
Laboratory-scale Synthesis of Mono-diacylglycrol from Palm Oil
Stearin using Glycerolysis
Didah Nur Faridah
1,2
, Nurhadi Rahmat Sumitra
1
, Purwiyatno Hariyadi
1,2
, Ria Noviar Triana
2
,
Andri J. Laksana
2
and Nuri Andarwulan
1,2
1
Department of Food Science and Technology, IPB University, Indonesia
2
Southeast Asian Food Science and Agricultural Science and Technology (SEAFAST) Center, IPB University, Indonesia
phariyadi@apps.ipb.ac.id, andarwulan@apps.ipb.ac.id,
Keywords: Glycerolysis, MDAG, Reaction Order, Stearin.
Abstract: CPO refining process generally consisted of degumming, bleaching, filtration, deodorizing and fractionation.
At the end of the process, the yield is divided into two products, namely olein (liquid fraction) and stearin
(solid fraction). The aims of this research was to study the difference of stirring speeds on synthesis of MDAG
using glycerolysis method at laboratory scale. The stirring speed was performed at scale 3 and 4, while MDAG
was synthesized at substrate ratio of stearin:glycerol (1:2.3), temperature reaction of 180°C, time reaction of
90 min, and addition of 0.5% NaOH. Formation of MAG and DAG, as well as decomposition of TAG, was
found to follow reaction order 0, with R
2
of 0.4053, 0.5833, dan 0.3588, respectively. In the use of scale 3,
formation of MAG and DAG followed reaction order 0, whereas decomposition of TAG followed reaction
order 1, resulting in R
2
of 0.6551, 0.6114, and 0.7708, respectively. At verification stage, the results
demonstrated high accuracy, with Coefficient of Variance (CV) of < 5%, resulting in MAG 0.56% and DAG
2.52%. Acylglycerol fractions of verified MDAG product showed a noticeable variety, i.e. MAG
46.68±0.26%, DAG 32.57 ± 0.82%, and TAG 6.78 ± 0.47%. Furthermore, physichochemical characteristics
of MDAG showed the greatest proportion of fatty acid as follows: palmitic acid (C16: 0) 55.71±0.41% and
oleic acid (C18: 1 cis) 29.48±0.15%, with moisture content 0.57±0.02%, FFA 1.91±0.07%, iod number
6.08±0.04 mg/g, and slip melting point at 48.5-50 °C.
1 INTRODUCTION
As a precious commodity in Indonesia, oil palm has
received economic importance to the country.
Economically, palm oil industrial sector significantly
contributed to the country's foreign exchange
(Ministry of Agriculture 2015). For this reason, total
area for oil palm farm has continuously increased and
currently reached 9.26 millions Ha in 2017, with the
production of 35.36 million tons at productivity rate
of 3.82 tons per Ha; this leads Indonesia as the world's
largest palm oil producer (Ministry of Agriculture
2017).
Palm fruit consists of two parts, namely CPO
(Crude Palm Oil) which is produced from palm fruit
flesh and PKO (Palm kernel oil) from its fruit core
(Larasati et al. 2016). CPO is rich in palmitic acid
(C16: 0), while PKO is rich in lauric acid (C12: 0) and
myristic acid (C14: 0) (Ketaren 2008). Conversion of
CPO into food products needs refining process,
including degumming, bleaching, filtration, and
deodorizing. The final result of refining was obtained
without refined palm oil (RBDPO) (Silalahi et al.
2017). Now, palm oil is used more for cooking oil,
oleochemical and biodiesel industries.
The fractionation stage is an advanced process to
separate the RBDPO into two fractions, namely the
solid fraction (stearin) by 25% and the liquid fraction
(olein) by 75% (Malik 2015). Stearin has melting
points in the range 33.4-46.2 °C, while olein is 13-23
°C; thus, stearin is at solid state room temperature, but
olein is liquid. Olein is widely appleid as cooking oil
due to its properties during frying, including low
oxidation and degradation rate. Meanwhile, stearin is
generally used as main ingredient for hard fat in
various products, such as shortening, pastry,
margarine. Stearin is often considered a by-product of
palm olein, which make it cheaper compared to olein
(GAPKI 2014). Therefore, there is a need to convert
112
Faridah, D., Sumitra, N., Hariyadi, P., Triana, R., Laksana, A. and Andarwulan, N.
Laboratory-scale Synthesis of Mono-diacylglycrol from Palm Oil Stearin using Glycerolysis.
DOI: 10.5220/0009977700002833
In Proceedings of the 2nd SEAFAST International Seminar (2nd SIS 2019) - Facing Future Challenges: Sustainable Food Safety, Quality and Nutrition, pages 112-117
ISBN: 978-989-758-466-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

RBDPStearin into high quality products, such as
mono-diacylglycerol (MDAG) as emulsifier.
MDAG is commonly known as emulsifying agent
in a variety of industries such as food, cosmetics and
pharmaceutical, estimated to reach 70% total
emulsifier use. In food industries, it is applied in
bakery products, margarine, and frozen dessert.
Generally, MDAG is used as part of fatty products
and is often combined with other types of emulsifiers.
To produce MDAG, chemical glycerolysis from oils
or fats was carried out at high temperature, with the
help of inorganic alkaline catalysts (Cheirsilp et al.
2009). These emulsifiers are available in various
forms, such as liquid, solid, semi-liquid, flakes,
grains, and powders. The emulsifier relates to some
advantageous features, including water-in-oil (w / o)
with HLB of 4-6, no smell and taste, not water-
soluble at room temperature (O'Brien 2009). In the
food industry, MDAG is widely used in bakery,
margarine and chocolate products. Based on CFR
Regulation (2002), MDAG with code 21 CFR
182.4505 has no ADI value (acceptable daily intake)
or not limited; therefore, it can be categorized as
GRAS (Generally Recognized as Safe).
As a result of synthesize a laboratory-based
MDAG using palm oil stearin, the MDAG was
properly made from ratio substrate (stearin: glycerol)
of 1: 2.3, 0.5%N NaOH catalyst, reaction temperature
180 °C for 90 min, and stirring speed on scale 3,
yielding product with a percentage of acylglycerol
MAG fraction, DAG, and TAG as follows: 50.33 ±
0.95%, 28.13 ± 0.63%, and 4.49 ± 2.08%,
respectively. The MDAG product posseseed ALB
value of 1.64 ± 0.00%, moisture content of 0.55 ±
0.02%, iodine number of 34.56 ± 0.01 mg / g, and a
slip melting point of 49.5-50 °C. In this reagard,
stirring speed became a crucial factor that affects
formation of MDAG. According to Baeza-Jiménez
(2013), higher rate of stirring leads to increase in
decomposition time of TAG into MAG and DAG.
Therefore, this current work investigated the effect of
stirring speeds on MDAG synthesis from palm oil
stearin.
2 MATERIAL AND METHODS
2.1 Materials
This research was conducted at Chemistry Laboratory
Center, SEAFAST, IPB University. Chemicals used
in this experiment included stearin, olein, and PKO,
glycerol, sodium hydroxide (NaOH), citric acid,
nitrogen gas, standard Fatty Acid Methyl Esters
(FAME) Mix C4-C24, N-Methyl-N-trymethylsilyl
trifluoroacetamide, 0.1 M Na
2
S
2
O
3
solution, 95%
neutral alcohol solution, 50% citric acid, glacial
acetic acid, chloroform, heptane, acetone, distillate
water, Wijs solution, KI solution, phenolphthalein
indicator, starch indicator, and other analytical
materials.
The main equipments included 250 mL-three
neck flask equipped with a condenser, oil bath,
stirring hotplate, magnetic stirrer. For chemical
analysis, equipment needed was parafilm, aluminum
foil, analytical balance, Erlenmeyer flask, biuret,
pipette mohr, oven, desiccator, Gas
Chromatoghraphy FID Hewlett Packard 6890 series
DB5 HT column, GC FID series DB 23 column
Shimadzu Co. and HPew RID Hewlett Packard Series
1100.
2.2 Characterization of Palm Oil Raw
Materials
The physicochemical conditions of raw materials can
affect the effectiveness of the glycerolysis; thus, raw
material needs to be ensured for meeting all
requirements. The physicochemical analysis included
water content (AOCS Official Method Aa 3-38 year
2003), free fatty acid (AOCS Official Method Ca 5a-
40 year 2003), peroxide number (AOCS Official
Method Cd 8- 53 year 2003), iodic number (AOCS
Official Method Cd 1-25 year 2003), fatty acid
composition (AOCS Official Method Ce 2-66 year
2003), acylglyserol fraction (AOCS Official Method
Cd 11b-91 year 2003, with modification), and profiles
of triacylglycerol (AOCS Official Method Ce 5b-89
year 2003).
2.3 Synthesis of MDAG at Laboratory
Scale
The laboratory-scale MDAG synthesis conformed to
method of Triana (2014). Sampling was carried out
each 30 min to measure the acylglycerol fraction. The
repetition of MDAG synthesis in laboratory scale as
a verification stage was carried out five times in a
series of tests aimed at finding out the consistency of
the formation of the MDAG.
2.4 Physicochemical Properties of
MDAG Products
The chemical characterization of MDAG included
water content, iod number, acylglycerol fraction, free
fatty acid value, as well as fatty acid profile, while
physical characterization included slip melting point
Laboratory-scale Synthesis of Mono-diacylglycrol from Palm Oil Stearin using Glycerolysis
113
(referring to Official Method Cc 3-25 AOCS year
2005) and visual color measurement.
3 RESULTS AND DISCUSION
3.1 Physicochemical Characteristics of
Raw Materials
RBDP stearin is a by-product of CPO fractionation
and is previously refined. The standard for RBDP
stearin conformed to SNI 01-0021-1998, i.e.
maximum level of free fatty 0.15%, maximum water
and impurities content of 0.1%, maximum iodine
number of 40 mg / g, and arsenic contamination of 0.1
mg / kg. The characteristics of palm oil stearin as raw
material included water content, free fatty acid (FFA),
iodine number, peroxide number, fatty acid
composition, acylglycerol fraction, and
triacylglycerol (TAG) profile.
Table 1: Physicochemical characteristics of stearin.
Parameters Value
Water Content (%) 0.020 ± 0.00
Free Fatty Acids (%) 0.073 ± 0.00
Iod Number (mg/g) 34.45 ± 0.68
Peroxide Number
(meq O
2
/kg)
1.225 ± 0.05
Slip melting point (°C) 50.0-50.5
The results showed that water content in stearin
reached 0.02%. As investigated by Triana (2014), the
high level of moisture content enabled to induce oil
damage due to hydrolysis process, resulting in
increased level of FFA as indicator of the reduced oil
quality. Besides, FFA level was found at 0.073%. The
presence of high free fatty acids allowed to react with
alkaline catalysts and caused saponification, thereby
reducing the effectiveness of the catalyst (Rousseau
et al. 2017).
Furthermore, stearin possessed iod number of
34.45 mg / g, while peroxide number of the stearin
was 1,225 meq O
2
/ kg. Based on the results, the
strearin used as raw material is chemically acceptable
since it fits the standards. Physically, slip melting
point (SMP) of the stearin ranged from 50.0-50.5 °C.
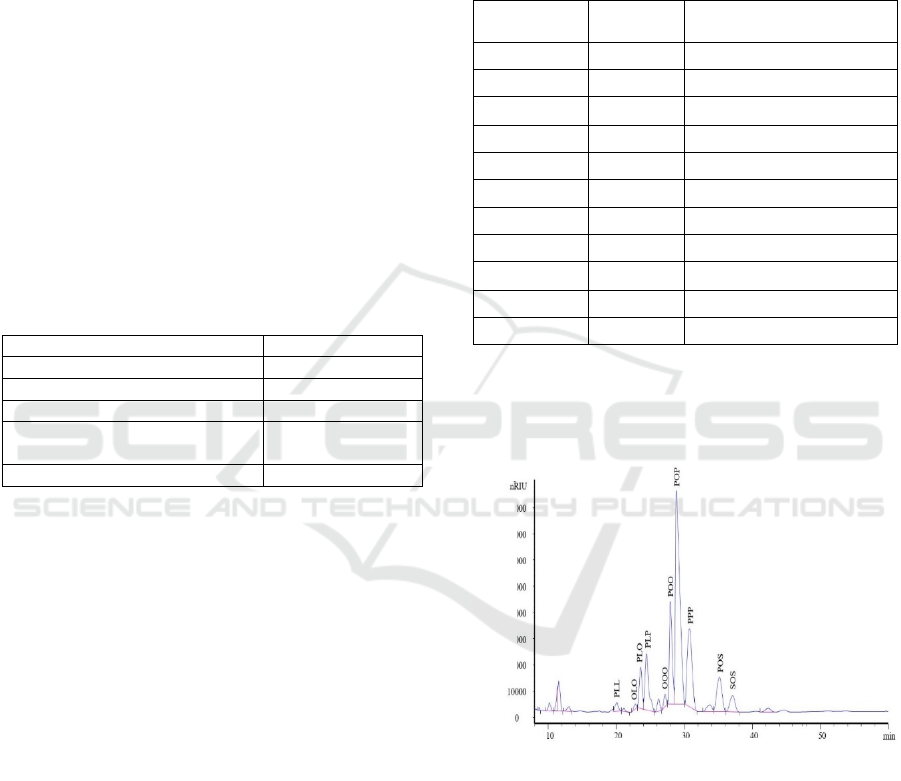
Profile of TAG demonstrated two main fractions:
POP (36.95%) and PPP (15.81%). According to
O'Brien (2009), when TAGs are saturated, the texture
of the raw material is hard; conversely, at high level
of unsaturated TAGs, the texture tends to be softer.
Thus, the stearin used as raw material has a soft
texture. TAG profile analysis was performed to
determine the highest TAG type as a reference for
stochiometric calculations in MDAG synthesis. The
calculation is based on POP TAG type as a mole base
because it is present at the highest proportion. TAG
profile of palm oil stearin can be seen in Table 2,
while TAG chromatograms is depicted in Figure 1.
Table 2: Profile of triacylglycerol in palm oil stearin (n=4).
TAG type ECN
a,b
Triglyceride Composition
(%)
PLL 44 1.24 ± 0.09
OLO 46 0.54 ± 0.02
PLO 46 4.56 ± 0.10
PLP 46 8.36 ± 0.21
OOO 48 1.32 ± 0.24
POO 48 11.67 ± 0.31
POP 48 36.94 ± 0.49
PPP 48 15.81 ± 0.67
POS 50 7.00 ± 0.19
SOS 52 3.42 ± 0.10
Others - 9.14 ± 0.12
Information:
P: Palmitate (C16: 0); S: Stearic (C18: 0); O: Oleat (C18:
1); L: Linoleic (C18: 2)
Source:
a
Costales-Rodriguez et al. (2009);
b
Adhikari et al.
(2009)
Figure 1: TAG profile chromatogram of raw material for
palm oil stearin with High Performance Liquid
Chromatography.
FFA analysis of the stearin is important since it
remarkably affect fatty acid characteristics of the
MDAG. The results indicated that saturated fatty
acids (SFA) dominated the composition with
proportion of 61.67 ± 0.25%, mainly consisting of
palmitic acid (C16: 0) at 55.03 ± 0.24%. Besides,
monounsaturated fatty acid (MUFA) was observed at
total amount of 30.57 ± 0.14, mainly composed of
Retention time (min)
Area response (nRIU)
2nd SIS 2019 - SEAFAST International Seminar
114
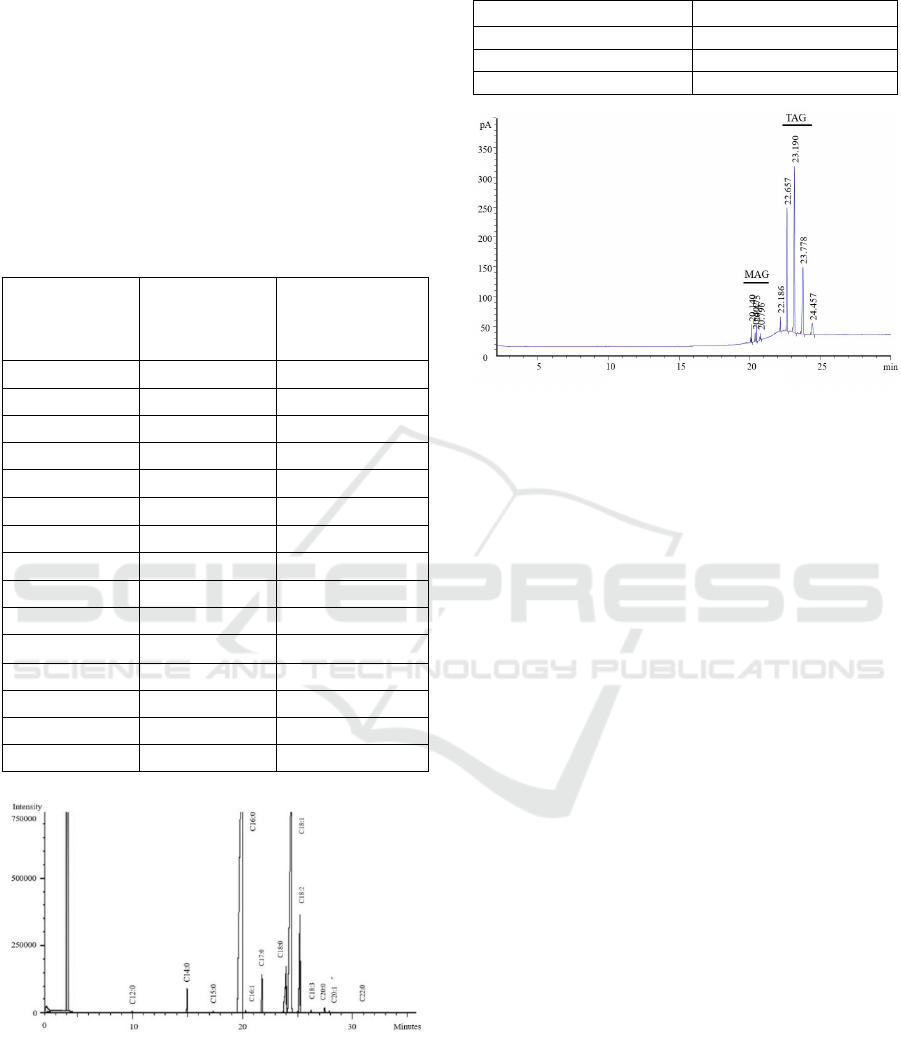
oleic acid (C18: 1cis) at percentage of 30.47 ± 0.14%.
The profile of fatty acid is presented in Table 3, while
chromatogram of the fatty acid composition of palm
oil stearin is exhibited in Figure 2.
Analysis of acylglycerol fraction was carried out
to determine the total percentage of initial MAG,
DAG and TAG fractions present in palm oil stearin.
The results exhibited that DAG and TAG fractions
became two major components, i.e. 4.60% and
95.40%, respectively. The chromatogram of the
acylglycerol fractions can be seen in Figure 3.
Table 3: Composition of fatty acids in palm oil stearin.
Fatty Acid
Average
concentration of
fatty acids g /
100g of oil
Average fatty
acids from total
fatty acids (%)
C12:0 0.11 ± 0.00 0.11 ± 0.00
C14:0 1.00 ± 0.01 1.04 ± 0.01
C16:0 52.76 ± 0.19 55.03 ± 0.24
C18:0 4.77 ± 0.02 4.97 ± 0.02
C20:0 0.32 ± 0.00 0.34 ± 0.00
C22:0 0.17 ± 0.00 0.18 ± 0.00
Total SFA 59.13 ± 0.21 61.67 ± 0.25
C16:1 0.10 ± 0.00 0.10 ± 0.00
C18:1 cis 29.21 ± 0.12 30.47 ± 0.14
Total MUFA 29.31 ± 0.12 30.57 ± 0.14
C18:2 cis 6.60 ± 0.02 6.89 ± 0.03
C18:3 0.14 ± 0.00 0.14 ± 0.00
Total PUFA 6.74 ± 0.02 7.03 ± 0.03
Unknown FA 0.71 ± 0.33 0.74 ± 0.35
Total area 95.88 ± 0.50 100
Figure 2: Chromatogram of fatty acid composition of palm
oil stearin analyzed by Gas Chromatography.
Table 4: The fraction of acylglycerol in palm oil stearin.
Acylglycerol fractions Value (%)
MAG 0.00
DAG 4.60
a
TAG 95.40
a
Figure 3: Chromatogram of acylglycerol fractions in palm
oil stearin detected by Gas Chromatography.
3.2 Glycerolysis for MDAG Synthesis
As depicted in Figure 4, formation of MAG and DAG
could be grouped into 2 phases. First, conversion of
TAG into MAG and DAG increased sharply within
30 min, reaching up to 42.95 ± 1.59% and 27.85 ±
2.18%, respectively. Second, after 30 min, sloping
phase occurred in which formation of MAG tended to
be stagnated, constantly at range of 42%. However,
formation of DAG continously increased during
glycerolysis, reaching up to 29-34%.
The optimum reaction condition was determined
according to the highest total percentage of MAG and
DAG, statistically evaluated using Duncan test in
SPSS statistical software. ANOVA test results
showed that production of MAG in reaction time of
90 min did not differ significantly compared to that in
reaction time of 30 and 180 min (p >0.05), but differ
significantly (p <0.05) in comparison with the
reaction time of 60, 120, and 150 min. Level of DAG
fraction in 90 min-reaction time was significantly
different (p <0.05) compared that in 60 min-reaction
time, but did not show significant difference
compared to subsequent reaction times (p> 0.05), i.e.
120, 150, and 180 min. For this reason, the most
desirable reaction time was achieved at 90 min.
Additionally, such condition was performed at
reaction temperature of 180 °C, substrate ratio
(stearub:glycerol) of 1:2.3, NaOH 18 N of 0.5%.
Further, the condition needs to be verified, ensuring
the consistency as well as determining the
physicochemical characteristics of the product.
Retention time (min)
Laboratory-scale Synthesis of Mono-diacylglycrol from Palm Oil Stearin using Glycerolysis
115
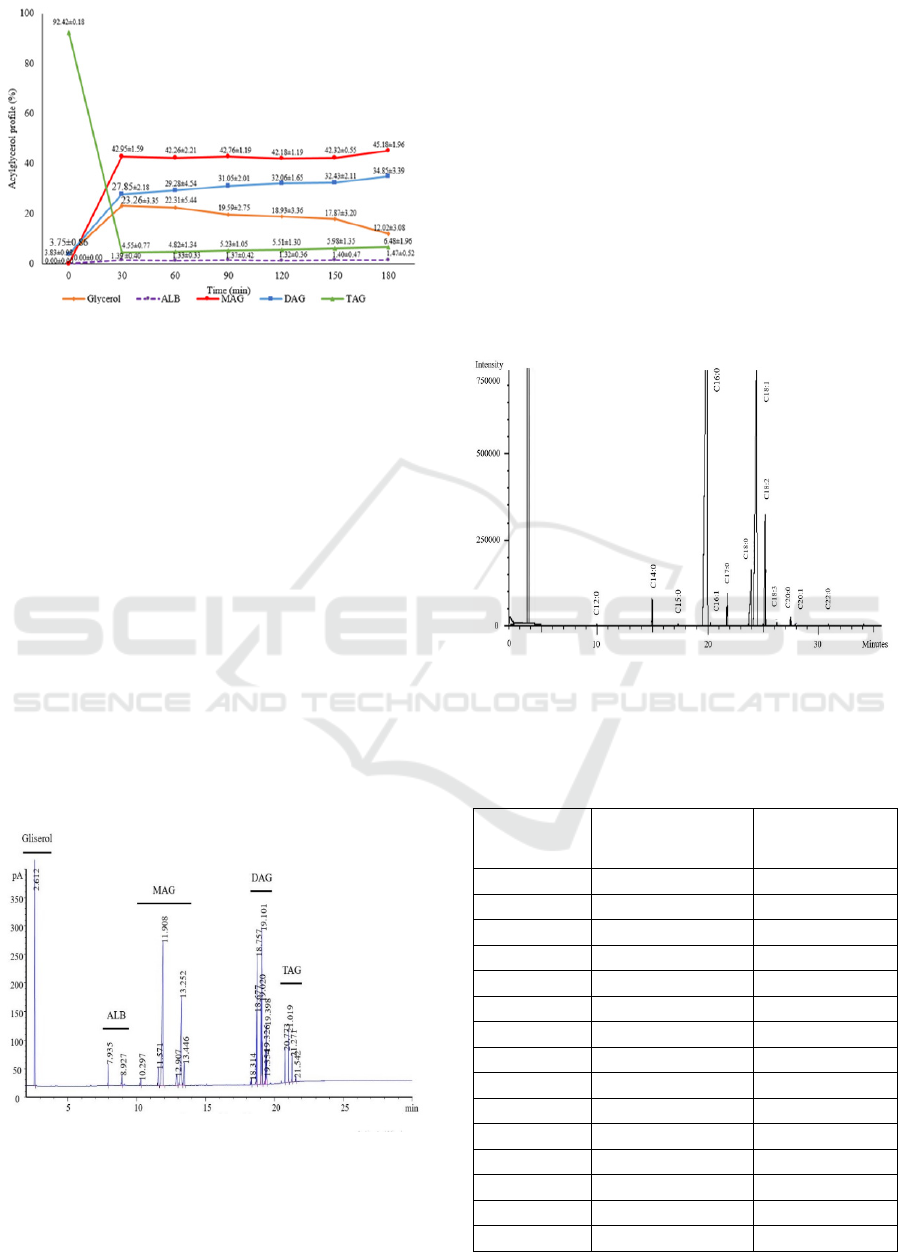
Figure 4: Average percentage of MDAG acylglycerol
fractions during glycerolysis for 180 min.
3.3 Characteristics of MDAG
The characterization of MDAG is necessary, enabling
to compare with current regulations. The results
exhibited that the resulting MDAG possessed total
fraction of acylglcerol MAG and DAG of 46.68 ±
0.26% and 32.57 ± 0.82%, respectively, as depicted
in Figure 5.
The results suggested that saturated fatty acids
(SFA) dominated the composition of fatty acids,
reaching up to 62.28 ± 0.42%, in which palmitic acid
(C16: 0) showed the greatest proportion at 55.71 ±
0.41%. In addition, monounsaturated fatty acids
(MUFA) also existed at appreciable quantity, i.e.
29.57 ± 0.15%, with oleic acid (C18: 1 cis) at 29.48 ±
0.15% as major fatty acid. Fatty acid composition of
MDAG is presented in Table 5, while the
chromatogram of the fatty acid composition in
MDAG is depicted Figure 6.
Figure 5: Chromatogram of acylglycerol fraction of MDAG
synthesized from palm oil stearin.
In terms of chemical properties, FFA level of
MDAG reached 1.91 ± 0.07%, with iod number of
6.08 mg / g. This suggests that iod number in MDAG
is lower than that in stearin as raw material, i.e. 34.56
mg /g. The significant depletion is caused by
glycerolysis which promotes reduction of unsaturated
fatty acids in MDAG products. Besides, moisture
content was recorded at 0.57 ± 0.02%.
Regarding to physical properties, slip melting
point (SMP) of MDAG ranged from 48.5 to 50 °C.
The product visually appeared as brownish yellow in
color. The color of product resulted from combination
of yellow in stearin and clear in glycerol.
Physicochemical characteristics of MDAG are
presented in Table 6.
Figure 6: Chromatogram of fatty acid composition of
MDAG products from palm oil stearin with Gas
Chromatography.
Table 5: Composition of fatty acids in MDAG made from
palm oil stearin.
Fatty Acid
Average
concentration of fatty
acids g / 100g of oil
Average fatty acids
from total fatty
acids (%)
C12:0 0.07 ± 0.00 0.09 ± 0.00
a
C14:0 0.83 ± 0.00 1.03 ± 0.01
a
C16:0 44.93 ± 0.18 55.71 ± 0.41
a
C18:0 3.99 ± 0.03 4.94 ± 0.04
a
C20:0 0.27 ± 0.00 0.33 ± 0.00
a
C22:0 0.14 ± 0.00 0.17 ± 0.00
a
Total SFA 50.23 ± 0.18 62.28 ± 0.42
a
C16:1 0.08 ± 0.00 0.09 ± 0.00
a
C18:1 cis 23.77 ± 0.25 29.48 ± 0.15
a
Total MUFA 23.85 ± 0.25 29.57 ± 0.15
a
C18:2 cis 5.25 ± 0.06 6.51 ± 0.03
a
C18:3 0.10 ± 0.00 0.13 ± 0.00
a
Total PUFA 5.35 ± 0.06 6.64 ± 0.03
a
Unknown FA 1.22 ± 0.29 1.51 ± 0.35
a
Total area 80.65 ± 0.63 100
Retention time
2nd SIS 2019 - SEAFAST International Seminar
116
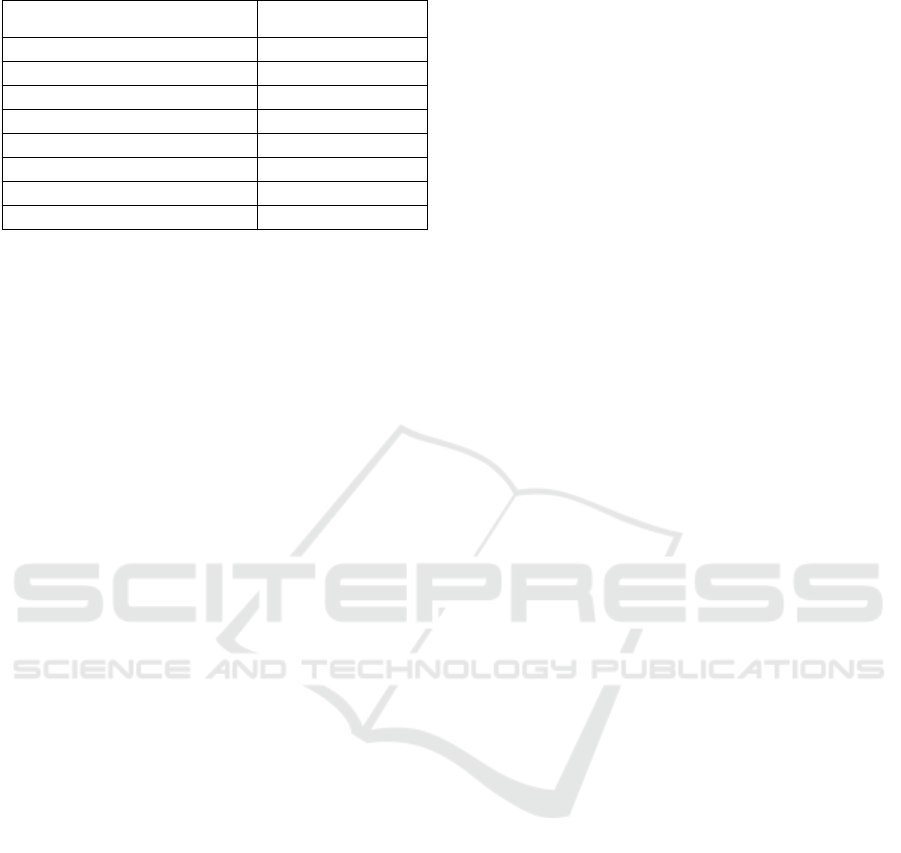
Table 6: Characteristics of MDAG products.
Characteristics Value
MAG (%) 46.68 ± 0.26
a
DAG (%) 32.57 ± 0.82
a
TAG (%) 6.78 ± 0.47
a
ALB (%) 1.91 ± 0.07
a
Water Content (%) 0.57 ± 0.02
a
Iod Number (mg/g) 6.08 ± 0.04
a
Slip melting point (°C) 48.5-50.0
a
Colour Brownish Yellow
4 CONCLUSION
Synthesis of mono-diacylglycerol (MDAG) could be
performed using different stirring speeds. The
formation of MAG and DAG and decomposition of
TAG at the stirring speed of scale 4 was evidenced to
follow order of reaction 0. At lower scale of speed, ,
the formation of MAG and DAG followed order of
reaction 0, but decomposition of TAG followed order
of reaction 1. Verification of MDAG synthesis
suggested high accuracy as represented by
Coefficient of Variance (CV) of <5%. Besides,
verification procedure successfully described
acylglycerol fractions of MDAG, resulting in MAG
(46.68 ± 0.26%), DAG (32.57 ± 0.82%), and TAG
(6.78 ± 0.47%). The physicochemical characteristics
of MDAG could be clearly stated as follows: palmitic
acid (C16:0) of 55.71 ± 0.41%, oleic acid (C18: 1 cis)
of 29.48 ± 0.15%, moisture content of 0.57 ± 0.02%,
FFA of 1.91 ± 0.07%, iod number of 6.08 ± 0.04 mg
/ g, and melting point at range of 48.5-50 °C.
REFERENCES
Adhikari P, Shin JA, Lee JH, Hu JN, Hwang KT, Lee KT.
2009. Enzymatic production of transfree hard fat
stock from fractionated rice bran oil, fully
hydrogenated soybean oil, and conjugated linoleic acid.
Journal of food science. 74(2): 87-96.
[AOCS] American Oil Chemist’s Society. 2003.
Official Methods and Recommended Practices of the
AOCS. Edisi ke-5. Illinois (US): AOCS.
[AOCS] American Oil Chemist’s Society. 2005.
Official Methods and Recommended Practices of the
AOCS. Edisi ke-6. Illinois (US): AOCS.
Baeza-Jiménez R, Miranda K, García, HS, Otero C. 2013.
Lipase-catalyzed glycerolysis of fish oil to obtain
diacylglycerols. Grasas y Aceites. 64(3): 237- 242.
[CFR] Code of Federal Regulations, Title 21, Vol. 2,
Section 182.4505. Washington (US): Office of the
Federal Register United States.
Cheirsilp B, Jeamjounkhaw P, H-Kittikun A. 2009.
Optimizing an alginate immobilized lipase for
monoacylglycerol production by the glycerolysis
reaction. Journal of Molecular Catalysis B: Enzimatic.
59: 206-211.
Costales-Rodríguez R. Gibon V, Verhé R, De Greyt W.
2009. Chemical and enzymatic interesterification of a
blend of palm stearin: soybean oil for low trans-
margarine formulation. Journal of the American Oil
Chemists' Society. 86(7): 681-697.
[GAPKI] Gabungan Pengusaha Kelapa Sawit Indonesia.
2014. Mengenal Minyak Sawit dengan Beberapa
Karakter Unggulnya. Jakarta (ID): GAPKI.
Ketaren S. 2008. Pengantar Teknologi Minyak dan Lemak
Pangan. Jakarta (ID): Universitas Indonesia Press.
Larasati N, Chasanah S, Machmudah S, Winardi S. 2016.
Studi analisis ekonomi pabrik CPO (Crude Palm Oil)
dan PKO (Palm Kernel Oil) dari buah kelapa sawit.
Jurnal Teknik ITS. 5(2): 212-215.
Malik A. 2015. Fraksinasi olein dan stearin minyak sawit
kasar menggunakan larutan dengan berat jenis antara.
Jurnal Edukasi dan Sains Biologi. 2(2): 18- 22.
Ministry of Agriculture. 2015. Rencana Strategis
Kementerian Pertanian Tahun 2015-2019. Jakarta (ID):
Kementerian Pertanian Republik Indonesia.
Ministry of Agriculture. 2017. Komoditas Pertanian Sub
Sektor Perkebunan: Kelapa Sawit. Jakarta (ID):
Kementerian Pertanian Republik Indonesia.
O’Brien RD. 2009. Fats and Oils: Formulating and
Processing for Applications. Third Edition. Florida
(USA): CRC Press.
Rousseau D, Ghazani SM, Marangoni AG. 2017. Chemical
Interesterification of Food Lipids: Theory and Practice,
editor: Akoh CC. Food Lipids: Chemistry, Nutrition,
and Biotechnology. Florida (US): CRC Press.
Silalahi RLR, Sari DP, Dewi IA. 2017. Pengujian free fatty
acid (FFA) dan colour untuk mengendalikan mutu
minyak goreng produksi PT. XYZ. Industria: Jurnal
Teknologi dan Manajemen Agroindustri. 6(1): 41-50.
Triana RN. 2014. Sintesis mono dan diasilgliserol (MDAG)
dari fully hydrogenated palm kernel oil (FHPKO)
dengan metode gliserolisis. [Tesis]. Bogor (ID): Institut
Pertanian Bogor.
Laboratory-scale Synthesis of Mono-diacylglycrol from Palm Oil Stearin using Glycerolysis
117
