch, modified taro starch by AC-1C, AC-2C, AC-3C, Annealing, and
HMT.
Note: Prebiotic effect and index are expressed in the different typescript letters of the bar chart, where the noticeable different
occurs at the p<0.05 level.
Figure 2: Prebiotic activity of native taro starch, modified taro starch by AC-1C, AC-2C, AC-3C, annealing, and HMT to
diarrhea-causal-bacteria.
Note: Prebiotic activity is expressed in the different typescript letters of the bar chart, where the noticeable different occurs
at the p<0.05 level.
a
b
b
b
c
d
e
a
b
b
b
c
d
e
2nd SIS 2019 - SEAFAST International Seminar
80

3 RESULTS AND DISCUSSION
3.1 In Vitro and Starch Digestibility
Profiles
The analysis identifies that the native taro starch had
the highest in-vitro digestibility up to 80.17%
compared to modified taro starches (Table 1). The
improvement of RS content might reduce the
digestibility of taro starch consistently. Starch
digestibility had a negative correlation with RS
content. This result was similar to analysis by Cheng
et al. (2019), Ashwar et al. (2016), Shi & Gao (2011),
and Zheng et al. (2018) showed that the HMT
treatment can reduce the vitro digestibility of rice
starch. Annealing, HMT, and autoclaving-cooling
cycles significantly reduced in-vitro digestion of taro
starch (p <0.05) (Table 1). HMT and autoclaving-
cooling cycle resulted the formation of double helix
structures, the increase of chain bonding between
amylose-amylose, amylopectin-amylopectin and
amylose-amylopectin, consequently taro starch was
more difficult to digest by α-amylase enzymes
(Cheng et al., 2019; Zheng et al., 2018).
The increasing number of autoclaving-cooling
cycles decreased the in-vitro digestibly of taro starch.
The AC-3C treatment showed the lowest digestibility
of taro starch (62.83%) compared to other treatments
(Table 1). The annealing treatment, autoclaving
cooling cycle and HMT also reduced the in-vitro
digestibility of starch due to the retrogradation
process, hence increased RS and SDS levels (Shah et
al., 2016; Chen et al., 2018; Lovera & Perez, 2017).
MTS with high RS content had low in-vitro starch
digestibility (Perera et al., 2010). The in-vitro
digestibility reduction due to the retrogradation
treatment (e.g. HMT and autoclaving-cooling cycle)
was also reported previously by Cheng et al. (2019),
Shah et al. (2016), Ashwar et al. (2016), Shi & Gao
(2011), Zheng et al. (2018), and Chen et al. (2018).
Annealing, HMT and autoclaving-cooling cycles
reduced the levels of VRDS and RDS significantly
(p<0.05) compared to the native taro starch (Table 1).
The more autoclaving-cooling cycles were applied,
the lower VRDS and RDS became. Taro starch with
autoclaving-cooling of 3 cycles (AC-3C) treatment
showed the lowest result of VRDS level (27.42%),
followed by AC-2C (29.29%), HMT (30.58%), AC-
1C (31.95%) and annealing (33.27%) (Table 1).
Moreover, taro starch with HMT showed the lowest
value of RDS level (18.54%). The VRDS and RDS
from annealing, HMT and autoclaving-cooling cycle
treatments showed significant decrease as their
internal structures were changed into the SDS and RS.
This is proved by the significant increase (p<0.05) of
SDS and RS levels in taro starch after annealing,
autoclaving-cooling cycles, and HMT (Table 1). The
more autoclaving-cooling cycles were applied, the
higher SDS and RS become. AC-3C treatment
showed the highest SDS levels (28.67%) while HMT
resulted the highest RS levels (23.62%) (Table 1).
These results were relatively higher than the research
from Cheng
et al. (2019) in which the HMT was
applied at 120
o
C condition (2 hours, 30% moisture
content) in corn, pea, and lentil starch. HMT
treatment led to the increase of resistant starches of
corn, pea, and lentil up to 7.7, 11.2, and 10.4%
respectively (Cheng et al., 2019).
3.2 Prebiotic Effect and Prebiotic
Index
The prebiotic effect is the increasing number of the
absolute probiotic bacteria without considering the
prebiotic concentration (Roberfroid, 2007; Huebner
et al., 2007). Meanwhile, the prebiotic index is the
increasing of probiotic bacteria population correlated
with the prebiotic concentration (Roberfroid, 2007;
Huebner et al., 2007). The highest prebiotic effect and
index were noticeable in L. plantarum SU-LS 36 in
the AC-2C treatment (Figure 1). The RS in AC-2C
taro starch accommodated the growth of probiotic
bacteria. The examination on prebiotic effect and
index were conducted directly to the taro starch
sample to explain its prebiotic properties. Huebner et
al. (2007) reported that a diet was a good prebiotic
source if it had more than 1.5 prebiotic effect and
index (Figure 1).
The AC-2C taro starch was a good prebiotic
candidate if it had more than 1.5 prebiotic effect and
index. This value was higher than the
fructooligosaccharide (FOS), as commercial
prebiotic. The resistant starch content in AC-2C taro
starch increased the probiotic growth of L. plantarum
SU-LS 36 (Figure 1). To increase the prebiotic effect
index could be achieved by isolating the RS from taro
starch or consuming the AC-2C taro starch in the
larger quantities (20 gram/day) as a functional diet.
The RS with 20 – 30 degree polymerization played an
important role as a prebiotic source, therefore it could
be fermented to form the short chains fatty acids
(especially the butyric acid) in the colon, using
probiotic bacteria assistance (Danneskiold-Samsøe et
al., 2019). The increase of butyric acid caused the
decrease of pH inside the colon. Therefore this
condition inhibited the pathogenic bacteria growth
and prevented the proliferation of cancer cells in the
colon (Sullivan et al., 2017; Luo et al., 2017).
Effect of Heat Process for Prebiotic Properties of Taro Starch (Colocasia Esculenta L. Schott)
81

3.3 Prebiotic Activity to
Diarrhea-Causal-Bacteria
The prebiotic activity is the prebiotic capability to
grow probiotic bacteria, which is related to its
selectivity towards pathogenic bacteria over glucose
(Vrese & Marteau, 2007). A diet had positive
prebiotic activity (over 0.25) if it was selectively
metabolized by probiotic bacteria’s such as
Bifidobacterium sp., L. acidophilus and L. plantarum,
and not metabolized by pathogenic bacteria such as
EPEC (Vrese & Marteau, 2007). Native taro starch,
AC-1C, AC-3C, annealing and HMT had negative
prebiotic activity values. These mean that they were
not potential as prebiotic candidates (Figure 2). The
AC-2C treatment was capable to produce a resistant
starch with a degree of polymerization (DP) of around
20-30. Resistant starch was a selective and specific
prebiotic source for probiotics L. plantarum SU-LS
36.
Furthermore, L. plantarum SU-LS 36 probiotics
utilized the resistant starch from the AC-2C MTS as
a carbon source for its growth. Meanwhile, EPEC
could not use it as a source of nutrition for its growth.
The AC-2C MTS had the highest prebiotic activity
and it was a positive growth medium for L.
plantarum- EPEC (0.072) (Figure 2). Positive
prebiotic activity was also produced by
fructooligosaccharide (FOS) as a commercial
prebiotic growth medium for L. plantarum-EPEC
(0.033) (Figure 2). Finally, the AC-2C MTC was the
best prebiotic candidate as it had the higher values of
prebiotic effect, index, and activity than any other
treatments.
4 CONCLUSIONS
Annealing, autoclaving-cooling cycle, HMT
decreased in-vitro starch digestibility, VRDS, RDS
significantly. On the other hand, all treatments
significantly increased SDS and RS levels from taro
starch. MTS with AC-2C had the potential as a
prebiotic candidate as it had the highest prebiotic
effect, index and activity against EPEC.
ACKNOWLEDGEMENTS
The authors would like to express their deepest
gratitude to the Research Center for Biology
(Indonesian Institute of Sciences) LIPI and the
Science & Technology Postgraduate Scholarship
from Ministry of Technology and Higher Education
in Republic of Indonesia which had funded this
research.
REFERENCES
Aboubakar, Njintang, N.Y., Scher, J., Mbofung, C.M.F.,
2009. Texture, microstructure and physicochemical
characteristics of taro (Colocasia esculenta) as
influenced by cooking conditions. Journal of Food
Engineering, 91, 373-379.
Airul, A., Yusof, M. S. M., Jamil, M. S., Abdullah, A.,
Yusoff, S. F. M., Arip, M. N. M., Lazim, A. M., 2014.
Physicochemical characterization of starch extracted
from Malaysian wild yam (Dioscorea hispida Dennst.).
Emirates Journal Food Agriculture, 26(8), 652–658.
Anderson, A.K., Guraya, H.S., James, C., Salvaggio, L.,
2002. Digestibility and pasting properties of rice starch
heat-moisture treated at the melting temperature.
Starch/Stärke, 54, 401–409.
Ashwar, B.A., Gani, A., Wani, I.A., Shah, A., Masoodi,
F.A., Saxena, D.C., 2016. Production of resistant starch
from rice by dual autoclaving retrogradation treatment:
In-vitro digestibility, thermal and structural
characterization. Food Hydrocolloids, 56, 108-117.
Chen, Y.F., Singh, J., Archer, R., 2018. Potato starch
retrogradation in tuber: Structural changes and gastro-
small intestinal digestion in vitro. Food Hydrocolloids,
84, 552-560.
Cheng, K., Chen, S., Yeh, A., 2019. Physicochemical
properties and in vitro digestibility of rice after
parboiling with heat moisture treatment. Journal of
Cereal Science, 85, 98-104.
Danneskiold-Samsøe, N.B., de Freitas Queiroz Barros,
H.D., Santos R., Bicas, J.L., Cazarin, C.B.R., Madsen
L., Kristiansen, K., Pastore, G.M., Brix, S. Junir,
M.B.M., 2019. Interplay between food and gut
microbiota in health and disease. Food Research
International, 115, 23–31.
Deka, D., Sit, N., 2016. Dual modification of taro starch by
microwave and other heat moisture treatments.
International Journal of Biological Macromolecules,
92, 416-422.
Demirkesen-Bicak, H., Tacer-Caba, Z., Nilufer-Erdil, D.,
2018. Pullulanase treatments to increase resistant starch
content of black chickpea (Cicer arietinum L.) starch
and the effects on starch properties. International
Journal of Biological Macromolecules, 111, 505-513.
Englyst, H.N., Kingman, S.M., Cummings, J.H., 1992.
Classification and Measurement of Nutritionally
Important Starch Fractions. European Journal of
Clinical Nutrition, 46, 533–550. H.N.
Hazarika, B.J., Sit, N., 2016. Effect of dual modification
with hydroxypropylation and cross-linking on
physicochemical properties of taro starch.
Carbohydrate Polymers, 140, 269-278.
Huebner, J., Wehling, R.L., Hutkins, R.W., 2007.
Functional activity of commercial prebiotics. Journal
2nd SIS 2019 - SEAFAST International Seminar
82

International Dairy, 17, 770-775.
Kaushal, P., Kumar, V., Sharma, H.K., 2012. Comparative
study of physicochemical, functional, antinutritional
and pasting properties of taro (Colocasia esculenta),
rice (Oryza sativa) flour, pigeonpea (Cajanus cajan)
flour and their blends. LWT - Food Science and
Technology, 48, 59-68.
Li, H., Dong, Z., Liu, X., Chen, H., Lai, F., Zhang, M.,
2018a. Structure characterization of two novel
polysaccharides from Colocasia esculenta (taro) and a
comparative study of their immunomodulatory
activities. Journal of Functional Foods, 42, 47-57.
Lovera, M., Pérez, E., Laurentin, A., 2017. Digestibility of
starches isolated from stem and root tubers of
arracacha, cassava, cush–cush yam, potato and taro.
Carbohydrate Polymers, 176, 50-55.
Luo, D., Li, Y., Xu, B., Ren, G., Li, P., Li, X., Han, S., Liu,
J., 2017. Effects of inulin with different degree of
polymerization on gelatinization and retrogradation of
wheat starch. Food Chemistry, 229, 35-43.
Muñoz-Cuervo, I., Malapa, R., Michaleta, S., Lebot, V.,
Legendrea, L., 2016. Secondary metabolite diversity in
taro, Colocasia esculenta (L.) Schott, corms. Journal of
Food Composition and Analysis, 52, 24-32.
Oyeyinka, S.A., Adeleke, O.F., Dauda, A.O., Abiodun,
O.A., Kayodea, R.M.O., Adejuyitan, J.A., 2018. Flour
composition and physicochemical properties of white
and yellow bitter yam (Dioscorea dumetorum) starches.
Industrial Crops & Products, 120,135-139.
Perera, A., Meda, V., Tyler, R.T., 2010. Resistant starch: A
review of analytical protocols for determining resistant
starch and of factors affecting the resistant starch
content of foods. Food Research International, 43,
1959–1974.
Roberfroid, M. 2007. Prebiotics: The Concept Revisited.
The Journal of Nutrition Effect of Probiotics and
Prebiotics, 137, 830-837.
Setiarto, R.H.B., Jenie, B.S.L., Faridah, D.N., Saskiawan,
I., Sulistiani., 2018. Effect of lactic acid bacteria
fermentation and autoclaving-cooling for resistant
starch and prebiotic properties of modified taro flour.
International Food Research Journal, 25(4), 1691-
1697.
Shah, A., Masoodi, F.A., Gani, A., Ashwar, B.A., 2016. In-
vitro digestibility, rheology, structure, and functionality
of RS3 from oat starch. Food Chemistry, 212,749-758.
Sharlina, M.S.E., Yaacob, W.A., Lazim, A.M., Fazry, S.,
Lim, S.J., Abdullah, S., Noordin, A., Kumaran, M.,
2017. Physicochemical Properties of Starch from
Dioscorea pyrifolia tubers.
Food Chemistry, 220, 225-
232.
Shi, M., Gao, Q., 2011. Physicochemical properties,
structure and in vitro digestion of resistant starch from
waxy rice starch. Carbohydrate Polymers, 84, 1151-
1157.
Sullivan, W.R., Hughes, J.G., Cockman, R.W., & Small,
D.M., 2017. The effects of temperature on the
crystalline properties and resistant starch during storage
of white bread. Food Chemistry, 228, 57-61.
Vrese, M.D., Marteau, P.R., 2007. Probiotics and
Prebiotics: Effects on Diarrhea. The Journal of
Nutrition, 22, 803-811.
Wang, X., Reddy, C.K., Xu, B., 2018. A systematic
comparative study on morphological, crystallinity,
pasting, thermal and functional characteristics of
starches resources utilized in China. Food Chemistry,
259, 81-88.
Yu, Z.Y., Jiang, S.W., Cai, J., Cao, X.M., Zheng, Z., Jiang,
S.T., Wang, H.L., Pan, L.J., 2018a. Effect of high
pressure homogenization (HPH) on the rheological
properties of taro (Colocasia esculenta (L). Schott)
pulp. Innovative Food Science and Emerging
Technologies, 50, 160-168.
Yu, Z., Wang, Y.S., Chen, H.H., Li, Q.Q., Wang, Q., 2018b.
The gelatinization and retrogradation properties of
wheat starch with the addition of stearic acid and
sodium alginate. Food Hydrocolloids, 81, 77-86.
Zheng, B., Wang, H., Shang, W., Xie, F., Li, X., Chen, L.,
Zhou, Z., 2018. Understanding the digestibility and
nutritional functions of rice starch subjected to heat-
moisture treatment. Journal of Functional Foods, 45,
165-172.
Zhu, G., Xiao, Z., Zhou, R., Lei, D., 2015. Preparation and
simulation of a taro flavor. Chinese Journal of
Chemical Engineering, 23, 1733-1735.
Effect of Heat Process for Prebiotic Properties of Taro Starch (Colocasia Esculenta L. Schott)
83
