
Synthesis of Rhodinol Ester from Citronella Oil Reduction Product
Ali Nurdin*
1
and Retno Yunilawati
2
1
Pusat Teknologi Sumberdaya Energi dan Industri Kimia, Badan Pengkajian dan Penerapan Teknologi, Puspiptek Serpong,
Indonesia
2
Badan Penelitian dan Pengembangan Industri, Kementerian Perindustrian, Indonesia
Keywords: Rhodinol Ester, Reduction, Citronella Oil, Esterification
Abstract: Rhodinol is a mixture of citronellol and geraniol that can be esterified using organic acids into citronellol
esters and geraniol esters to generate a specific odour as fragrances. Rhodinol esters in this study were
synthesized from citronella oil by first reducing to convert the citronellal in citronella oil into citronellol.
Reduction was carried out using NaBH
4
in conditions with ethanol as a solvent and without a solvent and the
variation of mole ratio. Esterification of reduction product (rhodinol) was done to produce rhodinol ester.
Reduction citronellal in citronella oil was efficient without solvent in the mole ratio of citronellal and NaBH
4
1:1, and successfully converted citronellal to citronellol with the rhodinol total (citronellol and geraniol) was
65.85%. Esterification of rhodinol produced 69.69% rhodinol ester which contains 55.16 % citronellyl acetate
and 14.53% geranyl acetate.
1 INTRODUCTION
Citronella oil is one type of essential oil which widely
exported by Indonesia with a production of 700MT-
800MT (Dewan Atsiri Indonesia, 2017).Citronella oil
is an essential product to produce the basic
ingredients of perfume in perfumery, cosmetics,
soaps, and detergent. Citronella oil also has
characteristic as insect and mosquito repellent.
Citronella oil contains three main components
consisting of citronellal, citronellol, and geraniol
(Simic et al., 2008) (Wany et al., 2014) (Eden et al.,
2018). Citronellal (3,7-dimethyl-6-octenal) is a
monoterpene that with an aldehyde group and has an
important role in the synthesis of fine chemicals as
terpene derivatives (Lenardão et al., 2007).
Citronellol and geraniol are alcohol monoterpene and
the mixture of both is commonly named rhodinol.
Rhodinol was known to have a much finer and
flowery rose odour than citronellol.
Rhodinol can be converted into rhodinol ester to
generate a specific odour as the raw material in
fragrance. Geranyl acetate presents a sweet fruity
flavour and rose and lavender aroma (Murcia et al.,
2018). The synthesis of rhodinol ester was an effort
to derivatize citronella oil thus increase the added
value of citronella oil.
Synthesis of rhodinol ester in this experiment
was done in two steps. The first step was the reduction
of citronellal in citronella oil directly without
separation. The reduction reaction was done using
NaBH
4
. This step was converted citronellal into
citronellol with the aim of increase the rhodinol
(citronellol and geraniol) content. The second step
was esterification of rhodinol to produce rhodinol
ester (citronellyl acetate and geranyl acetate). This
experiment was interesting because of the reduction
reaction and the esterification reaction were done in
citronella oil directly. Some of the previous study was
done these process (Yu et al., 2000) (Yadav and
Lande, 2006).
2 MATERIALS AND METHODS
2.1 Materials
Citronella oil was used in this experiment obtained
from a small industry in Yogyakarta. The chemical
materials used in this experiment were natrium
borohydride (NaBH4) (Merck), ethanol technical
grade, hydrochloric acid (HCl) technical grade,
sodium hydroxide (NaOH) technical grade,
anhydrous acetic acid (Merck), and anhydrous
sodium sulphate (Na
2
SO
4
).
Nurdin, A. and Yunilawati, R.
Synthesis of Rhodinol Ester from Citronella Oil Reduction Product.
DOI: 10.5220/0009973101650169
In Proceedings of the 2nd International Conference of Essential Oils (ICEO 2019), pages 165-169
ISBN: 978-989-758-456-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
165
2.2 Methods
2.2.1 Gas Chromatography Mass
Spectrometry Identification
The citronella oil and the product from the reaction
were identified by gas chromatography with a mass
spectrometer detector (GC-MS) Agilent 6890 series
with capillary column HP-5MS, 30 m x 0.25 mm id x
0.25 µm film thickness. Helium gas was used as the
carrier gas at a constant pressure of 65 kPa. The
sample was injected with a volume of 1 µL in a split
ratio of 1:25. The increasing of oven temperature was
programmed from 60-240°C with a step of 3°C per
minute until reaching 240°C.
2.2.2 Reduction of Citronella Oil
Reduction using Ethanol as the Solvent. The
reduction was carried out in round-bottomed flask
with reflux. The reaction contained NaBH
4
and
ethanol. NaBH
4
was dissolved with ethanol in flask
and the citronella oil was added with variation in the
mole ratio of the citronellal and NaBH
4
(1:1 and 1:3).
The reduction reaction was done at 78 °C for 3 hours.
The ethanol solvent was evaporated. The white solid
obtained from this reaction was diluted with water
and acidified with 20% HCl to pH reached 2, then
heated at 50°C for 1 hour. The reaction mixture was
extracted with ether, washed with water to neutral,
and dried with anhydrous Na2SO4. The product was
identified with GCMS.
Reduction without Solvent.
The reduction was
carried out in round-bottomed flask with reflux. The
citronella oil and NaBH
4
were added to the flask with
variation in a mole ratio of the citronellal and NaBH
4
(1:3; 1;1; 1: 0.5 and 1:0.025). The reduction reaction
was done for 3 hours at 150 °C. After completion, the
mixture was cooled, added H
2
O and stirred for half an
hour and added with the HCl 20% until pH reached 2.
The mixture was extracted with ether, washed with
water until neutral, and dried with anhydrous
Na2SO
4
. The product was identified with GCMS.
2.2.3 Esterification of Rhodinol
The optimum product reduction (rhodinol),
anhydrous acetic acid, and 5% of NaOH were
arranged in a round-bottomed flask with a mole ratio
of rhodinol and acetic acid was 1:3. The mixture was
stirred and heated at 180 °C for 3 hours. This was
followed by the neutralization with 1% of HCl
solution to separate it from the NaOH catalyst. The
rhodinol ester from this reaction was identified using
GCMS.
3 RESULTS AND DISCUSSION
3.1 Chemical Compounds Composition
of Citronella Oil
Characterization using GC-MS showed the
chromatogram profile detected several peaks in
citronella oil (Figure 1). The compounds identified
based on a comparison of the mass spectrum with
reference data from the database (Wiley 7) and the
results were presented in Table 1.
Figure 1: GCMS chromatogram of citronella oil.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
166

Table 1: Chemical compounds of citronella oil
No Retention time Identified compound Relative percentage area (%)
1 13.11 Citronellal
55.93
2 16.62 Citronellol
7.40
3 17.97 Geraniol
10.84
4 22.01 Citronellyl acetate
3.30
5 23.36 d-carene
2.27
6 23.51 β-elemene
2.59
7 24.61 Geranyl acetate
3.30
8 27.21 Germacrene
2.35
9 28.05 Methyl isoeugenol
2.28
10 29.04 d-cadinene
3.30
11 30.16 Elemol
4.29
The compounds were citronellal, citronellol,
geraniol, citronellyl acetate, d-carene, β-elemene,
geranyl acetate, d-cadinene, and elemol. The main
compounds in citronella were citronellal (55.93%),
geraniol (10.74%), and citronellol (7.40%. These
results appropriate with the previous finding in the
literature, citronellal, geraniol, and citronellol has
been described as the main compounds of citronella
oil (Simic et al., 2008) (Wany et al., 2014) (Eden et
al., 2018).
3.2 Reduction of Citronella Oil using
Ethanol as the Solvent
The reduction of citronellal to citronellol was carried
out using NaBH
4
with the reaction in Figure 2.
Borohydrides are very routinely used for selective
reduction in preparatory synthesis and also on a
commercial scale (Yadav and Lande, 2006). The
results of reducing citronellal to citronellol in
citronella oil was shown in Table 2.
Table 2. Reaction products of citronella oil reduction using
NaBH
4
with ethanol solvent.
Compounds Initial
Reduction product
in mole ratio citronellal
and NaBH
4
1:1 1:3
Citronellal 55.93 26.48 -
Citronellol 7.40 38.66 50,42
Based on Table 2, there was a change in the
amount of citronellal and citronellol at the end of the
reaction when compared to the initial amount, both at
a mole ratio of 1: 1 and 1: 3. This means that the
reaction under these conditions successfully reduced
citronellal to citronellol. In the 1: 3-mole ratio there
was no citronellal at the end of the reaction which
means that the citronellal has been converted
completely. However, in this condition, citronellal
was not completely converted into citronellol as
indicated by the amount of citronellol formed. The
imperfect citronellal reduction in this experiment was
predicted because of the ethanol solvent used.
Figure 2: Reduction of citronellal to citronellol
This experiment used technical ethanol which
still contains a lot of water thus there was NaBH
4
which reacted with water before reacting with
citronellal. The possibility of NaBH
4
reacting with
water was observed with the appearance of foam
when dissolving NaBH
4
in ethanol. So, the use of
solvents will require expensive costs because the
solvent must be free of water. For this reason, it is
necessary to try hydrogenation without ethanol as a
solvent.
3.3 Reduction without Solvent
Aldehyde reduction using NaBH
4
can be carried out
in the absence of a solvent (Zeynizadeh and Behyar,
2005). To improve the efficiency and effectiveness of
the reduction process, the citronella oil reduction
reaction was carried out with NaBH
4
without the use
of a solvent. The results of reducing citronellal to
citronellol without solvent were shown in Table 3 and
the GCMS chromatogram of reduction product were
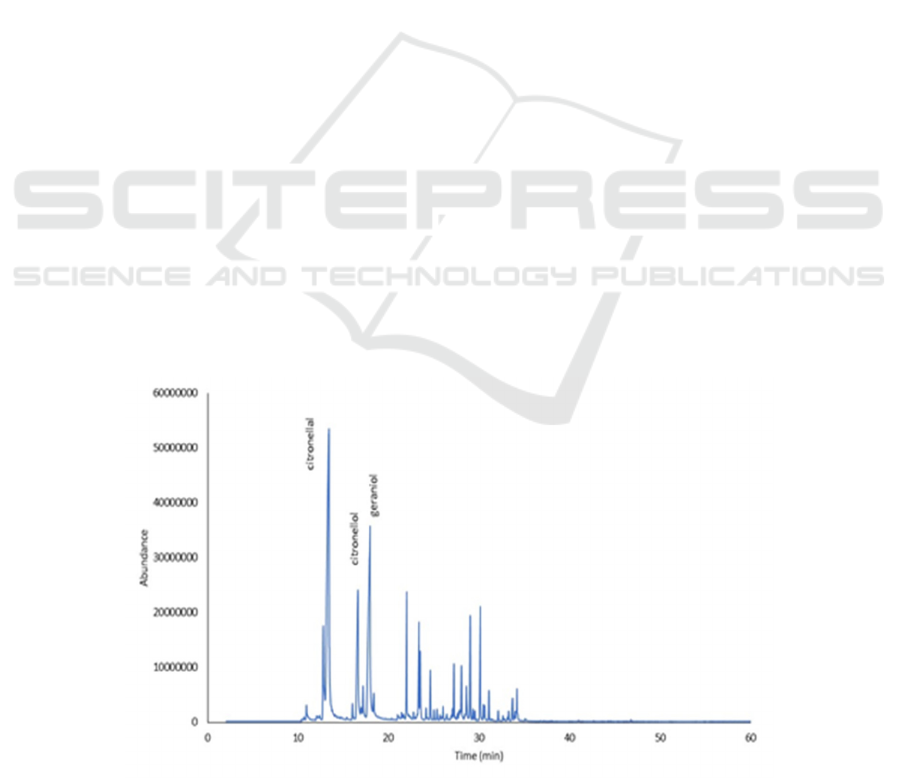
described in Figure 3.
Synthesis of Rhodinol Ester from Citronella Oil Reduction Product
167

Figure 3: GCMS chromatogram of citronella oil reduction product
Figure 4. GCMS chromatogram of rhodinol ester
Table 3. Reaction products of citronella oil reduction using
NaBH
4
without solvent
Mole ratio of
citronellal
and NaBH
4
Citronellal Citronellol Geraniol
Initial 55.934 7.40 10.84
1 : 3 - 59.73 16.67
1 : 1 2,14 51.56 14.29
1 : 0,5 1,98 46.51 13.43
1 : 0,25 1,76 40.21 13.60
Table 3 showed that citronellal can be converted
into citronellol with NaBH
4
without the use of
solvents as indicated by decreasing levels of
citronellal and increasing levels of citronellol in
reaction products. In the variation of the mole ratio,
the higher the mole of NaBH
4
, the reduced citronellal
was higher. Citronellal was reduced completely at
1:3-mole ratios. The product contained 59.73%
citronellol and 16.67% geraniol so the amount of
rhodinol was 76.4%. Although optimal, this process
was inefficient because it required a large number of
moles of NaBH
4
. Therefore, for the next process used
rhodinol from the reduced mole ratio of 1: 1 because
need less NaBH4 and this was considered more
efficient. The rhodinol from this process contains
51.56% citronellol dan 14.29% geraniol with
rhodinol total was 65.85%.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
168

3.4 Esterification of Rhodinol
Esterification of rhodinol was conducted to obtained
rhodinol ester that has a specific smell. Geranyl
acetate presents a sweet fruity flavour and rose and
lavender aroma (Murcia et al., 2018). Rhodinol ester
(citronellyl acetate, geranyl acetate) can be isolated
by vacuum fractionation, but the availability of these
natural raw materials was limited. However, this
method was not suitable for large-scale industrial
production. For the alternative, these esters may be
produced by chemical synthesis and enzymatic
extraction or catalysis (bio catalysis) (Paroul et al.,
2012) (Wu et al., 2018) (Murcia et al., 2018).
Chemical synthesis was often performed using acetic
acid anhydride or direct acetic acid esterification (Jian
et al., 2014). This method was the traditional
chemical synthesis and commonly used in large-scale
industries. Rhodinol ester in this experiment was
synthesized using acetic acid.
Table 4. Rhodinol ester product from esterification
Compounds Rhodinol (%) Rhodinol ester
(%)
Citronellol 51.56 -
Geraniol 14.29 -
Citronellyl acetate 3.00 55.16
Geranyl acetate - 14.53
The GCMS analysis showed that all rhodinol
(citronellol and geraniol) have changed to rhodinol
acetate esters. This result was observed with the loss
of the rhodinol peak and the appearance of the
rhodinol acetate peak, as shown in Figure 4. The
complete data on the results of the experiment are
shown in Table 4.
4 CONCLUSIONS
Reduction citronellal in citronella oil was
successfully converted citronellal to citronellol with
the rhodinol total (citronellol and geraniol) was
65.85%. Esterification of rhodinol produced 69.69%
rhodinol ester which contains 55.16 % citronellyl
acetate and 14.53% geranyl acetate.
REFERENCES
Dewan Atsiri Indonesia (2017) ‘Indonesian Essential Oil
Output’.
Eden, W. T. et al. (2018) ‘Fractionation of Java Citronella
Oil and Citronellal Purification by Batch Vacuum
Fractional Distillation’, IOP Conference Series:
Materials Science and Engineering, 349(1). doi:
10.1088/1757-899X/349/1/012067.
Jian, X. et al. (2014) ‘Lipase-Catalyzed Transesterification
Synthesis of Geranyl Acetate in Organic Solvents and
Its Kinetics’, Food Science and Technology Research,
20(2), pp. 207–216. doi: 10.3136/fstr.20.207.
Lenardão, E. J. et al. (2007) ‘Citronellal as key compound
in organic synthesis’, Tetrahedron, 63(29), pp. 6671–
6712. doi: 10.1016/j.tet.2007.03.159.
Murcia, M. D. et al. (2018) ‘Kinetic modelling and kinetic
parameters calculation in the lipase-catalysed synthesis
of geranyl acetate’, Chemical Engineering Research
and Design. Institution of Chemical Engineers, 138, pp.
135–143. doi: 10.1016/j.cherd.2018.08.025.
Paroul, N. et al. (2012) ‘Solvent-free production of
bioflavors by enzymatic esterification of citronella
(Cymbopogon winterianus) essential oil’, Applied
Biochemistry and Biotechnology, 166(1), pp. 13–21.
doi: 10.1007/s12010-011-9399-4.
Simic, A. et al. (2008) ‘Essential oil composition of
Cymbopogon winterianus and Carum carvi and their
antimicrobial activities’, Pharmaceutical Biology,
46(6), pp. 437–441. doi: 10.1080/13880200802055917.
Wany, A. et al. (2014) ‘Extraction and characterization of
essential oil components based on geraniol and
citronellol from Java citronella (Cymbopogon
winterianus Jowitt)’, Plant Growth Regulation, 73(2),
pp. 133–145. doi: 10.1007/s10725-013-9875-7.
Wu, T. et al. (2018) ‘Engineering Saccharomyces
cerevisiae for the production of the valuable
monoterpene ester geranyl acetate’, Microbial Cell
Factories. BioMed Central, 17(1), pp. 1–11. doi:
10.1186/s12934-018-0930-y.
Yadav, G. D. and Lande, S. V (2006) ‘Novelties of kinetics
of chemoselective reduction of citronellal to citronellol
by sodium borohydride under liquid – liquid phase
transfer catalysis’, Journal of Molecular Catalysis A :
Chemical, 247, pp. 253–259. doi:
10.1016/j.molcata.2005.11.015.
Yu, W. et al. (2000) ‘Selective hydrogenation of citronellal
to citronellol over polymer-stabilized noble metal
colloids’, Reactive and Functional Polymers, 44(1), pp.
21–29. doi: 10.1016/S1381-5148(99)00073-5.
Zeynizadeh, B. and Behyar, T. (2005) ‘Fast and Efficient
Method for Reduction of Carbonyl Compounds with
NaBH 4 /Wet SiO 2 Under Solvent Free Condition’, J.
Braz.Chem.Soc., 16(6), pp. 1200–1209.
Synthesis of Rhodinol Ester from Citronella Oil Reduction Product
169
