
Antimicrobial Effect of Concord Paper Containing with Lemongrass
Oil against Escherichia coli and Staphylococcus aureus
Bunda Amalia
3
, Retno Yunilawati
1
, Windri Handayani
2
, Agustina Arianita C.
3
and Cuk Imawan
1
1
Department of Physics, Faculty of Mathematics and Natural Sciences (FMIPA) Universitas Indonesia, 16424 Depok,
Indonesia
2
Department of Biology, Faculty of Mathematics and Natural Sciences (FMIPA) Universitas Indonesia, 16424 Depok,
Indonesia
3
Badan Penelitian dan Pengembangan Industri, BBKK, Kementerian Perindustrian, Indonesia
Keywords: Lemongrass Oils, Concord Paper, Antimicrobial Activity.
Abstract: The use of an antimicrobial label in food packaging as a form of active packaging is an interesting to
investigate. This label can be used to extend the shelf life of food. Lemongrass oil is one of essential oil that
is potential used as an antimicrobial agent. In this study, the antimicrobial effect of label made from concord
paper which incorporated with lemongrass oil was prepared and tested against the bacteria Escherichia coli
and Staphylococcus aureus using disk inhibition zone method. This antimicrobial label was tested using FTIR
to investigate the interaction between essential oil and the matrix. The lemongrass oil was tested using Gas
Chromatography-Mass Spectrometry to determine the levels and presence of compounds suspected of having
antimicrobial activity. The labels have antibacterial activity against E. coli with the diameter of inhibition
zone maximum about 47.85 mm but not active toward the S. aureus. From the results of the antibacterial test
can be seen that the use of antibacterial label is promising when used for food safety with a prolonged shelf
life.
1 INTRODUCTION
Contamination of food can occur during the process
of harvesting, food processing, packaging and
distribution. Packaging is one of the effective ways to
protect food from contamination from the outside
environment such as air, dust, physical, chemical and
biological impacts such as microbes that cause food
spoilage. Conventional packaging which is widely
used today, cannot actively control the reactions that
occur in food (Mousavi et al., 2018). One of the
packaging technologies that have been developed to
maintain the quality of food and extend the shelf life
of food is to use active packaging. The use of
antimicrobial labels on active packaging is now an
interesting technology for research.
With the aim to reduce the use of additional
chemical substances in food, one way is to use natural
ingredients to inhibit the growth of microbes that
cause food spoilage that does not have a negative
effect on human health (Chiralt and Atar, 2016).
Essential Oil is one of the antimicrobial agents
derived from plant extracts that have antimicrobial
properties. However, this essential has a strong
enough odour that it is rarely used to be added directly
to food because it will damage the taste of the food
itself. Because that reason, it is interested to combine
essential oils into a matrix to reduce the strong odour
as antimicrobial label.
Several studies have been carried out by
combining essential oil such as oregano with cassava
starch/chitosan with oregano essential oil (Pelissari et
al., 2009), alginate with lemongrass oil (Chiralt and
Atar, 2016), and coated paper with Cuminum
cyminum L. and Prubus mahaleb L. in terms of
improving antimicrobial properties (Ezel and Dal,
2018). In this research, lemongrass oil is combined
into a paper matrix. The paper used is Concord paper.
Concord paper which has another name namely
Japanese linen paper is textured paper. By combining
lemongrass oil into the concord, it is hoped that it can
make an effective antimicrobial label which effective
against Eschericha coli and Staphylococcus aureus
which can be used to extend the shelf life of food.
54
Amalia, B., Yunilawati, R., Handayani, W., Arianita C., A. and Imawan, C.
Antimicrobial Effect of Concord Paper Containing with Lemongrass Oil against Escherichia coli and Staphylococcus aureus.
DOI: 10.5220/0009956200540059
In Proceedings of the 2nd International Conference of Essential Oils (ICEO 2019), pages 54-59
ISBN: 978-989-758-456-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 MATERIALS AND METHOD
2.1 Materials
Lemongrass oil was used in this experiment obtained
from Nusaroma, a local essential oils company in
Indonesia. The matrix used in this study is concord
paper with a gramatur of 220 gr / mm
2
produced by
PT. Parisindo Pratama.
2.2 Preparation of Antimicrobial
Labels
The antimicrobial label was prepared by dropping of
25 µL lemongrass oil using a micro pipette onto the
surface of the concord paper with a size of 1 cm x 3
cm, then allowed at room temperature for 5 minutes.
2.3 Characterization
2.3.1 Lemongrass Oil Characterization
Characterization Lemongrass Oil using GC- MS.
Lemongrass oil compounds were identified by gas
chromatography with a mass spectrometer detector
(GCMS) Agilent 6890 series with capillary column
HP-5MS, 30 m x 0.25 mm id x 0.25 µm film
thickness. Helium gas was used as the carrier gas at
constant pressure of 65 kPa. The lemongrass oil was
injected with a volume of 1 µL in split ratio of 1:25.
The increasing of oven temperature was programmed
from 60-240°C with step of 3°C per minute until
reaching 240°C.
Antimicrobial Activities Assay of Lemongrass Oil:
Direct Contact Agar Diffusion Tests. The
antimicrobial activities determined by the paper disc
diffusion method using type strain of Staphylococcus
aureus NBRC 100910 and Escherichia coli NBRC
3301 in The Mueller Hinton Agar. 10 ml of molten
media poured into sterile Petri plates (d=90 mm) and
allowed to solidify for 5 minutes. After that, in a tube,
10 µl of bacteria culture 10
-6
CFU/mL added with 10
ml of medium and mixed gently with the inoculate
before poured on the top of molten media before and
allowed to dry for 5 minutes. The negative control
(sterile distilled water), positive control (tetracycline
15 µg/mL), lemongrass oil with concentration 1000
µg/mL loaded on 6 mm disc, whereas the volume for
each disc was 10 µl. The loaded disc placed on the
surface of the medium then incubates at 35° C for 18
hours. After the end of incubation, a clear zone
formed around the disc was measured.
2.3.2 Label Characterization
Antimicrobial Activities Assay of Labels. The
antimicrobial activities of labels were tested in vapour
phase agar diffusion test, because in its application as
label antimicrobial will used vapour phase. The
vapour phase method follows the method used by
(Wang et.al, 2016).
Labels are cut in a circle with a diameter of 0.6 cm
and then placed in a petri dish to test antimicrobial
activity. The vapour phase agar diffusion test was
technically similar to the direct contact diffusion test.
However, the filter discs were placed at the top in
centre of the inner side of the Petri dish cover. The
dishes were then sealed using laboratory parafilm to
avoid evaporation of the test compounds, followed by
incubation at 32° C for 24h. The diameter of the
inhibition zone was recorded.
Efficacy Test of Label on the Product. The efficacy
of the antimicrobial label was evaluated by placing
the label with a size of 1x3 cm above the surface of a
plastic package containing chicken meat (10 g)
purchased from the local market in Depok. Then the
chicken meat is kept at room temperature for 5 days
to see the visual changes found in the chicken meat.
Fourier Transform-Infra Red (FTIR) Analysis.
The spectra of the antimicrobial label (control paper
and paper that had been dropped with lemongrass oil)
were test using Fourier Transform Infrared (FTIR)
using a double beam spectrophotometer (Thermo
Nicolet iS5) to determine functional groups. FTIR
analysis was carried out on blank label before and
after it was used to store chicken breast filled.
UV-Vis Analysis. UV- vis spectrophotometer was
used to measure the reflectance of the antimicrobial
label (control paper and paper that had been dropped
with lemongrass oil) with a size of 1x3 cm. The brand
of UV-Vis apparatus is Shimadzu UV-2450.
3 RESULT AND DISCUSSION
3.1 Chemical Compounds of the
Lemongrass Oil
Characterization using GC-MS showed the
chromatogram profile detected 6 peaks in lemongrass
oil (Figure 1) which indicated there were 6
compounds in lemongrass oil. The compounds were
identified based on comparison of mass spectrum
Antimicrobial Effect of Concord Paper Containing with Lemongrass Oil against Escherichia coli and Staphylococcus aureus
55
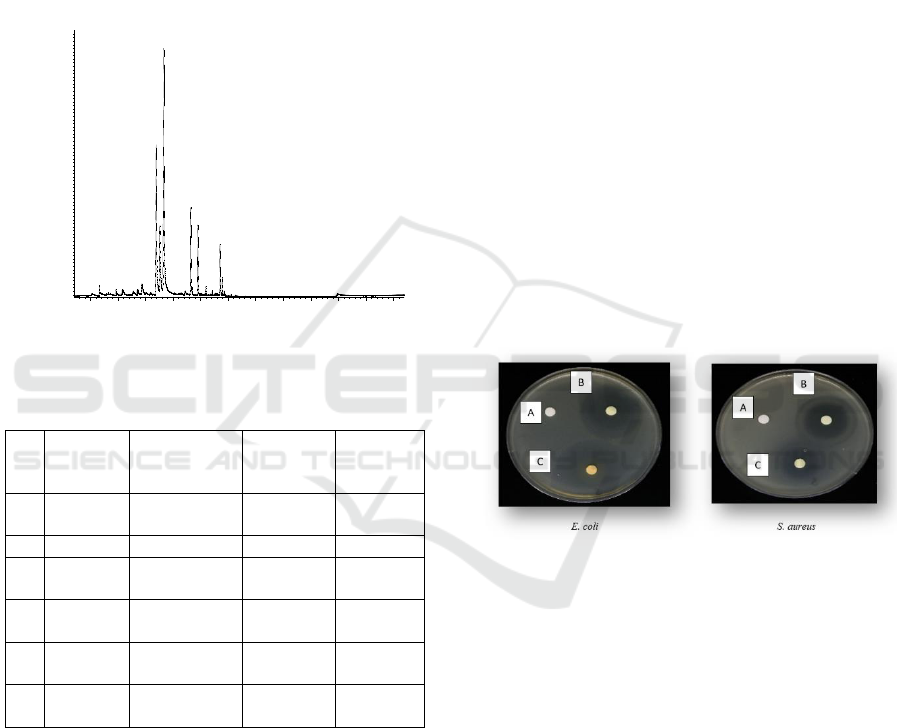
with reference data from the database (Wiley 7).
Based on this, lemongrass oil was known contain 6
compounds, namely neral (beta-citral), geraniol,
geranial (alpha-citral), geranyl acetate, beta-
caryophyllene and gamma cadinene (Table 1) with
the main compounds being citral and geraniol. These
results appropriated with previous finding reported in
literature, citral and geraniol has been described as the
main compounds of lemongrass oil (Ganjewala,
2009).
Figure 1: GC-MS chromatogram of the lemongrass oil.
Table 1: Chemical compound identified of lemongrass oil
with GC-MS.
No
Retention
time
Identified
compound
Molecular
formula
Relative
percentage
area (%)
1
17.101
Neral (beta-
citral)
C
10
H
16
O
29.00
2
17.753
Geraniol
C
10
H
18
O
10.80
3
18.524
Geranial
(Alpha citral)
C
10
H
16
O
44.21
4
23.302
Geranyl
acetate
C
12
H
20
O
2
6.50
5
24.588
Beta-
caryophyllene
C
15
H
24
5.67
6
28.589
Gamma-
cadinene
C
15
H
24
3.83
Citral (3,7 dimethyl-2-6-octadienal) is an
unsaturated aldehyde, the most common flavour in
citrus oil and widely used in food and beverages.
Citral is the mixture of two isomers geometric, neral
(beta-citral) and geranial (alpha-citral) which are
monoterpene aldehyde. Citral has an activity
antibacterial against Gram-positive bacteria and
Gram-negative bacteria, both on oil form and vapour
form (Argyropoulou et al., 2007) It is revealed the
presence of C = O bond for aldehyde from indicates
the presence of citral compounds. Antimicrobial
activity of cinnamaldehyde was found against E. coli
and staphylococcus aureus. Citral that have aldehyde
function group plays a role in disrupting bacterial cell
membranes (Firmino et al., 2018)
3.2 Antimicrobial Activities of
Lemongrass Oil and the Label
The bacteria is one of indicator used for examination
of spoilage to meat products (Pranoto et al., 2005).
Meat and processed products that are perishable food
because they are very vulnerable to contamination by
microorganisms. Spoilage meat can contain
pathogenic bacteria such as S. Aureus and E. coli.
Therefore, in this research an Antimicrobial activity
of lemongrass oil against test was carried out against
E. coli and S. aureus (Figure 2). The inhibitory
activity is measured based on the clear zone that
occurs around the label. The measurement of the clear
zone diameter is calculated including the diameter of
the label. The diameter produced will be greater than
the diameter of the label if a clear zone is detected. If
no clear zone is formed around the label, then it is
assumed that there is no inhibitory region and the
diameter is declared zero.
Figure 2: Antimicrobial activities of lemongrass oil using
paper disk method against Gram-positive bacteria S. aureus
and Gram negative bacteria E. coli; A = negative control;
B=positive control; C=sample.
In Figure 2 it can be seen that the negative control
in the form of sterile distilled water does not form a
clear zone which means it does not show an inhibitory
effect on E. coli and S. aureus bacteria. Inhibition
diameter can be seen in Table 2. For positive control
in the form of antibiotic tetracycline, a clear zone with
a diameter of 1.9 cm can be seen for E. coli bacteria
and 3.1 cm for A. aureus bacteria. As for the
lemongrass oil, a clear zone with a diameter of 4.7 cm
is formed for E. coli bacteria and 2.5 cm for A. aureus
bacteria. The diameter of the clear zone formed in
lemongrass oil against E. coli bacteria (gram -) is
greater than that of S. aureus (gram +), which means
that lemongrass oil is more effective against E. coli
bacteria (gram -). This is because Gram positive has
5 . 0 0 1 0 . 0 0 1 5 . 0 0 2 0 . 0 0 2 5 . 0 0 3 0 . 0 0 3 5 . 0 0 4 0 . 0 0 4 5 . 0 0 5 0 . 0 0 5 5 . 0 0 6 0 . 0 0
1 0 0 0 0 0 0
2 0 0 0 0 0 0
3 0 0 0 0 0 0
4 0 0 0 0 0 0
5 0 0 0 0 0 0
6 0 0 0 0 0 0
7 0 0 0 0 0 0
8 0 0 0 0 0 0
9 0 0 0 0 0 0
1 e + 0 7
1 . 1 e + 0 7
1 . 2 e + 0 7
1 . 3 e + 0 7
1 . 4 e + 0 7
T im e - - >
A b u n d a n c e
T I C : 1 3 0 3 . D \ d a t a . m s
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
56

a cell wall structure that is different from gram
negative bacteria. In addition, gram-positive bacteria
have cell walls composed of a thicker layer of
peptidoglycan (20 to 80 nanometres), while gram-
negative bacteria have a thinner layer of
peptidoglycan.
Table 2: Diameter of inhibition zone of lemongrass oil.
S. aureus(cm)
E.coli (cm)
Sample
Control
(-)
Contro
l (+)
Sample
Contro
l (-)
Control
(+)
2,5
-
3,1
4,7
-
1,9
Besides seeing the antimicrobial activity of
Lemongrass oil, it was also carry out an antimicrobial
test from the label. For the label antimicrobial test, the
vapour method is used to match the application used.
The antimicrobial activity of the label can be seen in
Figure 3.
Figure 3: Antimicrobial activities of antimicrobial labels
with lemongrass oil concentration 10% using vapour
method against Gram-positive bacteria S. aureus and Gram-
negative bacteria.
From Figure 3 it can be seen that the label
provides antimicrobial effect on E. coli (gram
negative bacteria) but not on S. aureus (gram positive
bacteria). It can be seen from a clear zone or the
diameter of the inhibition zone of E. coli is around
47.85 cm, while the S.aureus bacteria do not form a
clear zone, then it is assumed that there is no
inhibitory region and the diameter is zero, this is
possible because the antimicrobial testing of the label
was use the vapour method. The effectiveness of
lemongrass oil on E.coli is also similar as that of other
researchers (Faleiro, 2019) and (Naik et al., 2010).
Other research which states that lemongrass essential
oil also has antimicrobial properties against other
bacteria such as A. baumannii (Adukwu et al., 2016).
3.3 Efficacy Test of Label on the
Product
The efficacy of the antimicrobial label was evaluated
within 5 days using chicken breast filled. From Figure
4 it can be seen that there is a change in the colour,
texture and odour of the chicken breast filled. On the
fifth day, Figure 4 (A) is chicken breast filled without
using a label. Figure 4 (A) shows the colour of
chicken breast filled is paler compared to Figure 4
(B). In addition to colour observation, the texture of
chicken breast filled in Figure 4 (A) is also soggier
when compared to Figure 4 (B), this indicates that the
label application can maintain the freshness of
chicken breast filled. In addition to investigating the
colour and texture, an investigation was also
conducted on odours. In this experiment, the odour of
lemongrass oil still affected the odour of the food in
the packaging.
Figure 4: Label application on the chicken breast filled, (a)
label without application, (b) label with application.
3.4 FTIR Analysis of Label
Functional group analysis is performed to determine
changes in functional groups that occur during
efficacy tests on the labels. The performance test of
labels on chicken breast was carried out for five days.
During this time, functional group analyses are
carried out using FTIR. Figure 5 displays the spectra
of concord paper and label.
Figure 5: FT-IR Spectra.
500 1000 1500 2000 2500 3000 3500 4000
O=H
(3200-3600)
% Transmittance
Wave namber (cm-1)
Concord+EO H-5
Concord+EO H-4
Concord+EO H-3
Concord+EO H-2
Concord+EO H-1
Concord+EO H-0
Concord
C=O
(1690-1760)
Day 1
Day 5
Antimicrobial Effect of Concord Paper Containing with Lemongrass Oil against Escherichia coli and Staphylococcus aureus
57

Fingerprint for lemongrass oil is mostly in the
range of 1800-600 cm-1 (Li, 2013). In the IR spectra,
it is shown that the absorbance band at 1690-1760 cm
-
1
revealed the presence of C=O bond for aldehyde
from indicates the presence of citral compounds
(Adinew 2014). Besides that, it is shown that the
absorbance band at 3200-3600 cm-1 revealed the
presence of O=H, which indicates the presence of
compounds geraniol. From the Figure, the C=O
intensity of citral is decreasing. It is because citral has
to be released from the label and the presence of this
citral compound was strengthened by GC MS results
and the result of antimicrobial assay.
3.5 UV-VIS Analysis
UV-Vis analysis was carried out to see the Reflect
ants from the label before and after the addition of
essential oil. From Figure 6 there is a change in the %
reflectance intensity of the label. The color change
occurred from blue to green can be seen in Figure 7.
The green color change occurred after the addition of
lemongrass oil followed by a decrease in the intensity
value of % Reflectance at wavelength around 600-
650 nm.
400 450 500 550 600 650 700
0.0
0.1
0.2
0.3
0.4
0.5
% Reflectance
wavelenght (nm)
Blanko Concord
Concord+EO H0
Concord+EO H2
Concord+EO H5
Figure 6: Reflectance spectra of antimicrobial labels.
Figure 7: Discoloration of label.
4 CONCLUSIONS
In this study, it can be concluded that labels made
from Concorde paper added with lemongrass oil have
the potential to become antimicrobial label. The
labels have antibacterial activity against E. coli with
the diameter of inhibition zone maximum about 47,85
mm but not active toward the S. aureus. However, the
application will depend on the type of food where
flavour is not a problem.
ACKNOWLEDGEMENTS
This research supported by PSNI (Penelitian Strategis
Nasional Institusi) from Kementerian Riset,
Teknologi, dan Perguruan Tinggi Republik Indonesia
No NKB-1798/UN2.R3.1/HKP.05.00/2019. We also
thank the Center of Excellence Biology Resources
Genome Study (CoE IBR-GS) FMIPA UI and the
Center for Chemical and Packaging (CCP) for the
facilities and equipment to support this research.
REFERENCES
Adukwu, E. C., Bowles, M., Jones, V, W., Bone, H., 2016.
Antimicrobial Activity , Cytotoxicity and Chemical
Analysis of Lemongrass Essential Oil (Cymbopogon
flexuosus) and Pure Citral, Applied Microbiology and
Biotechnology. Applied Microbiology and
Biotechnology, 9619–9627.
Argyropoulou, C., Daferera, D., Tarantilis, P, A., Fesseas,
C., Polissiou, M., 2007. Chemical Composition of the
Essential Oil from Leaves of Lippia citriodora H.B.K.
(Verbenaceae) at Two Developmental Stages,
Biochemical Systematics and Ecology, 35(12), 831–
837.
Chiralt, A., Atar, L., 2016. Trends in Food Science &
Technology Essential Oils as additives in
Biodegradable Films and Coatings for Active Food
Packaging, 48.
Ezel, A., Dal, B., 2018. Strength Properties of Coated Paper
with Cuminum cyminum L. and Prunus mahaleb L,
14(2), 247–249.
Gago, C, M, L., Artigas, M, A., Antunes, M, D, C., Faleiro,
M, L., Miguel, M, G., Belloso, O, M., 2019.
Effectiveness of Nanoemulsions of Clove and
Lemongrass Essential Oils and Their Major
Components Against Escherichia coli and Botrytis
cinerea’, 56, 2721–2736.
Firmino, D, F., Cavalcante, T, T, A., Gomes, G, A.,
Firmino, N, C, S., Rosa, L, D., de Carvalho, M, G.,
Junior, F, E, A, C., 2018. Antibacterial and Antibiofilm
Activities of Cinnamomum Sp . Essential Oil and
Cinnamaldehyde : Antimicrobial Activities, 2018.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
58

Ganjewala, D., 2009. Cymbopogon Essential Oils :
Chemical Compositions and Bioactivities,
International Journal of Essential Oil Therapeutics, 3,
56–65.
Khaneghah, A, M., Hashemi, S, M, G., Limbo, S., 2018.
Food and Bioproducts Processing Antimicrobial
Agents and Packaging Systems in Antimicrobial Active
Food Packaging : An Overview of Approaches and
Interactions, Food and Bioproducts Processing.
Institution of Chemical Engineers, 111, 1–19.
Naik, M. I., Fomda, B, A., Jaykumar, E., Bhat, J, A., 2010.
Antibacterial Activity of Lemongrass (Cymbopogon
citratus) Oil Against some Selected Pathogenic
Bacterias, Asian Pacific Journal of Tropical Medicine,
3(7), 535–538.
Pelissari, F. M., Grossmann, M, V, E., Yamashita, F.,
Pineda, E, A, G., 2009. Antimicrobial, Mechanical, and
Barrier Properties of Cassava Starch-Chitosan Films
Incorporated with Oregano Essential Oil, Journal of
agricultural and food chemistry, 7499–7504.
Pranoto, Y., Rakshit, S, K, A., Salokhe, V, M., 2005.
Enhancing Antimicrobial Activity of Chitosan Films by
incorporating garlic oil , Potassium Sorbate and Nisin,
38, 859–865.
Antimicrobial Effect of Concord Paper Containing with Lemongrass Oil against Escherichia coli and Staphylococcus aureus
59
