
Characterization of Seedlac Hydrolysis from Kesambi (Schleicera
oleosa Merr) as an Intermediate Compound for Fragrance Synthesis
Retno Yunilawati
1
, Dwinna Rahmi
1
, Chicha Nuraeni
1
, Arief Riyanto
1
, Novinci Muharyani
2
, Pujo
Sumantoro
2
, Murgunadi
2
, and Nur Hidayati
1
1
Badan Penelitian dan Pengembangan Industri, Kementerian Perindustrian, Indonesia
2
Pusat Penelitian dan Pengembangan Perum Perhutani, Indonesia
Keywords: Seedlac, Kesambi, Hydrolysis, Aleuritic Acid.
Abstract: Seedlac is the organic resin obtained from secretion of female insect Laccifer lacca Kerr on a selected plant,
one of them is Kesambi (Schleicera oleosa Merr). Seedlac contains almost 80% polyester which can be
hydrolysed to ester compounds such as aleuritic acid which is an intermediate compound for the fragrance
synthesis of the perfume industry. One of the problems in the seedlac hydrolysis is the presence of natural
dyes (laccaic acid) which interfered with the hydrolysis process and affect the purity of the hydrolysis
products. In this research, hydrolysis was carried out by first removing the natural dyes of shellac (decolorized
process). The hydrolysis results were characterized using Gas Chromatography-Mass Spectrometry to
determine the type of ester and its composition. The decolorized process of seedlac before hydrolysis in this
experiment could improve the percentage of aleuritic acid up to 56%. Therefore, seedlac hydrolysis by
decolorized process before hydrolysis can be considered for the production of esters from seedlac, especially
aleuritic acid.
1 INTRODUCTION
Lac is an organic resin secreted by the insect Laccifer
lacca Kerr on a selected plant (Sutherland and Río,
2014) (Nagappayya and Gaikar, 2010). In Indonesia,
Kesambi (Schleicera oleosa Merr) is a plant
prioritized for use as the host plant in the cultivation
of the insects (Taskirawati et al., 2017). Lac forms a
solid material on the branches of host plants attacked
by the insects, and when collected in this form it is
referred to as sticklac. The sticklac is crushed and
sieved to remove impurities to get seedlac. Further
processing in the refining of seedlac produced
shellac.
Lac in Indonesia is developed by Perhutani
(Probolinggo) and the insect’s cultivation has spread
evenly in Nusa Tenggara Barat and Nusa Tenggara
Timur (Taskirawati et al., 2017). Pehutani produced
lac in the form of seedlac to fulfil domestics and
foreign market and used mainly as varnish. So far
there was no diversification of other lac products
were done by Perhutani.
Shellac consists of 68% resin, 6% wax, and 1-2%
dyes (such as laccaic acid and erythrolaccin). The
resin of seedlac is a mixture of cross-linked polyester
or cyclic aliphatic polyhydroxy acid with
sesquiterpenic acid (Biswas, 2014) (Sutherland and
Río, 2014) (Nagappayya and Gaikar, 2010). The
composition varies depending on the insect species
and the host plant where seedlac is obtained (Farag
and Leopold, 2009). The main compositions of
polyester in shellac consists of aleuritic acid, butolic
acid, shellolic acid and jalaric acid (Farag, 2010).
Aleuritic acid (9,10,16-trihydroxyhexadecanoic
acid) was used as the starting material because of its
multi functionalities (Ravi, Padmanabhan and
Mamdapur, 2001). Aleuritic acid is mainly used in the
perfumery industry, as a starting material for
preparation isoambritolite is the main ingredient
fragrance compounds "musk" (Biswas, 2014).
Derivatization of shellac to aleuritic acid can increase
shellac added value up to 15 times (Prasad, 2014).
The most common method in the isolation of
aleuritic acid is alkaline hydrolysis of lac resin,
separation, and purification. Besides containing
polyester, seedlac also contains natural dyes which
the presence influences the isolation of aleuritic acid.
It is possible that polyester could be transferred into
the colorant matrix (Berbers et al., 2019) and so the
otherwise that colorant matrix could have been
86
Yunilawati, R., Rahmi, D., Nuraeni, C., Riyanto, A., Muharyani, N., Sumantoro, P., Murgunadi, . and Hidayati, N.
Characterization of Seedlac Hydrolysis from Kesambi (Schleicera oleosa Merr) as an Intermediate Compound for Fragrance Synthesis.
DOI: 10.5220/0009957200860090
In Proceedings of the 2nd International Conference of Essential Oils (ICEO 2019), pages 86-90
ISBN: 978-989-758-456-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

transferred when attempting the separation, it can
prolong the purification process. In this experiment,
seedlac was hydrolysis after decolorized. The dyes
were obtained in the decolorized process ca be used
as natural dyes. Product hydrolysis was compared
with seedlac hydrolysis product without the
decolorized process.
2 MATERIALS AND METHOD
2.1 Materials
Seedlac was used in this experiment obtained from
Perhutani. The chemical materials used in this
experiment were methanol (Merck), potassium
hydroxide (Merck), ethyl acetate (Merck),
hydrochloric acid (Merck), n-hexane, and activated
charcoal.
2.2 Method
2.2.1 Seedlac Characterization
The characterization of seedlac includes moisture
contents, ash contents, and acid value. A Fourier
Transform Infrared (FTIR) spectra were collected for
seedlac to determine the functional group.
2.2.2 Extraction of Natural Dyes
(decolorized process)
Seedlac that have been crushed macerated using
water with a ratio of seedlac: water is 1:10.
Maceration is carried out for 3-4 hours at room
temperature while stirring (Yaqub et al., 2014),
2014). After maceration, filter the products, take the
filtrate as natural dyes and the pulp for hydrolysis.
2.2.3 Hydrolysis of Seedlac
There are 2 types of hydrolysed seedlac, one is
seedlac from the 2.3 process, namely decolorized
seedlac, and the second is pure seedlac. To a reflux
apparatus add 20 g of seedlac granules, 80 mL of
methanol and 11 g of potassium hydroxide dissolved
in 100 mL water, reflux for 15 minutes. Then the
methanol is distilled off completely and the solution
is then neutralized until pH 5 is reached. Then add 4
g of activated charcoal, filter while hot and let stand
for 3 days. Then the solution is filtered and the filtrate
is added to a beaker with boiling water. Add just
enough of ethyl acetate until all of the crude product
dissolves. 800 mg of activated charcoal and 2-4 g of
sodium sulphate is added and the solution is brought
to boil. The solution is filtered and few drops of n-
hexane are added, the solution is then allowed to
stand for 24 h and it is then filtered and dried.
2.2.4 Characterization of Seedlac Hydrolysis
A Fourier Transform Infrared (FTIR) spectra were
collected for seedlac hydrolysis to determine the
functional groups. Seedlac hydrolysis compounds
were identified by gas chromatography with a mass
spectrometer detector (GCMS) Agilent 6890 series
with capillary column HP-5MS, 30 m x 0.25 mm id x
0.25 µm film thickness. Helium gas was used as the
carrier gas at constant flow mode at 1.5 mL/min. The
sampel was injected with a volume of 2 µL in splitless
mode. The increasing of oven temperature was
programmed from 50-320°C with step of 10°C per
minute until reaching 320°C and hold 12 min.
3 RESULTS AND DISCUSSION
3.1 Characterization of Seedlac
Moisture content, ash content and acid value in this
experiment are presented in Table 1. The acid value
(AV) is a good indicator of the quality of seedlac
(Farag and Leopold, 2009). AV indicates the content
of acid available in the seedlac. AV was expressed as
the weight of KOH in mg needed to neutralize the
organic acids. Some studies reported seedlac has
various AV, ranging from 55 to 85 (Prasad, 2014).
During storage, polymerization induced by
esterification takes place, resulting in a decrease in
the AV (Farag and Leopold, 2009). AV in this study
is very low compared to AV in the literature already
mentioned.
The seedlac used in this experiment may have
been stored for a long time. Aldehydes are
susceptible to oxidation and the aldehyde groups in
seedlac are converted to carboxylic acid groups over
time (Shearer, 1989). The polymerization of
carboxylic acid can occur over time during storage.
There are also a large number of free hydroxyl groups
which are susceptible to further esterification during
storage. Therefore, AV has decreased.
Characterization of Seedlac Hydrolysis from Kesambi (Schleicera oleosa Merr) as an Intermediate Compound for Fragrance Synthesis
87
Tabel 1: Water content, ash content and acid value of
seedlac
Specification Unit Result
Water content % (wt) 3.60
Ash content % (wt) 6.60
Acid value mg KOH / g 13.14
4000 3500 3000 2500 2000 1500 1000 500
80
90
100
%T
Wavenumber (cm-1)
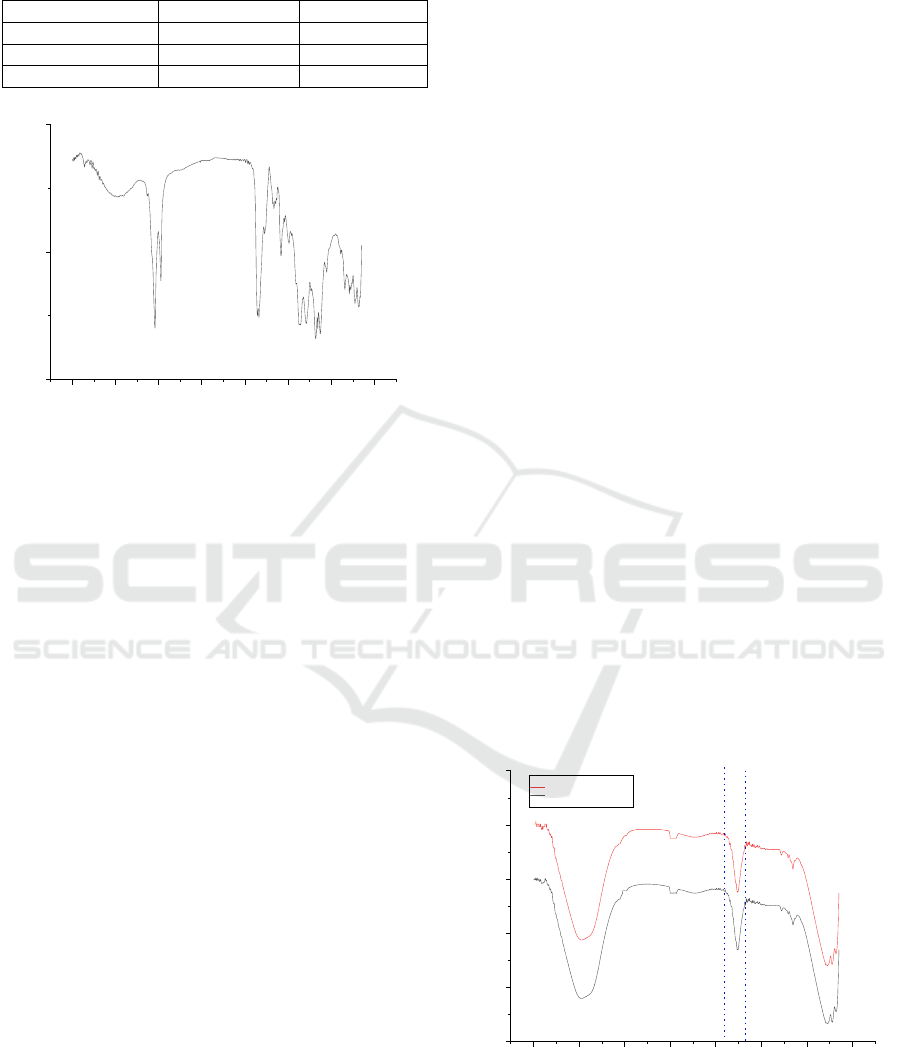
Figure 1: FTIR spectra of seedlac
FTIR spectroscopy was performed to determine
the functional groups in seedlac, the result was shown
in Figure 1. The carbonyl groups absorption of
seedlac has three shoulder in the region of 1640 cm
-1
,
1610 cm
-1
and 1550 cm
-1
. A broad peak in the range
between 3400 cm
-1
-3300 cm
-1
indicated the stretching
vibration of hydroxyl group (O-H), and bands at 2934
cm
-1
-2920 cm
-1
and 2857 cm
-1
was the C-H stretching.
The carbonyl band from ester formation was visible
at 1730 cm
-1
, and the band at 1715 cm
-1
corresponds
to acid groups. An olefinic band from C=C stretching
was present at 1630 cm
-1
, while C-O bands from ester,
acid and alcohol groups are present at 1240 cm
-1
,
1163 cm
-1
, and 1040 cm
-1
, respectively (Derry, 2012).
The region between 1500 cm
-1
and 900 cm
-1
was very
characteristic for shellac.
3.2 Seedlac Hydrolysis
Aleuritic acid (9,10,16-Trihydroxyhexa-decanoic
acid) was a major constituent acid of lac resin and
founded in the lac resin about 35% (Prasad, 2014).
The terminal hydroxyl and carboxyl functional
groups on aleuritic acid made it an excellent starting
material for the synthesis of perfumery chemicals like
macrocyclic lactones such as civetone, ambrettolide,
isoambrettolide (Nagappayya and Gaikar, 2010).
Aleuritic acid in the seedlac was in the form of
polyester.
Aleuritic acid was obtained from seedlac through
four steps. The first step was the hydrolysis of seedlac
by sodium or potassium hydroxide. The second step
involves the filtration of hydrolysate and washing of
the precipitates with saturated saltwater to yield
sodium aleuritate. The third step was acidified
sodium aleuritate using hydrochloric acid or
sulphuric acid to yield aleuritic acid. The last step was
the purification of aleuritic acid.
Lac contains natural dyes, namely erythrolaccin
and laccaic acid which are still present in seedlac.
Erythrolaccin forms a violet coloured salt when
reacted with alkali. This could interfere with the
purification process. In this experiment, seedlac was
decolorized to reduce interference. Decolorized of
seedlac was carried out by maceration at room
temperature and get natural dyes. The residue of
maceration was used in hydrolysis to yield aleuritic
acid. From this process, the natural colour was
obtained beside the aleuritic acid.
3.3 FTIR Spectrum of Product
Hydrolysis
Several analytical techniques have been applied to
study the resin of lac, and spectroscopic methods are
most widely used (Sutherland and Río, 2014). The
FTIR spectroscopy was used to investigate the
hydrolysis product of seedlac and decolorized seedlac
in this experiment. When they were compared, FTIR
spectrum of hydrolysis product from seedlac and
decolorized seedlac showed the same pattern, there
was no significant difference (Figure 2.). The main
band at 1702 cm
-1
corresponded to the C=O of
carboxylic acid groups (Heredia-Guerrero et al.,
2010). If the spectra were compared with seedlac
spectra, there were some differences.
4000 3500 3000 2500 2000 1500 1000 500
1702cm
‐1
%T
Wavenumber (cm-1)
seedlac hydrolysis
decolorized seedlac hydrolysis
Figure 2: FTIR spectra of hydrolysis product from seedlac
and decolorized seedlac
Characterization of the hydrolysis product of
seedlac and decolorized seedlac using GCMS was
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
88
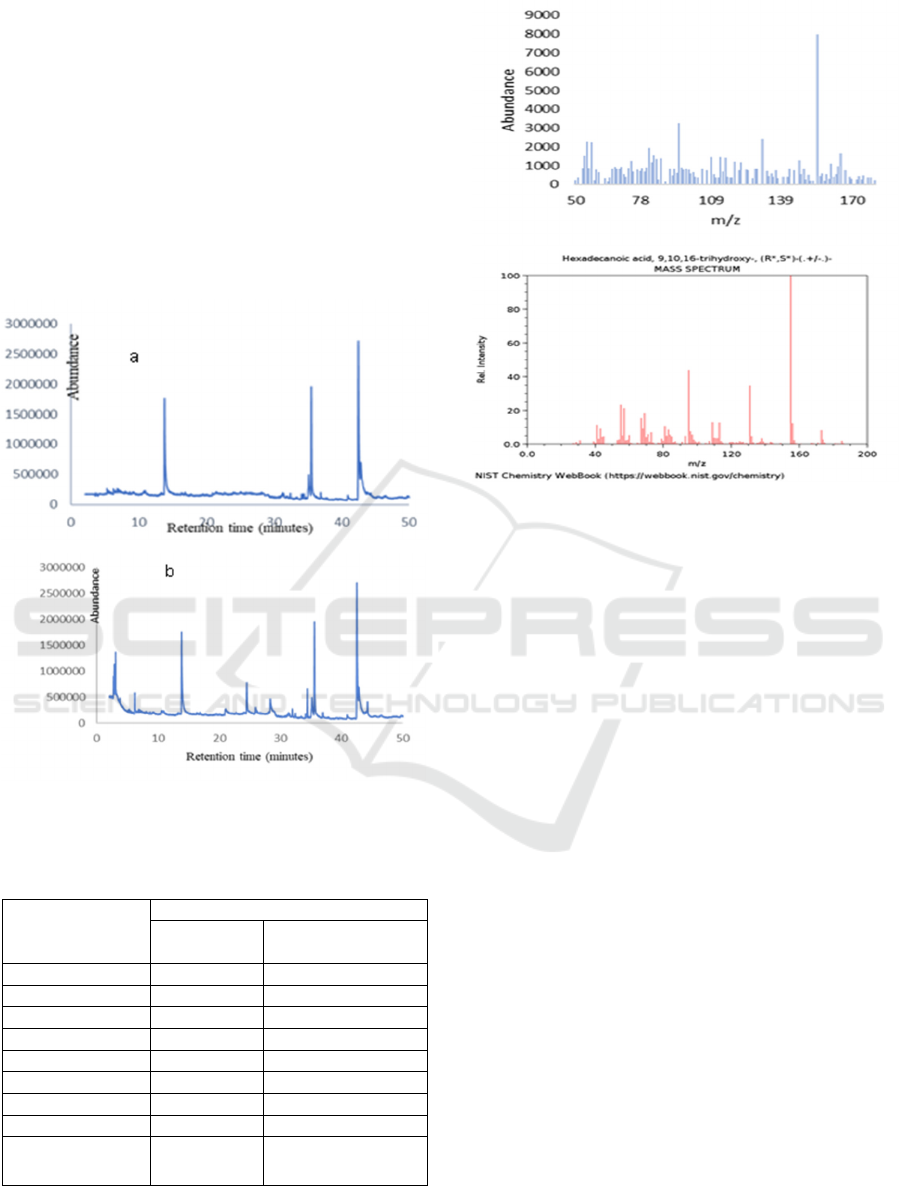
shown in Figure 3. The chromatogram of decolorized
seedlac has three dominant peaks in retention time
13,87 minutes; 35,73 minutes and 42,80 minutes. The
chromatogram of seedlac has more peaks but not all
of the peaks were dominant peaks. Several peaks in
the chromatogram of seedlac indicated the impurities
of hydrolysis products. Decolorized process of
seedlac could eliminate the natural dyes which
interfere with the purification process, therefore the
chromatogram of decolorized seedlac has fewer
peaks. The aleuritic acid was suspected in retention
time 42,80 minute by comparing mass spectrum with
reference (NIST Chemistry WebBook).
Figure 3: GCMS Chromatogram of product hydrolysis a:
decolorized seedlac; b: seedlac
Tabel 2: Relative percentage area of peaks on GCMS
chromatogram
Retention time
(minutes)
Relative percentage area (%)
seedlac
hydrolysis
Decolorized
seedlac hydrolysis
3.48 2.29 -
6.23 1.76 -
13.86 13.87 20.07
24.52 5.56 -
28.38 6.31 -
34.45 3.20 -
35.16 4.44 6.83
35.58 11.31 19.43
42.51 (aleruritic
acid)
28.43 44.30
Figure 4: Mass spectrum of peak in retention time 42,80
minutes and reference
The relative percentage area of each
chromatogram peak was summarized in Table 2. The
percentage area of aleuritic acid from decolorized
seed hydrolysis (44.30%) was greater than aleuritic
acid from seedlac hydrolysis (28.43). The decolorized
process of seedlac before hydrolysis in this
experiment could improve the percentage of aleuritic
acid up to 56% (from 28.43% to 44.30%).
4 CONCLUSIONS
Characterization of seedlac hydrolysis with the
decolorized process before hydrolysis showed that
the percentage of aleuritic acid as a hydrolysis
product could be improved from 28.43 % to 44.30%.
This method could be considered in the production of
aleuritic acid from seedlac.
REFERENCES
Berbers, S. V. J. et al. (2019) ‘Historical formulations of
lake pigments and dyes derived from lac : A study of
compositional variability’, Dyes and Pigments.
Elsevier, 170(March), p. 107579. doi:
10.1016/j.dyepig.2019.107579.
Biswas, S. (2014) ‘Preparation of Environment Friendly
Composites from Effluent of Aleutitic acid Industry and
Modified Betel-Nut Fiber’,
Int.J.Curr.Res.Chem.Pharma.Sci, 1(6), pp. 50–55.
Characterization of Seedlac Hydrolysis from Kesambi (Schleicera oleosa Merr) as an Intermediate Compound for Fragrance Synthesis
89

Derry, J. (2012) ‘Investigating shellac: documenting the
process, defining the product. A study on the processing
methods of shellac, and the analysis of selected physical
and chemical characteristics’, The Institute of
Archeology, Conservation and History, Faculty of
Humanities, Master The.
Farag, Y. (2010) Characterization of Different Shellac
Types and Development of Shellac-Coated Dosage
Forms, Fakultät für Mathematik, Informatik und
Naturwissenschaften.
Farag, Y. and Leopold, C. S. (2009) ‘Physicochemical
Properties of Various Shellac Types’, Dissolution
Technologies, (May), pp. 33–39.
Heredia-Guerrero, J. A. et al. (2010) ‘Aleuritic (9,10,16-
trihydroxypalmitic) acid self-assembly on mica’,
Physical Chemistry Chemical Physics, 12(35), pp.
10423–10428. doi: 10.1039/c0cp00163e.
Nagappayya, S. K. and Gaikar, V. G. (2010) ‘Extraction of
Aleuritic Acid from Seedlac and Purification by
Reactive Adsorption on Functionalized Polymers’,
Ind.Eng.Chem.Res, 49, pp. 6547–6553.
Prasad, N. (2014) ‘Final Report of NAIP sub-project on A
Value Chain on Lac and Lac based Products for
Domestic and Export Markets’, . Indian Institute of
Natural Resins and Gums, Namkum, Ranchi.
Ravi, S., Padmanabhan, D. and Mamdapur, V. R. (2001)
‘Macrocyclic musk compounds: Synthetic approaches
to key intermediates for exaltolide, exaltone and
dilactones’, Journal of the Indian Institute of Science,
81(3), pp. 299–312.
Shearer, G. L. (1989) ‘An Evaluation of Fourier Transform
Infrared Spectroscopy for the Characterzation of
Organic Compounds in Art and Archaeology’,
(October), pp. 1–399.
Sutherland, K. and Río, J. C. (2014) ‘Characterisation and
discrimination of various types of lac resin using gas
chromatography mass spectrometry techniques with
quaternary ammonium reagents’, Journal of
Chromatography A. Elsevier B.V., 1338, pp. 149–163.
doi: 10.1016/j.chroma.2014.02.063.
Taskirawati, I. et al. (2017) ‘Peluang investasi dan strategi
pengembangan usaha budidaya kutu lak (Laccifer lacca
Kerr): studi kasus di KPH probolinggo, perum
perhutani unit II jawa timur’, Jurnal Entomologi
Indonesia, 4(1), p. 42. doi: 10.5994/jei.4.1.42.
Yaqub, A. et al. (2014) ‘Isolation and its Purification of
Laccaic Acid Dye from Stick Lac and study of its (
Colour Fastness ) Properties and Reflactance on Silk
Fabric Dyed with Heavy Metal Mordants’, Technical
Journal, University of Engineering and Technology
Taxila, 19(I), pp. 6–12.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
90
