
Release Profile of the Antimicrobial Agent from Clove Oil
Encapsulated in a Polyurethane Shell
Chicha Nuraeni
1
, Dwinna Rahmi
1
, Retno Yunilawati
1
, Emmy Ratnawati
1
, Tiara Mailisa
1
, Trisny
Andrianty
1
, Irwinanita
1
, Bunda Amalia
1
and Arief Riyanto
1
1
Balai Besar Kimia dan Kemasan, Badan Penelitian dan Pengembangan Industri
Kementerian Perindustrian, Jakarta, Indonesia
Keywords: Clove Oil, Encapsulation, Polyurethane Shell, Release Profile.
Abstract: The essential oil has been known for its antimicrobial properties and has the potency to be utilized as an
active agent in food preservative, packaging, and textile. Eugenol and caryophyllene are a major
antimicrobial component in the clove oil, which is proved against several bacterial and fungal strains. Due
to the clove oil is easily oxidized and have a strong smell, it needs to be encapsulated so it can be used for
long-term application. The encapsulation of the clove oil in the polyurethane shell was prepared by
polymerization in an oil-in-water emulsion. The FTIR spectra of the microcapsules showed that the clove
oil was successfully encapsulated. The release profile of the antimicrobial agent from the microcapsules was
measured using Headscape GC. From the prediction based on the release profile showed that the
microcapsules could emit the eugenol for 59 days and the caryophyllene for 15 days. Therefore, it could be
concluded that the microcapsules of clove oil in the polyurethane shell is suitable for long term application.
1 INTRODUCTION
Clove (Syzygium aromaticum) is one of the native
Indonesian plants that are well known worldwide.
Clove oils collected from the distillation of the
clove’s leaves have proved against several bacterial
and fungal strains (Cortés-Rojas et al., 2014). The
antimicrobial agent from natural plants such as the
clove oil is considered safe, so it has more consumer
preference than the chemical antimicrobial agent
(Han, 2003). Therefore, the oils have been
developed for widespread applications such as food
preservatives (Cui et al., 2015), active packaging
(Hosseini et al., 2009), and textiles (Kim and
Sharma, 2011). However, clove oils are easily
oxidized and have a strong smell, so they are not
suitable for long-term use. The encapsulation
process had been known could reduce those
weaknesses (Kfoury et al., 2016).
Encapsulation is the process through which one
substance or a combination of materials is coated or
trapped in another material or system. The coated
material is referred to as active or core material, and
the coating material is referred to as a shell, wall
material, carrier, or encapsulant (Madene et al.,
2006). Chemical encapsulation using polymerization
technique has been known as easy to be scaled-up,
generically fast, and provides high encapsulation
efficiency (Carvalho et al., 2016).
Many lists of researches regarding encapsulation
by polymerization method, but only a few were
using the essential oil, especially clove oil, as core
material. Scarfato et al. (2007) provided
encapsulation of essential oils by interfacial
polymerization in o/w emulsion between
polyfunctional isocyanates and diamines. They used
essential oils from lemon balm (Melissa officinalis
L.), lavender (Lavandula angustifolia Miller), sage
(Salvia officinalis L.), and thyme (Thymus vulgaris
L.). Liu et al. (2015) proposed a process for
nanocapsules containing cologne essential oil for
textile applications. Methyl methacrylate (MMA)–
styrene (St) copolymer was used as a shell material
to prepare nanocapsules containing cologne essential
oil as a core material by miniemulsion
polymerization. Chung et al. (2013) encapsulated
thyme oil using melamine–formaldehyde
prepolymer.
Nuraeni, C., Rahmi, D., Yunilawati, R., Ratnawati, E., Mailisa, T., Andrianty, T., Irwinanita, ., Amalia, B. and Riyanto, A.
Release Profile of the Antimicrobial Agent from Clove Oil Encapsulated in a Polyurethane Shell.
DOI: 10.5220/0009954900290036
In Proceedings of the 2nd International Conference of Essential Oils (ICEO 2019), pages 29-36
ISBN: 978-989-758-456-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
29

Gezundhait and Pelah (2017) used polyurethane
as the shell for the encapsulation of essential oils in
which the polyurethane shell made from the reaction
of TDI (toluene diisocyanate) and polyethylene
glycol 4000. In this paper, polyurethane from
methylene diphenyl diisocyanate (MDI) was used as
an encapsulation shell for the clove oil. According to
Allport et al. (2003), MDI is less hazardous
compared to TDI. Polyurethane was chosen as shell
material because it is inexpensive and has high
durability, then it is possible for a broad application
(Engels et al., 2013).
The release profile is an important thing for the
application of the encapsulated clove oil as an
antimicrobial agent. The active compounds are
expected can be released at a specific rate for a long
time period as possible. The aim of this research is
to study the release of antimicrobial compounds
from clove oil encapsulated in a polyurethane shell.
According to the literature, antimicrobial agents in
clove oil are eugenol and caryophyllene. Marchese
et al. (2017) described the mechanism of action of
eugenol on bacteria and fungi. The antimicrobial
activity of eugenol can be attributed to the presence
of a free hydroxyl group in the molecule that able to
bind to proteins, preventing enzyme action. The
eugenol also disrupts the cytoplasmatic membrane
and alters the permeability of the membrane, which
leads to cell death. Dahham et al. (2015) proved that
β-caryophyllene showed antimicrobial activity
against Staphylococcus aureus and showed better
antifungal activity than Kanamycin (a common
antifungal drug on the market).
2 MATERIAL AND METHOD
Materials used in this study were methylene
diphenyl diisocyanate (MDI) prepolymer, obtained
from PT Covestro Polymers Indonesia; polyethylene
glycol 400 (PEG) “Bratachem”; sodium lauryl
sulphate (SLS) “Emal 10 N” Kao Chemicals;
xanthan gum and clove oil. All materials were used
without further purification.
Clove oil was obtained from Java area. The
composition of the clove oil was analysed using GC-
MS (Gas Chromatography–Mass Spectrometry)
“Agilent” 7890B coupled to “Agilent” 5977. The
analysis was performed using a non-polar capillary
column (DB-5MS, 30 m × 250μm, film thickness
0.25 µm). Then, the compounds were identified by
matching their mass spectra with GC-MS libraries
(Wiley Registry).
Encapsulated clove oils were prepared by
polymerization of polyurethane in an oil-in-water
emulsion. At room temperature, the mixture of 150
mL of water and 25 grams of PEG were mixed at
400 rpm. Simultaneously, 8 grams of MDI and 25
grams of clove oil were mixed and then was added
to the mixture. After the “clump” of polyurethane-
clove oil was formed, add SLS and xanthan gum as
much as 1 gram, respectively, while continuously
stirring for 2 hours. The resultant microcapsules
were strained from the liquid phase and then were
rinsed with water twice. The process was conducted
at room temperature. At last, the microcapsule
powders were stored in chiller around 10
o
C before
analysed.
The microcapsules were examined its
morphology using the microscope “Olympus BX53”
and were characterized using FTIR (Fourier
transform infrared) “Nicolet iS5” with an iD5 ATR
diamond tip adapter.
The release properties of the clove oil
encapsulated in polyurethane shells were
qualitatively and quantitatively analysed by
headspace-analysis technique using a Perkin Elmer
Headspace GC Clarus® 680 (column 30.0m x
250μm). Sample as much as 2 grams of
microcapsules was equilibrated at 40°C in the
headspace unit before the injection. The carrier gas
was helium; the detector temperature was 300 °C;
the oven temperature was programmed from 40 °C
(5 min hold) to 250 °C (10 min hold) increasing at
20°C/min. A split injector was used at 200° in split
mode at a ratio of 1:50. The measurements were
conducted in 0 day, 1
st
day, 3
rd
day, 8
th
day, and 10
th
day.
3 RESULTS AND DISCUSSION
3.1 The Composition of the Clove Oil
The clove oil analysis performed by GC-MS shows
10 (ten) peaks (Figure 1). From the chromatogram,
was obtained major components clove oil that are
81.64% eugenol, 15.86% trans-caryophyllene,
1.15% alpha-caryophyllene, 0.47% caryophyllene
oxide, 0.29% trans-anethole (Table 1).
3.2 The Morphology of the Clove Oil
Encapsulated in a Polyurethane
Shell
The creation of microcapsules of clove oil in
polyurethane shell due to the reaction of a diol with
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
30

a diisocyanate (Figure 2). In this study, a diol is
referred to as PEG, whereas the diisocyanate is
referred to as MDI. The diol is dissolved in the
aqueous phase and the diisocyanate in the organic.
The reaction at the oil–water interface produces the
encapsulating shell (Yow & Routh, 2006).
In the process of encapsulation by emulsion
polymerization, surfactants play major roles such as
solubilising of highly water-insoluble monomers,
determining the mechanism of particle nucleation,
determining the number of particles nucleated and
therefore the rate of polymerization, maintaining
colloidal stability during the particle growth stage,
and controlling average particle size and the size
distribution of the final system (El-Aasser, 1990). In
this study, sodium lauryl sulphate (SLS) was chosen
because SLS is an anionic surfactant and a very
strong type of surfactant and is a common emulsifier
for most heterogeneous systems. SLS also helps
reduce the size of the capsules by lowering the
surface tension in the matrix (Lakkis, 2016)
Figure 3 shows the appearance of clove oil
encapsulated in polyurethane shell. At four times
magnification, it shows that the capsules gave
spherical shape with size range from 246 m to 832
m. The size is larger compared to other researches,
because this study uses a lower stirring rate. The
increase in the stirring rate led to the formation of
smaller particles and narrower distributions
(Leimann et al., 2009), but the higher stirring rate
might less efficient in scale-up manufacturing.
Mamaghani and Naghib (2017) demonstrated that
the stirring rate at 400 rpm is affordable for
production regarding the energy consumed.
Figure 1: Chromatogram of the clove oil.
Table 1: Compounds identified from clove oil using GC-MS.
Peak
No.
RT
(min)
Area %
Name
CAS
% Sim
1
12.115
81.64
Eugenol
97-53-0
98
2
12.283
0.14
Alpha-copaene
3856-25-5
98
3
12.904
15.86
trans-caryophyllene
87-44-5
99
4
13.304
1.15
alpha-caryophyllene
753-98-6
99
5
14.111
0.14
1-S-cis-calamenen
483-77-2
97
6
14.501
0.07
cis-jasmone
488-10-8
64
7
14.879
0.47
Caryophyllene oxide
1139-30-6
81
8
16.505
0.12
3-methoxycinnamic acid
6099-04-3
46
9
23.563
0.29
Trans-anethole
4180-23-8
46
10
23.802
0.13
6-Nitro-2,4-diphenylquinoline
138432-74-3
53
RT (min): retention times in minutes; Area%: relative area counts; CAS: CAS numbers; %Sim: % similarities to
reference library spectrum
Release Profile of the Antimicrobial Agent from Clove Oil Encapsulated in a Polyurethane Shell
31

Figure 2: Synthesis of polyurethane.
Figure 3: The morphology of clove oil microcapsule in a polyurethane shell.
The parameters that affect the morphology and
size of the microcapsules have been reported by
previous researches. Bouchemal et al. (2004)
reported that the increase in the molecular weight of
polyol tends to increase the mean size of capsules.
Zhenxing et al. (2011) showed that the
microcapsules from emulsion polymerization were
influenced by the concentration of surfactant SLS.
The higher concentrations of the surfactant, the
smaller particle size would be created.
The increase of the SLS concentration means
more surfactants can be adsorbed, and hence the
surface charge density should increase. Therefore, it
will lead to an increase in the particle number
density, along with the decrease of particle size.
3.3 The IR Spectrum of the
Microcapsules
The FTIR spectra of the clove oil encapsulated in
polyurethane shell, clove oil, and polyurethane as
the shell material are presented in Figure 4. As is
shown in Figure 4, all the absorption peaks in the
curve (b) could be found in the curve (a), it means
that the clove oil was successfully encapsulated by
polyurethane.
The peaks at ≈1700 cm
-1
, 2250 cm
-1
, and 3310
cm
-1
correspond to C=O, excess isocyanate C=N=O,
and -NH, respectively, are associated group in
polyurethane. A small amount of polyurethane
existed in clove oil microcapsule, which is showed
by C=O and –NH in both curves (a) and (c).
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
32
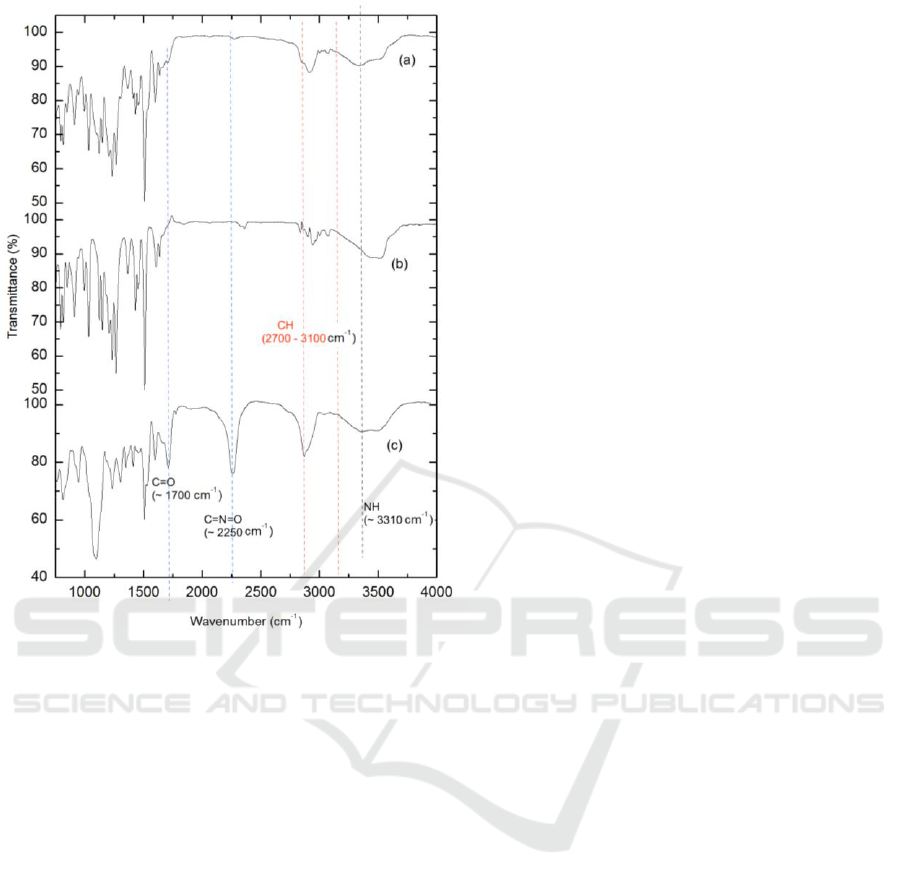
Figure 4: IR spectrum: (a) clove oil encapsulated in
polyurethane shell, (b) clove oil, and (c) polyurethane.
3.4 The Release Profile of
Antimicrobial Agent from the
Microcapsules
According to Attaei (2017), the release of active
ingredients can occur due to diffusion or rupture
(due to thermal or mechanical) or dissolution. In this
study, the release of antimicrobial agent due to
thermal activity in which the microcapsule was
heated 40
o
C prior to the measurement of release
using GC headscape.
GC headspace chromatogram of clove oil encapsulated in
polyurethane shell is presented in
Figure 5. The chromatogram indicated the major
compounds that are eugenol, caryophyllene and
some fatty acid (hexadecanoid acid, octadecenoid
acid and oleic acid). The fatty acid was not detected
through the GC-MS analysis of the clove oil but
occurred in the headspace analysis of the
microcapsule. The reason is might due to the
reaction of fatty acid with polyol resulting fatty acid
in an ester form during the process of
polymerization. Free fatty acids in the essential oil
suspected because of the hydrolysis reaction during
storage (Minhal et al., 2017)
In this case, the occurrence of fatty acid can be
an advantage because fatty acid and fatty acid ester
had been identified their antimicrobial bioactivities
(Arora et al., 2017; Nakayama et al., 2015). Fatty
acids have known modulate immune responses by
acting directly on T cells so they have antibacterial
and antifungal properties (Aparna et al., 2012).
The release of eugenol and caryophyllene in each
day was summarized then were plotted and added
with a trend line using Microsoft Excel®. The
equation and R-square (R
2
) were calculated based on
the trend line. R
2
higher than 0.98 indicates the
equation fits with the data. As presented in Figure 6,
the concentration of caryophyllene were released
based on the equation C = 0.1922x
3
- 2.698x
2
+
11.372x, while the equation of released eugenol is C
= 0.0436x
3
- 0.5741x
2
+ 2.3489x - 0.1681 with x
refers to number of days.
Based on the equation of the release profile, it
can be predicted the percentage of weight ratio for
the next days (after the 10
th
day). The release
concentrations were compared with concentration of
eugenol or caryophyllene in the clove oil that is
81.64% and 17.01% respectively (Table 1). The
prediction is presented in Figure 7. It shows that the
eugenol could release for 59 days but the
caryophyllene only for 15 days.
Release Profile of the Antimicrobial Agent from Clove Oil Encapsulated in a Polyurethane Shell
33
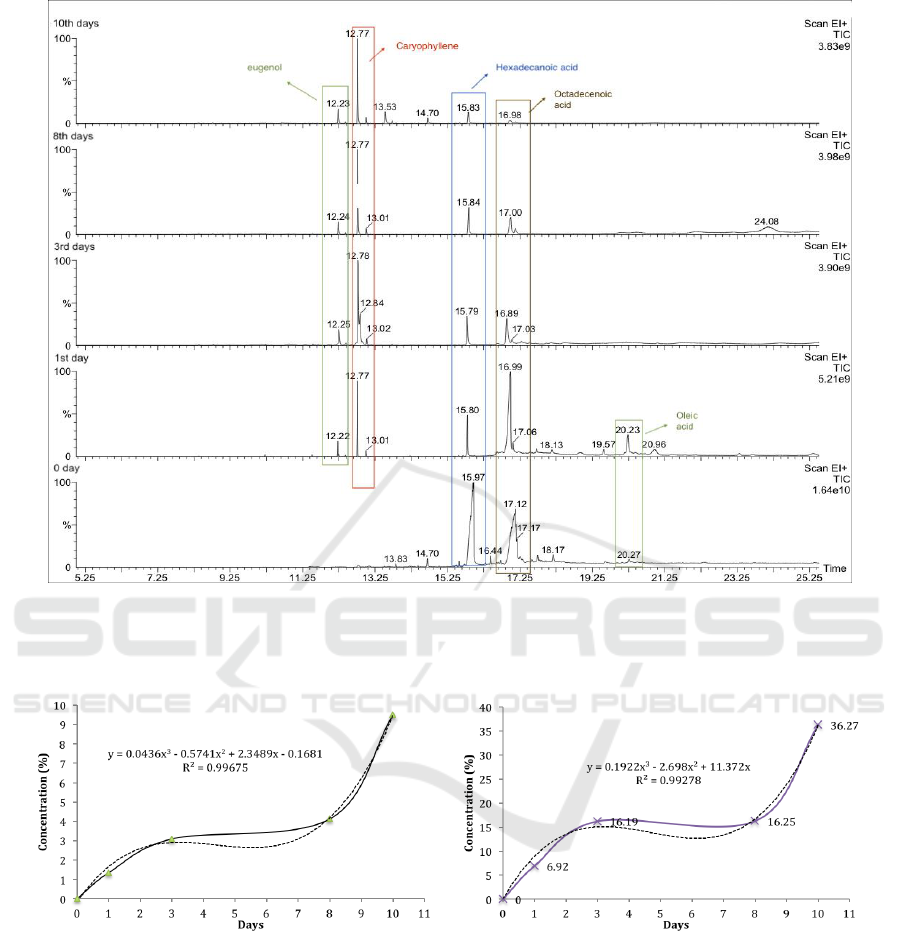
Figure 5: GC headspace chromatogram of clove oil encapsulated in polyurethane shell.
Figure 6: Release profile of clove oil encapsulated in polyurethane shell: (a) eugenol, (b) caryophyllene.
(a)
(b)
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
34
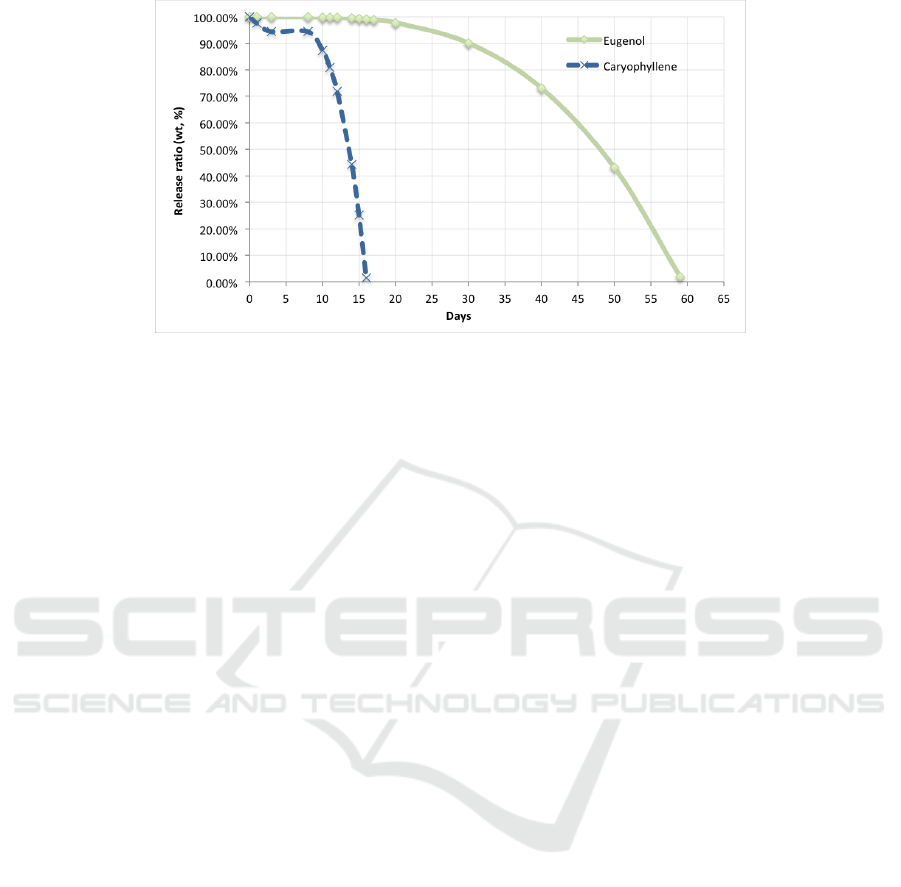
Figure 7: Predicted release of eugenol and caryophyllene.
4 CONCLUSIONS
The concentration of eugenol was released based on
the equation C = 0.0436x
3
- 0.5741x
2
+ 2.3489x -
0.1681, whereas the equation of released
caryophyllene is C = 0.1922x
3
- 2.698x
2
+ 11.372x
with x refers to number of days. From this release
profile, it was found that the clove oil encapsulated
in polyurethane shell could emit eugenol for 59 days
and caryophyllene for 15 days. Therefore, it could
be concluded that the microcapsules of clove oil in
polyurethane shell is suitable for long term
application.
ACKNOWLEDGEMENTS
We would like to thank to PT Covestro Polymers
Indonesia who gave us the sample of MDI as
material for this research.
REFERENCES
Allport, D, C., Gilbert, D, S., Outterside, S., 2003. MDI
and TDI: Safety, Health and the Environment: A
Source Book and Practical Guide. John Wiley &
Sons. West Sussex, England.
Aparna, V., Dileep, K.V., Mandal, P.K., Karthe, P.,
Sadasivan, C., Haridas, M., 2012. Anti-Inflammatory
Property of n-Hexadecanoic Acid: Structural Evidence
and Kinetic Assessment. Chemical Biology & Drug
Design, 80(3), 434-439.
Arora, S., Kumar, G., Meena, S., 2017. Screening and
Evaluation of Bioactive Components of Cenchrus
ciliaris L. by GC-MS Analysis. International Research
Journal of Pharmacy, 8(6), 69-76.
Attaei, M., 2017. Microencapsulation of Isocyanate
Compounds for Autoreactive, Monocomponent
Adhesive. Técnico Lisboa. Portugal.
Bouchemal, K., Briançon, S., Perrier, E., Fessi, H.,
Bonnet, I., Zydowicz, N., 2004. Synthesis and
Characterization of Polyurethane and Poly(Ether
Urethane) Nanocapsules Using A New Technique of
Interfacial Polycondensation Combined to
Spontaneous Emulsification. International Journal of
Pharmaceutics, 269, 89-100.
Carvalho, I.T., Estevinho, B, N., Santos, L., 2016.
Application of Microencapsulated Essential Oils in
Cosmetic and Personal Healthcare Products–A
Review. International journal of cosmetic science,
38(2), 109-119.
Chung, S.K., Seo, J.Y., Lim, J.H., Park, H.H., Yea, M, J.,
Park, H, J., 2013. Microencapsulation of Essential Oil
for Insect Repellent in Food Packaging System.
Journal of Food Science, 78(5), E709-E714.
Cortés-Rojas, D.F., de Souza, C, R, F., Oliveira, W, P.,
2014. Clove (Syzygium aromaticum): A Precious
Spice. Asian Pacific Journal of Tropical Biomedicine,
4(2), 90-96.
Cui, H., Zhao, C., Lin, L., 2015. The Specific
Antibacterial Activity of Liposome-Encapsulated
Clove Oil and Its Application in Tofu. Food Control,
56, 128-134.
Dahham, S, S., Tabana, Y, M., Iqbal, M, A., Ahamed, M,
B, K., Ezzat, M, O., Majid, A, S, A., Majid, A, M, S,
A., 2015. The Anticancer, Antioxidant and
Antimicrobial Properties of the Sesquiterpene β-
Caryophyllene from the Essential Oil of Aquilaria
crassna. Molecules, 20(7), 11808.
Release Profile of the Antimicrobial Agent from Clove Oil Encapsulated in a Polyurethane Shell
35

El-Aasser, M, S., 1990. Emulsion polymerization. In:
Candau, F. & Ottewill, R.H. (eds.). An Introduction to
Polymer Colloids. Springer. Dordrecht. 1
st
edition.
Engels, H-W., Pirkl, H-G., Albers, R., Albach, R, W.,
Krause, J., Hoffmann, A., Casselmann, H., Dormish,
J., 2013. Polyurethanes: Versatile Materials and
Sustainable Problem Solvers for Today’s Challenges.
Angewandte Chemie International Edition,
52(36):9422-9441.
Gezundhait, Y., Pelah, A., 2017. Non-woven fabric
containing microencapsulated essential oils for
preservation of crops. US 20170245493A1.
Han, J, H., 2003. Antimicrobial Food Packaging. In:
Ahvenainen, R. Cambridge: Woodhead Publishing
Ltd.
Hosseini, M, H., Razavi, S, H., Mousavi, M, A., 2009.
Antimicrobial, Physical and Mechanical Properties of
Chitosan-Based Films Incorporated With Thyme,
Clove and Cinnamon Essential Oils. Journal of Food
Processing and Preservation, 33(6):727-743.
Kfoury, M., Hadaruga, N, G., Hadaruga, D, I.,
Fourmentin, S., 2016. 4 - Cyclodextrins as
Encapsulation Material for Flavors and Aroma.
London. Academic Press.
Kim, J, R., Sharma, S., 2011. Acaricidal Activities of
Clove Bud Oil and Red Thyme Oil using
Microencapsulation Against HDMs. Journal of
Microencapsulation, 28(1):82-91.
Lakkis, J, M., 2016. Encapsulation and Controlled
Release Technologies in Food Systems. Blackwell
Publishing. USA. 1
st
edition.
Leimann, F, V., Gonçalves, O, H., Machado, R, A, F.,
Bolzan, A., 2009. Antimicrobial Activity of
Microencapsulated Lemongrass Essential Oil and the
Effect of Experimental Parameters on Microcapsules
Size and Morphology. Materials Science and
Engineering, 29(2):430-436.
Liu, C., Liang, B., Shi, G., Li, Z., Zheng, X., Huang, Y.,
Lin, L., 2015. Preparation and Characteristics of
Nanocapsules Containing Essential Oil for textile
Application. Flavour and Fragrance Journal,
30(4):295-301.
Madene, A., Jacquot, M., Scher, J., Desobry, S., 2006.
Flavour Encapsulation and Controlled Release – a
Review. International Journal of Food Science &
Technology, 41(1):1-21.
Mamaghani, K, R., Naghib, S, M., 2017. The Effect of
Stirring Rate on Electrodeposition of Nanocrystalline
Nickel Coatings and Their Corrosion Behaviors and
Mechanical Characteristics. Int. J. Electrochem. Sci.,
12:5023-5035.
Marchese, A., Barbieri, R., Coppo, E., Orhan, I.E., Daglia,
M., Nabavi, S.F., Izadi, M., Abdollahi, M., Nabavi, S,
M., Ajami, M., 2017. Antimicrobial Activity of
Eugenol and Essential Oils Containing Eugenol: A
Mechanistic Viewpoint. Critical Reviews in
Microbiology, 43(6):668-689.
Minhal, L., Harahap, L, A., Daulay, S, B., 2017. Uji Suhu
Uap Pada Alat Penyuling Minyak Atsiri Cengkeh Tipe
Uap Langsung (Temperature Test on Clove Oil
Distillator Direct Steam Type). Jurnal Rekayasa
Pangan dan Pertanian, 5(2):375-378.
Nakayama, M., Tomiyama, D., Ikeda, K., Katsuki, M.,
Nonaka, A., Miyamoto, T., 2015. Antibacterial Effects
of Monoglycerol Fatty Acid Esters and Sucrose Fatty
Acid Esters on Bacillus spp. Food Science and
Technology Research, 21(3):431-437.
Scarfato, P., Avallone, E., Iannelli, P., De Feo, V.,
Acierno, D., 2007. Synthesis and Characterization of
Polyurea Microcapsules Containing Essential Oils
with Antigerminative Activity. Journal of applied
polymer science, 105(6):3568-3577.
Yow, H.N., Routh, A.F., 2006. Formation of Liquid Core–
Polymer Shell Microcapsules. Soft Matter, 2(11):940-
949.
Zhenxing, H., Xiaowei, Y., Junliang, L., Yuping, Y., Ling,
W., Yanwei, Z., 2011. An Investigation of the Effect
of Sodium Dodecyl Sulfate on Quasi-Emulsifier-Free
Emulsion Polymerization for highly Monodisperse
Polystyrene Nanospheres. European Polymer Journal,
47(1):24-30.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
36
