
Optimizing the Pyrolysis Process and Modelling the Calorific Value of
Sawdust Charcoal as Composing Materials of Quality Briquettes
Musabbikhah
1
, Samsul Bakri
2
1
Mechanical Engineering Department, Akademi Teknologi Warga Surakarta
2
Accounting Department, Universitas Widya Mataram Jogjakarta
Keywords:
Optimization, Pyrolysis, calorific value, sawdust charcoal, model, briquettes.
Abstract:
This study aims to optimize the pyrolysis process and build calorific value model of sawdust charcoal as
composing materials of quality briquettes to fulfill the need of renewable fuels. The results showed that the
calorific value of sawdust increased by 2344 Cal/g after pyrolysis. The optimum conditions of calorific value
were achieved at drying temperature parameters of 60°C, pyrolysis temperature of 600°C, holding time of
120 minutes, and particle size of 100 mesh. The linearity test results between the value of R, R Square, and
Adjusted R Square of the calorific value showed there is a strong correlation between drying temperature,
pyrolysis temperature, holding time, and particle size. Based on validation test, the calorific value model
showed that the residual normality distribution (P-value) was > 0.05 which did not form a certain pattern
on the assumption of homoscedasticity, no multicollinearity (T OL > 1; V IF < 10) and the DW value was
between the specified range. The model was declared valid. Based on the feasibility test model, P-value was
(0.000) < α (0.05). This means the model of proper calorific value was reliable to be used to predict the
sawdust charcoal calorific value.
1 INTRODUCTION
Energy demand is increasing every year with increas-
ing population. One of the main energy sources
needed by humans is the energy source from fossils.
The availability of this fossil energy source will grad-
ually run out, so that it becomes a serious problem be-
cause it cannot be renewed. High dependency on fos-
sil resources combined with the need to reduce CO
2
emissions due to the climate change force people to
utilize renewable energy sources, including biomass.
Biomass is a renewable energy source required to
meet the energy needs, and is also used for carbon
neutrality as a means of preventing climate change.
Vargas (Vargas-Moreno et al., 2012) states that ad-
vanced societies have replaced the use of fossil fuels
with biomass. Wisakha (Wisakha, 2015) explains that
biomass is able to produce continuous heat, therefore
it can be used to replace fossil fuel. One of the renew-
able energy from biomass as a constituent material for
briquettes is sawdust (Lela et al., 2016).
Pyrolysis is needed by sawdust used to make bri-
quettes. Pyrolysis is a thermal degradation process of
solids in the absence of oxygen which allows the oc-
currence of several thermochemical conversion path-
ways so that the solid changes into gas, liquid, then
back to its solid form (Blasi, 2008). Furthermore,
Basu (Basu, 2013) explains that the pyrolysis reaction
from biomass is as follows:
CnHmOp
∑
liquid → CxHyOz +
∑
gasCaHbOc +
H
2
O +C (1)
Heat pyrolysis (thermolysis) decomposition is of
organic matter, such as coal heated more than 300
C°without atmospheric air. The selection of biomass
materials to produce carbon is based on the avail-
ability of materials, costs and the ability to be con-
verted into porous carbon powder after carbonization
(Kalyani and Anitha, 2013). In this study, the qual-
ity of sawdust charcoal is in terms of calorific value
which indicates the energy contained in the fuel per
unit mass of fuel (cal/g). This research is in accor-
dance with the development of solid bio-fuel using a
wood pellet model (Giacomo and Taglieri, 2013).
The research conducted (Lela et al., 2016) con-
cerning the physical, mechanical and thermal proper-
ties of sawdust briquettes is used as a reference for
fuel quality. The optimal value obtained for the bri-
quette making process parameters is the compression
strength of 588.6 KN, sawdust mass of 46.66% and
drying temperature of 22 C°. Based on the mathemat-
Musabbikhah, . and Bakri, S.
Optimizing the Pyrolysis Process and Modelling the Calorific Value of Sawdust Charcoal as Composing Materials of Quality Briquettes.
DOI: 10.5220/0009882402630267
In Proceedings of the 2nd International Conference on Applied Science, Engineering and Social Sciences (ICASESS 2019), pages 263-267
ISBN: 978-989-758-452-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
263
ical model, the optimal values generated are calorific
values increased to 17.41 MJ/kg, ash content de-
creased to 6.62% and maximum compressive strength
is of 149.54 N/mm2. Research on sawdust briquettes
was also carried out by (Stolarski et al., 2013) which
showed that the highest calorific value in sawdust bri-
quettes was 18.144 MJ/kg. Moreover, sawdust bri-
quettes have an effect of 0.40% on ash content.
2 MATERIAL AND METHOD
2.1 Material
The material used in pyrolysis is sawdust powder
from teak wood.
2.2 Method
Data collection of pyrolysis process and calorific
value refers to orthogonal L9(3)4 arrays. The in-
dependent variables used in the study were drying
temperature, pyrolysis temperature, holding time, and
particle size. The dependent variable to determine
the quality of sawdust charcoal is the calorific value.
The method for optimizing pyrolysis parameters is
Taguchi, while for modelling of calorific value is mul-
tiple linear regression model (MLRM) analysis.
3 RESULTS AND DISCUSSION
The effect of level factor differences on the sawdust
charcoal calorific value which has the highest aver-
age calorific value of 6231 cal/g was achieved at dry-
ing temperatures of 60 C°, pyrolysis temperature of
600 C°, holding time of 120 minutes, and particle
size of 100 mesh. The calorific value is influenced
by water content and carbon content. This study is
in line with the research of (Sundaram et al., 2016)
which states the water content of the particles changes
with the variation of drying time in fluidized bed dry-
ing. The same study was also carried out by (Wilk
et al., 2016) who stated that the carbonization of wood
residue into charcoal occurred during the low temper-
ature process.
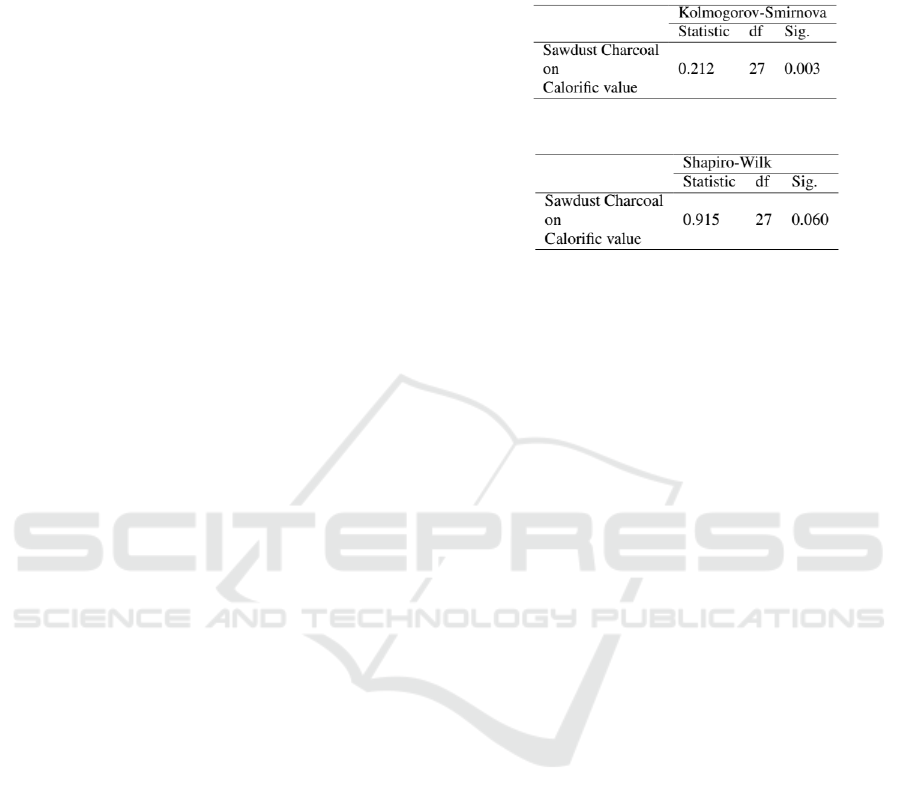
3.1 Normality Test
The normality test of the Sawdust Charcoal calorific
value variable uses a significance level of α = 0.05
and the Shapiro-Wilk statistical test shows that P −
value = 0.915. Therefore, H0 is accepted. It means
that Calorific value of sawdust charcoal variables are
normally distributed and are presented in Figure 1 and
Figure 2.
Figure 1: Normality Test of calorific value.
Figure 2: Normality Test of calorific value (extension).
a Lilliefors Significance Correction
3.2 ANOVA
Based on ANOVA calculations, the four variables
namely drying temperature, pyrolysis temperature,
holding time, and particle size have a significant in-
fluence on the sawdust charcoal calorific value with
the percentage value of the contribution consecutively
is 7.877%; 54.77%; 11.534%; and 25.817%. In this
case, the pyrolysis temperature has the greatest influ-
ence on the sawdust charcoal calorific value because
the higher the pyrolysis temperature is, the lower the
water content and the higher the calorific value. The
results of this study was in line with the research of
(Mandala et al., 2016) which states that pyrolysis tem-
perature is varied from 200 C°to 500 C°and in using
the random wood powder size, there is 29% bio-oil
yield occurs at a temperature of 400 C°.
3.3 Optimization
The optimum conditions of calorific value on sawdust
charcoal obtained were material drying temperature
of 60 C°, pyrolysis temperature of 600 C°, holding
time of 120 minutes, and particle size is of 100 mesh
as shown in Figure 3 and Figure 4. The difference in
mean values between factor effects is shown in Figure
5 and Figure 6. Research on optimization using the
Taguchi method was also conducted by ((Azadi et al.,
2011) and (Roy, 2010).
ICASESS 2019 - International Conference on Applied Science, Engineering and Social Science
264

space
Figure 3: Optimum condition of calorific value.
Figure 4: Optimum condition of calorific value (extension.).
Figure 5: Factor Effects of calorific valu.
Figure 6: Factor Effects of calorific value (extension).
The average sawdust calorific value obtained be-
fore pyrolysis was 3887 cal/g, and after pyrolysis the
average sawdust charcoal calorific value was 6231
cal/g. The effect of drying and pyrolysis are very sig-
nificant in increasing the sawdust calorific value. This
sawdust charcoal has fulfilled the Indonesia National
Standard so that sawdust charcoal is suitable as a high
quality briquette maker. This study is in accordance
with the results of (Wilk et al., 2016) which used py-
rolysis of wood waste material by varying carboniza-
tion temperatures of 230, 260 and 290 C°and car-
bonization times of 0.5, 1.0 and 1.5 hours. The same
study was also carried out by (Al-Refaie et al., 2010)
who explained that the HHV from torrefied samples
increased with increasing temperature. The highest
HHV was found at 26.09 MJ/kg obtained at 60 min-
utes and 300 C°.
3.4 Building a Calorific Value Model
Linearity test is a procedure to find out whether linear
data distribution is or not. The relationship of the re-
sponse variable sawdust charcoal calorific value and
predictor variables is shown in Figure 7 and Figure 8.
Figure 7: Relation between independent variable and
calorific value
Figure 8: Model Summary
b
.
Based on Figure 8, the value of R=0.935 shows a
fairly close degree of linear relationship between the
response variable of the calorific value and the pre-
dictor variable. The R Square=0.875 and Adjusted R
Square=0.852 showed that 85.2% of the variance in
the calorific value variable can be explained by the
independent variable. Meanwhile, St. Error=1.682
states the magnitude of the variance of the regression
model. Thus, there is a linear relationship between the
variables sawdust charcoal calorific value with drying
temperature, pyrolysis temperature, and holding time
and particle size. This modelling research is in accor-
dance with the results of (Sundaram et al., 2016) that
describe the process of drying materials using tem-
perature variations of 55, 60 and 65 C°; speeds of 2.2,
2.4 and 2.6 m/s and moisture content of 27.5, 30 and
32.5% of the total weight.
Optimizing the Pyrolysis Process and Modelling the Calorific Value of Sawdust Charcoal as Composing Materials of Quality Briquettes
265

3.5 Overall Test (Model Feasibility)
The feasibility test of multiple regression models at
the level of significance: α0.05 using the F test is
shown in Figure 9 and Figure 10. Based on ANOVA
in Figure 9, P-value (0.000) < α (0.05). H0 is re-
jected, meaning the sawdust charcoal calorific value
model is suitable for use.
Figure 9: ANOVA
a
.
Figure 10: ANOVA
a
(Extension).
a Dependent Variable: calorific value (cal/g)
b Predictors: (constant), drying temperature (°C),
pyrolysis temperature (°C), holding time (min-
utes) and particle size (mesh)
3.6 Coefficient Feasibility Test
The coefficient feasibility test is used to determine the
level of feasibility of the independent variable coeffi-
cients in the formation of a model of calorific value.
The output of the coefficient feasibility test of the
sawdust charcoal calorific value is presented in Fig-
ure 11 and Figure 12.
Figure 11: Coefficient feasibility test
a
.
space
Figure 12: Coefficient feasibility test
a
(extension).
a Dependent Variable: calorific value (cal/g)
Based on Figure 11 and 12, all the independent
variables in the model significantly affect the sawdust
charcoal calorific value variable. The mathematical
model to predict sawdust charcoal calorific value as
a function of drying temperature, pyrolysis tempera-
ture, holding time and particle size are:
ˆ
Y (cal/g) = 4708.58–33.23X
1
+ 42.71X
2
+
5.70X
3
+ 12.32X
4
(2)
Based on the above equation (2), the higher of
pyrolysis temperature, holding time and particle size
are significant to the higher the calorific value. This
happens because the higher the pyrolysis tempera-
ture, the higher the water content lost, the material
becomes dry so that the water content becomes low
and the carbon is bound as high which results in a
high calorific value. The results of this study is in
accordance with (Lela et al., 2016) which states that
the mathematical model and optimal value produces a
calorific value increased to 17.41 MJ/kg, ash content
decreased 6.62% and maximum compressive strength
of 149.54 N/mm
2
. The same study was alsocarried
out by (Al-Refaie et al., 2010) which explained that
the optical mal parameter design with regression tech-
nique and grey relational analysis. Research on op-
timization and regression modelling were also con-
ducted by (Vishwakarma et al., 2012).
3.7 Model Validation
Residual analysis is a way to validate the sawdust-
charcoal calorific value model. The results of resid-
ual analysis summary to determine the validity of
the response model to the sawdust charcoal calorific
value is presented in Figure 13. The model validation
test results show that the residual normality distribu-
tion (P-value) is 0.563> 0.05, no particular pattern is
formed on the assumptions of homoscedasticity, no
multicollinearity (T OL > 1;V IF < 10) and the DW
value is in the range of 0.878<1.456 <1.514.
ICASESS 2019 - International Conference on Applied Science, Engineering and Social Science
266

space
Figure 13: Residual normality test of calorific value.
Figure 14: Residual normality test of calorific value (exten-
sion).
Based on Figure 13 and Figure 14, it can be con-
cluded that the sawdust charcoal calorific value model
has met the eligibility requirements, and model vali-
dation, so that the resulting model is declared feasible
and valid and can be used to predict the sawdust char-
coal calorific value. The drying and pyrolysis treat-
ment has a positive effect on increasing the calorific
value. It is proved because the initial sawdust calorific
value of 3887 cal/g increased to 6231 cal/g after dry-
ing and pyrolysis. Therefore, there was an increase in
the calorific value of 2344 cal/g.
4 CONCLUSIONS
The optimum condition of the pyrolysis process
which can increase the calorific value of sawdust is
the drying temperature of 60 C°, the pyrolysis temper-
ature of 600 C°, holding time of 120 minutes, and par-
ticle size of 100 mesh. The linearity test results show
that the value of R; R Square; and Adjusted R Square
at the calorific value to have a strong correlation with
drying temperature, pyrolysis temperature, holding
time, and particle size. The result calorific value
model is
ˆ
Y(cal/g) = 4708.58–33.23X1 + 42.71X2 +
5.70X3 + 12.32X4. The validation test results of the
heat value model show that the residual normality dis-
tribution (P-value)> 0.05 does not form a certain pat-
tern on the assumption of homoscedasticity, no mul-
ticollinearity (TOL> 1; VIF <10) and the DW value
is within the specified range so the model is declared
valid. The model feasibility test results in a P-value
(0.000) < α (0.05) so that the calorific value model
is declared feasible and can be used to predict the
calorific value of sawdust charcoal to produce a qual-
ity briquette.
ACKNOWLEDGEMENTS
This research was funded by the Directorate Gen-
eral of Research and Development, Ministry of Re-
search, Technology and Higher Education for the pe-
riod 2017-2019.
REFERENCES
Al-Refaie, A., Al-Durgham, L., and Bata, N. (2010). Op-
timal parameter design by regression technique and
grey relational analysis. volume 3. Proceedings of the
World Congress on Engineering.
Azadi, M. M., Kolahan, F., and Golmezerji, R. (2011).
Multi objective optimization of turning process using
grey relational analysis and simulated annealing algo-
rithm. pages 2926–2932.
Basu, P. (2013). Biomass Gasification, Pyrolysis and Tor-
refaction: Practical Design and Theory. Elsevier Inc,
2nd edition.
Blasi, C. D. (2008). Modeling chemical and physical pro-
cesses of wood and biomass pyrolysis. 34:47–99.
Giacomo, G. D. and Taglieri, L. (2013). Modeling chemical
and physical processes of wood and biomass pyroly-
sis. 2(3):255–260.
Kalyani, P. and Anitha, A. (2013). Biomass carbon
& its prospects in electrochemical energy systems.
38:4034–4045.
Lela, B., Bari
ˇ
sic, M., and Nizˇetic, S. (2016). Card-
board/sawdust briquettes as biomass fuel: Physical–
mechanical and thermal characteristics. 47:236–245.
Mandala, W. W., Cahyono, M. S., Sukarjo, S., and War-
doyo, H. B. (2016). Influence of temperature to yield
and caloric value of plastic waste pyrolysis oil. 1.
Roy, R. K. (2010). A primer on the taguchi method: Society
of manufacturing engineers. 21.
Stolarski, J. M., Szczukowski, S., Tworkowski, J., Zaniak,
K., Gulczynski, P., and Mleczek, M. (2013). Compar-
ison of quality and production cost of briquettes made
from agricultural and forest origin biomass. 57:20–26.
Sundaram, P., Sudhakar, P., and Yogeshwaran, R. (2016).
Experimental studies and mathematical modeling of
drying wheat in fluidized bed dryer. 9(36):1–8.
Vargas-Moreno, J. M., Callej
´
on-Ferre, A. J., P
´
erez-
Alonsoa, J., and Vel
´
azquez-Mart
´
ı, B. (2012). A review
of the mathematical models for predicting the heating
value of biomass materials. 16:3065–3083.
Vishwakarma, M., Parashar, V., and Khare, V. (2012).
Regression analysis and optimization of material re-
moval rate on electric discharge machine foren-19 al-
loy steel using tungsten copper electrode. 2(6):785–
792.
Wilk, M., Magdziarz, A., Kalemba, I., and Gara, P. (2016).
Carbonisation of wood residue into charcoal during
low temperature process. pages 507–513.
Wisakha, P. (2015). Sustainability approach for energy pro-
duction using biomass at household and community
levels. a case study in thailand. 5(3):859–872.
Optimizing the Pyrolysis Process and Modelling the Calorific Value of Sawdust Charcoal as Composing Materials of Quality Briquettes
267
