
Effect of Addition of Zeolite and Sodium Chloride on Changes in
Bacterial Content and Turbidity in Industrial Wastewater Treatment
into Drinking Water using Electrocoagulation Process
Sutanto
1
and Nanang Rohadi
2
1
Department of Electrical Engineering, State Polytechnic of Jakarta, Depok, Jakarta
2
Department of Electrical Engineering, Faculty of Mathematics and Natural Sciences, Padjajaran
University,Jatinangor, Indonesia
Keywords: zeolite, bacterial, turbidity, electrocoagulation, wastewater
Abstract: The treatment wastewater of electrical industry was investigated through electrocoagulation process. The
study was conducted by flowing of 4,5 liters of wastewater into the three cells of electrocoagulation process
tank. Each cell is filled 1.5 liters of waste water. The electrocoagulation process is carried out at a voltage of
12 V and interval time for observation of bacterial content and turbidity in the water is done every 10
minutes. Subsequently, the same procedure was performed with added 100 g of the zeolite and at the end of
study was added 100 g of zeolite + 0.5 g of NaCl. To determine of bacterial content and turbidity was done
using Pour Plate Methode and turbidity meter, respectively. The electrocoagulation process for 120 minutes
can be reduced the bacterial content from 5125 CFU/mL to 2769 CFU/mL or equal of 45,97 % and
turbidity from 44,10 NTU to 18,24 NTU or equal 58,64 %. The electrocoagulation process for 120 minutes
with added of 100 g of zeolite can be reduced the bacterial content from 5125 CFU/mL to 2629 CFU/mL
or equal of 48,70 % and turbidity from 44,10 NTU to 16,34 NTU or equal 62,95%. The electrocoagulation
process for 120 minutes with added of 100 g of zeolite + 0.5 g NaCl can be reduced the bacterial content
from 5125 CFU/mL to 1429 CFU/mL or equal of 72,12 % and turbidity from 44,10 NTU to 1,34 NTU or
equal 96,96%.
In conclusion the electrocoagulation process with added 100 g zeolite and 0.5 g NaCl is
the best condition
compared to the other processes.
1 INTRODUCTION
Industrial or domestic wastewater generally contains
organic pollutants and heavy metals and has the
potential to be reused into clean or drinking water
through many methode processes. The water
parameters in water drinking that made from
wastewater must be accordanced with the
regulation of Indonesian Ministry of Health No. 492
/ Menkes / Per / IV / 2010. On the Minister of Health
regulation No. 492 / Menkes / Per / IV / 2010
mentioned that the water parameters in water
drinking are 5 NTU for turbidity, 0 per 100 mL for
Coliform bacteria and 0 per 100 mL for Escherichia
Coli (E Coli) bacteria.
One methode in water treatment from waste
water into drinking water or clean water is
electrocoagulation process. Anode and cathode
made from aluminum or iron plates are needed to
operate of the electrocoagulation process (Kourdali
et al, 2018).
When the electrocoagulation process is operated,
a coagulant compound will be generated in the
wastewater. Coagulant compounds are used as an
adsorbant material to absorb organic and inorganic
pollutants in wastewater (
Díaz et al, 2018)
In generally, pollutants in wastewater are formed
from organic and inorganic materials. As a result of
mixed organic and inorganic pollutants can reduce
the electrical conductivity in the water. If the
electrical conductivity process is very low, then the
process of forming coagulant compounds in
wastewater becomes less and less. To increase the
electrical conductivity in wastewater can be added
sodium chloride (NaCl) into wastewater (
Daniel,
2018). Sodium chloride has has the ability to kill
microorganisms ( bacteria) and can produce a strong
electrolyte solution. Zeolite also can be added in
wastewater to increase speed up of reducing
Sutanto, . and Rohadi, N.
Effect of Addition of Zeolite and Sodium Chloride on Changes in Bacterial Content and Turbidity in Industrial Wastewater Treatment into Drinking Water using Electrocoagulation Process.
DOI: 10.5220/0009873700002905
In Proceedings of the 8th Annual Southeast Asian International Seminar (ASAIS 2019), pages 67-73
ISBN: 978-989-758-468-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
67

pollutant in the wastewater. Because zeolite is easy
to adsorb pollutants in wastewater.
The combination of zeolite and NaCl into
wastewater is intended to help reduce the content of
bacteria and pollutants in wastewater (Thanasia,
2019).
Anode and cathode made from aluminum or iron
plates are needed to operate of the
electrocoagulation process (Ghalwa et al, 2018). If in
electrocoagulation process using anode made from
aluminum, then the equation of reaction as follows (
Kobya et al, 2013):
anode (oxidation process ):
2Al 2Al
+3
+ 6e
-
(1)
cathode (reduction process) :
6H
2
O+6e
-
6OH
-
+3H
2
(2)
in overall :
2Al+6H
2
O2Al(OH)
3
+3H
2
(3)
The coagulant Al(OH)
3
in equation 3 is formed
that has a function as an absorbent compound for
pollutants or bacteria in wastewater.
The results of the study (Karicappan et al,
2014) showed that in domestic wastewater treatment
using electrocoagulation process was able to reduce
the total solid content (TS) to 98.45% and Coliform
bacteria to 96.34%.
The results of a study on the electrocoagulation
process in liquid waste showed that process can
reduce lead content (Pb) over of 90% (Eiband et al,
2014).
The equation for calculating the weight of Al
+ 3
metal ions that formed in the continuous
electrocoagulation process as follows (Kurmar et al,
2010):
m =(S)(A)(a
r
)(I)/[(Q)(96.500)(n)] (4)
where m: mass Al + 3 released by the anode (gram),
S: process bath height (cm), A: cross-sectional area
of process bath (cm
2
), a
r
: relative atomic mass, I:
electric current (ampere), Q: discharge wastewater
(cm
3
/sec) and n: change in oxidation number. Based
on equation 4 can be explained that in use of high
electric currents, so more will be generated of Al
+3
ions.
The equipments for the electrocoagulation
process consists of process tank, DC source (direct
current), anode and cathode. Process tank must be
made from insulator materials, anodes and cathode
can be made from aluminum or iron (Shanthi et al,
2011).
If the electrocoagulation process is added
Na
2
SO
4
(Sodium Sulfate) or NaCl (Sodium
Chloride) in wastewater, then can be formed strong
electrolyte. Therefore the solution is easy to conduct
electric current which can increase the coagulant
compound to absorb pollutant in wastewater (Rios
et al, 2014).
The electrocoagulation process with the addition
of NaCl as much as 740 mg/L can be reduced the
chemical oxygen demand (COD) up to 98% and
total suspended solid (TSS) up to 93%
(Thirugnanashambandam et al., 2013). Hypochlorite
(HOCl) compounds as byproducts will be produced
at during occur of process. Hypochlorite is an
oxidizing agent which can kill bacteria in waste
water. The mechanism of hypochlorite formation
can be explained by reaction equation as follows :
NaClNa
+
+Cl
-
(5)
2Cl
-
Cl
2
+2e (6)
Cl
2
+H
2
OHOCl+Cl
-
+H
+
(7)
HOCl OCl
-
+ H
+
(8)
The electrocoagulation process that added NaCl
can be increased the dissolution of Al from the
anode (Eskibalci et al, 2018). In studies conducted
with the addition of 0.5 gr/L of NaCl on stirring
speed of 180 rpm and a residence time of 55 seconds
can be reduced total suspended solid (TTS),
detergents, oils and fats, total phosphate and
turbidity up to 100% (Agustin et al, 2008).
The process of adsorption of metal ion by
zeolites can be explained as follows
(Meng et al,
2017
) :
Na
2
-Z+M
+
MZ+Na
+
(9)
Z-H + M
+
MZ + H
+
(10)
where M
+
is a metal ion that absorbed by zeolite.
The approach equation of Freundlich isotherm in ion
exchange process as follows (Hong et al,2019):
c
e
= k (q
e
)
n
(11)
where
c
e
: M
+
concentration in solution at equilibrium
(meq/L), k: constant,
q
e
: the amount of absorbed M
+
/
zeolite weight at equilibrium (meq/g), n: constant. If
n is between 2 and 10, the adsorption process is
very faster and can be approached in
isoterm
Langmuir equation as follows (
Li et al, 2018):
q
e
=q
0
kc
e
/(1+kc
e
) (12)
where q
e
: the amount of absorbed M
+
/zeolite weight
at equilibrium (meq/g),
q
0
: maximum absorption
ASAIS 2019 - Annual Southeast Asian International Seminar
68
capacity on the surface/weight of zeolite (meq/g),
k :
constant,
c
e
: M
+
concentration in solution at
equilibrium (meq/L)
. Equation 12 changed to as
follows (Munagapati et al, 2017):
1/q
e
=(1/q
0
k)(1/c
e
)+1/q
0
(13)
By making a curve of the relationship between 1/q
e
to 1/c
e
, can be obtained slope 1/q
0
k and intercept
1/q
0
. The constant value and maximum absorption
capacity (q
0
) of zeolite can be estimated easily
(Padilla et al, 2018).
2 METHOD
The research method consists of materials,
equipments and work procedures.
2.1 Materials
The materials needed are the Aluminum HTC 16-35
as an electrode, zeolite, NaCl and industrial
electronics wastewater
2.2 Equipments
The equipments needed are process tanks, DC
sources, ampermeters, flow meters, AAS (Atomic
Absorption Spectrophotometer), Soxhlet, pH meters,
turbidity meters and colony counter
2.3 Procedure
2.3.1 Measuring Wastewater Quality
The parameters of wastewater measured were metal
concentration using AAS, turbidity using
turbidimeter and bacterial using colony counter.
The measurement results shown as follows:
bacterial is 5125 CFU/mL and turbidity
is 44,10 NTU.
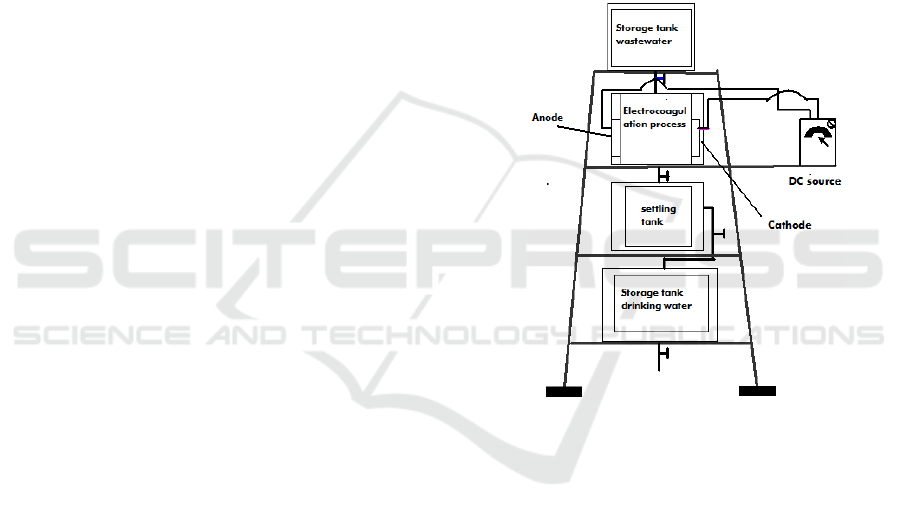
2.3.2 Constructing Research Equipments
The series of research tools can be seen in figure 1.
The equipments needed are a DC source, an
avometer, a wastewater storage tank, an
electrocoagulation process tank, a sewage settling
tank and a water reservoir.
2.3.3 Research Implementation
The resaerch was conducted by flowing 4.5 liters of
wastewater from storage tank into an
electrocoagulation tank which was divided into three
cells. The DC source is turned on at a voltage of 12
V and the electric current can be read on the ampere
meter. The process is turn off after 10 minutes.
Water from the electrocoagulation process tank was
drained into the sewage settling tank. After a few
minutes sediment are formed at the bottom of the
tank. Bacterial content in the water of sewage
settling tank is measured by the Pour Plate Method
and turbidity by a turbidimeter. Re-measurements
were carried out with a processing time of 20, 30,
40, 50, 60, 70, 80, 90, 100, 110 and 120 minutes.
Subsequenty, research was carried out by adding
100 g of zeolite and a mixture of 100 g of zeolite
with 0.5 g of NaCl.
Figure 1: The series of research tools
3 RESULT AND ANALYSIS
The results measurements of changes in bacterial
content and water turbidity showed based on the
elctrocoagulation process (Elc), elctrocoagulation
process with added zeolite (Elc + zeolite) and
elctrocoagulation process with added zeolit and
NaCl (Elc + zeolite+Nacl)
3.1 The Effect of Electrocoagulation
(Elc) Processes on Changes in
Bacterial Content and Water
Turbidity
The results of measurements of bacterial content and
water turbidity from electrocoagulation wastewater
treatment processes are shown in table 1. Based on
Effect of Addition of Zeolite and Sodium Chloride on Changes in Bacterial Content and Turbidity in Industrial Wastewater Treatment into
Drinking Water using Electrocoagulation Process
69
table 1, it can be explained that the
electrocoagulation process can reduce bacterial
content and water turbidity. Bacterial content can be
reduced from 5125 CFU / mL to 2769 CFU / mL or
equivalent to 45.97% at 120 minutes of processing
time. While with the same time the turbidity can be
reduced from 44.10 NTU to 18.24 NTU or
equivalent to 58.64%. The electrocoagulation
process until to 120 minutes did not produce
drinking water standard, because the bacterial
content was more than 0 CFU/mL and the turbidity
of the water was more than 5 NTU.
The coagulant compound of Al (OH)
3
will be
generated when the electrocoagulation process is
carried out using aluminum as anode. The
compounds of AlOH)
3
is an adsorbant material that
can absorb bacteria and pollutants in the water.
Therefore bacterial content and turbidity will be
reduced from the wastewater.
Table 1: Results of measurements of bacterial content and
water turbidity from the electrocoagulation process (Elc)
Time Bacterial content Turbidity
(minute) (CFU/mL) (NTU)
0 5125 44.10
10 5000 44.02
20 496 5 43.20
30 4876 42.52
40 4629 40.14
50 4560 38.02
60 4365 36.50
70 4100 34.32
80 3845 31.23
90 3676 28.52
100 3423 25.14
110 3156 22.32
120 2769 18.24
3.2 The Effect of Electrocoagulation
Process by Addition of Zeolite (Elc
+ Zeolite) on Changes in Bacterial
Content and Water Turbidity
The results of measurements of bacterial content and
turbidity from the treatment wastewater by
electrocoagulation process which added zeolite,
shown in table 2. Based on table 2, it can be
explained that the electrocoagulation process can
reduce bacterial content and turbidity in the water.
Bacterial content can be reduced from 5125
CFU/mL to 2629 CFU/mL or equivalent to 48.70%
with 120 minutes processing time. While with the
same time the turbidity of water can be reduced
from 44.10 NTU to 16.34 NTU or equivalent to
62.95%. The addition of 100 g zeolite in the
electrocoagulation process was able to increase
bacterial removal by 2.73% and increase turbidity
removal by 4.31%. However, this process also
cannot produce drinking water, because the bacterial
content was more than 0 CFU/mL and the turbidity
of the water was more than 5 NTU.
The reducing of bacterial content and turbidity
in the water was accelerated by added of zeolites.
Because zeolite has the property of easily absorbing
bacterial and pollutants in the wastewater
Table 2: Results of measurements of bacterial content and
water turbidity from the electrocoagulation process (Elc)
added zeolite (Elc + zeolite)
Time Bacterial content Turbidity
( menit) (CFU/mL) (NTU)
0 5125 44.10
10 4978 43.82
20 4875 42.40
30 4776 41.52
40 4519 39.74
50 4369 37.42
60 4125 35.90
70 3990 33.22
80 3755 28.83
90 3426 26.52
100 3213 24.34
110 2936 21.52
120 2629 16.34
3.3 The Effect of Electrocoagulation
Process by Addition of Zeolite and
Sodium Chloride (Elc + zeolite +
NaCl) to Changes in Bacterial
Content and Water Turbidity
The results of measurements of bacterial content
and water turbidity from the treatment wastewater
by electrocoagulation process which added zeolite
and NaCl, are shown in table 3. Based on table 3, it
can be explained that the electrocoagulation process
added by zeolite and NaCl can be reduced bacterial
content and turbidity in the water. The bacterial
content can be reduced from 5125 CFU/mL to 1429
CFU/mL or equivalent to 72.12% with 120 minutes
processing time. While with the same time the
turbidity of water can be reduced from 44.10 NTU
to 1.34 NTU or equivalent to 96.96%. The addition
of 100 g zeolite and 0.5 g NaCl in the
electrocoagulation process was able to increase
bacterial removal by 26.15% and increase turbidity
removal by 50.99%. In this treatment the turbidity
ASAIS 2019 - Annual Southeast Asian International Seminar
70
value is below 5 NTU, but the bacterial content is
still more than 0 CFU/mL.Therefore this treatment
also has not been able to produce drinking water
standard.
The reducing of bacterial content and turbidity
in the water was accelerated by added of zeolites and
NaCl. Because zeolite has the property of easily
absorbing bacterial and pollutants in the wastewater
and NaCl easily killing of bacterial.
The addition of NaCl can also increase the
electrical conductivity of wastewater, so that the
current in the electrocoagulation process becomes
even greater. The greater of electric current will be
accelerated the formation of Al (OH)
3
coagulant.
The compounds of Al (OH)
3
easily to absorb
bacterial and pollutants in the water, so that the
bacterial content and water turbidity was decreased
from the water.
Table 3: Results of measurements of bacterial content and
water turbidity from the electrocoagulation process (Elc)
added zeolite (Elc + zeolite +NaCl)
Time Bacterial content Turbidity
(menit) (CFU/mL) NTU
0 5125 44.10
10 4888 41.22
20 4665 38.20
30 4446 36.52
40 4129 32.54
50 3869 27.92
60 3575 25.70
70 3196 21.26
80 2765 18.93
90 2526 15.52
100 2018 11.34
110 1836 6.52
120 1429 1.34
3.4 Comparison Curves of
Wastewater Treatment
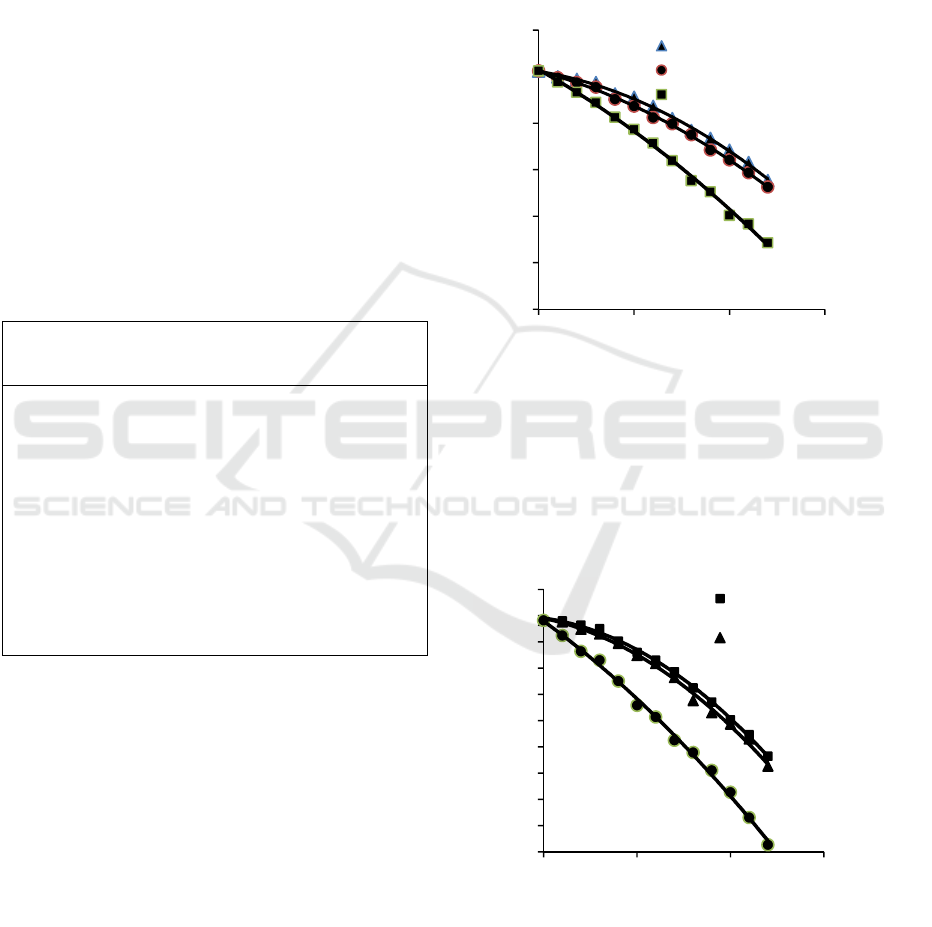
Figure 2 is curve for result of measurement of
bacterial content based on the results of the
electrocoagulation process (Elc), the
electrocoagulation process added zeolite (Elc +
zeolite) and the electrocoagulation process added
zeolite and NaCl (Elc + zeolite + NaCl) based on tables
1, 2 and 3.
Based on figure 2, shows that the
electrocoagulation process (Elc) is able to reduce
bacterial content in water. Zeolite 100 g added to
the electrocoagulation process (Elc + zeolite) is able
to accelerate of reduce bacterial content in water
compared to the electrocoagulation process only. If
the electrocoagulation proses was added zeolite 100
g and NaCl 0.5 g, then the decrease in bacterial
content is faster than the only electrocoagulation
process or the electrocoagulation process which is
added zeolite. In be concluded that the most rapid to
reduce bacterial content in the water was found in
the electrocoagulation process which was added 100
g zeolite and 0.5 g NaCl.
Figure 2: Curve changes of bacterial content in water
Figure 3 is curve for result of measurement of
turbidity based on the results of the
electrocoagulation process (Elc), the
electrocoagulation process added zeolite (Elc +
zeolite) and the electrocoagulation process added
zeolite and NaCl (Elc + zeolite + NaCl) based on tables
1, 2 and 3.
Figure 3: Curve changes of turbidity in water
Based on figure 3, shows that the electrocoagulation
process (Elc) is able to reduce turbidity in water.
Zeolite 100 g added to the electrocoagulation
0
1000
2000
3000
4000
5000
6000
0 50 100 150
Concentration of bacteria, CFU
Time, minute
Elc
Elc+Zeolite
Elc+zeolite+NaCl
0
5
10
15
20
25
30
35
40
45
50
0 50 100 150
Turbidity, NTU
Time, minute
Elc
Elc+Zeolite
Effect of Addition of Zeolite and Sodium Chloride on Changes in Bacterial Content and Turbidity in Industrial Wastewater Treatment into
Drinking Water using Electrocoagulation Process
71

process (Elc + zeolite) is able to accelerate of reduce
turbidity in water compared to the only
electrocoagulation process. If the electrocoagulation
proses was added zeolite 100 g and NaCl 0.5 g, then
the decrease in turbidity is faster than the only
electrocoagulation process or the electrocoagulation
process which is added zeolite. In be concluded that
the most rapid to reduce turbidity in the water was
found in the electrocoagulation process which was
added 100 g zeolite and 0.5 g NaCl
4 CONCLUSION
The addition of zeolites and NaCl to the
electrocoagulation process can be reduced bacterial
content and turbidity in the water.
Electrocoagulation process at 12 V for 120 minutes
by adding zeolite 100 g and NaCl 0.5 can be
reduced bacterial content from 5125 CFU/mL to
1429 CFU / mL or equivalent to 72.12% and
turbidity from 44.10 NTU to 1.34 NTU or
equivalent to 96.96%.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Ministry of
Research and Technology Directorate General of
Higher Education of the Republic of Indonesia for
the financial support awarded to carry out this re-
search for the third year, also Afiliation-Laboratory
FMIPA University of Indonesia UI those have
helped a lot in the data collection required.
REFERENCES
Agustin , M.B., Sengpracha, W.P. and W. Phutdhawong
W., 2008. Electrocoagula tion of Palm Oil Mill
Effluent, Int. J. Environ. Res. Public Health.
Athanasia,A.Z.,Tekerlekopouloub,G.and Vayenasac, D.V.,
2019. a Hybrid System for Groundwater Denitrification
using Electrocoagulation and Adsorption, Journal of
Environmental Management
Daniel, B., Sara, W., Couperthwaiteab, J. and Millarab,
G.J.,2018.
I
nfluence of operating parameters during
electrocoagulation of sodium chloride and sodium
bicarbonate solutions using aluminium electrodes,
Journal of water process engineering.
Díaz, J.C.,
Silva, T.P.,
Segura, E. G.
and
Cruz, A.C.,2017.
Electrocoagulation-Adsorption
to Remove Anionic
and
Cationic
Dyes from Aqueous Solution by
PV-
Energy,
Journal of
C
hemistry
Eiband, M.M.S, Trindade, K.C.D.A, Gama, K., Melo,
J.V.D., Huitle C.A.M, and Ferro ,S., 2014 .Elimination
of Pb
2+
Through Electrocoagulation Applicability
of Adsorptive Stripping Voltammetry for Monitoring
The Lead Concentration During its Elimination,
Journal of Electroanalytical Chemistry.
Eskibalci, M.F. and Ozkan, M.F., 2018. An investigation
of the effect of NaCl concentration on the
electrocoagulation of coal preparation plant tailings,
Physicochem. Probl. Miner. Process.
Ghalwa, N.M.A, Romia, R.S. and Farhat, N.B., 2018.
Application of Electrocoagulation on Adsorption of
Styrene Acrylate Polymer from Aqueous Solutions,
International Journal of Scientific Research in
Environmental Science and Toxcicology
Hong,M.,Yu,L., Wang, Y., Jiahang, J. Zhuwen Chen, Z.,
Dong, L., Zan, Q. and Li, R., 2019. Heavy metal
adsorption with zeolites: The role of hierarchical pore
architecture, Chemical Engineering Journal.
Karichappan, Venkatachalam, S. and P.M. Jeganathan,
P.M.,2014.Optimation of Electrocoagulation Process
to Treat Grey Waste wate in Bach Mode using
Response Surface Methodology, Journal of
Environmental Health Science and Engineering.
Kobya, M., Akyol. A, Demirbas E., and Oncel,M.S.S,
2013. Removal of Arsenic from Drinking Water
by Batch and Continuous Electrocoagulation
Processes Using Hybrid Al-Fe Plate Electrode,
American Institute of Chemical Engineers Environ
Prog.
Kourdali,S., Badis,A., Boucherit,A.and Boudjema,
K., 2018. Electrochemical Disinfection of Bacterial
Contamination: Effectiveness and Modeling Study
of E. Coli inactivation by Electro-Fenton,Electro-
Peroxi-Coagulation and Electrocoagulation, Journal of
Environmental Management.
Kumar,N.S, and Goel, 2010. Factors Influencing Arsenic
and Nitrate Removal from Drinking Water in a
Continuous Flow Electrocoagulation (EC) Process.
Journal of Hazardous Materials.
Li,Z.,Wang,L.,Meng,J.,Liu,X.,Xu, J.,Wang, F.,Brookes,P.,
2018. Zeolite-supported nanoscale zero-valent iron:
New findings on simultaneous adsorption of Cd (II),
Pb (II), and As (III) in aqueous solution and soil,
Journal of Hazardous Materials.
Meng, Q., Chen, H., Lin, J. Zhang Lin, Z. and Sun, J.,
2017. Zeolite A synthesized from alkaline assisted pre-
activated halloysite for efficient heavy metal removal
in polluted river water and industrial wastewater,
Journal of Environmental Sciences.
Munagapati, S.V. ,Yarramuthi, V. and Kim,D.S.,2017.
Methyl orange removal from aqueous solution using
goethite, chitosan beads and goethite impregnated with
chitosan beads, Journal of Molecular Liquids
Padilla, I., Andrés, S.L. and Delgado, A.L., 2018. Al-
Waste-Based Zeolite Adsorbent Used for the Removal
of Ammonium from Aqueous Solutions, International
Journal of Chemical Engineering.
Rios, K.C., Ocampo,G.T. and Palma, R.A.T., 2014.
Experimental Design to Measure Escherichia Coli
ASAIS 2019 - Annual Southeast Asian International Seminar
72

Removal in Water Through Electrocoagulation,
International Journal of Electrocemical Science.
Shanthi, V., Ramanathan, K., and Basha, A., 2011.
Domestic Sewage Treatment Using Batch Stirred Tank
Electrochemical Reactor, International Journal of
ChemTech Research.
Thirugnanashambandam,K.,Sivakumar,V. and Maran,J.P.,
2013. Optimization of Electrocoagulation to Treat
Biologically Pretreated Bagasse Effluent, Journal of
the Serbian Chemical Society.
Effect of Addition of Zeolite and Sodium Chloride on Changes in Bacterial Content and Turbidity in Industrial Wastewater Treatment into
Drinking Water using Electrocoagulation Process
73
