
The Differences of Electrophoretic Profile and Snake Venom
Phospholipase A2 (svPLA
2
) Activity from the Venom of Javan
Spitting Cobra, Naja sputatrix, based on Body Scales Color and
Storage Condition
Nia Kurniawan
1
*, Dea Jolie Chrestella
1
, Dinda Sherlyndra Hapsari
1
, Fatchiyah
1
1
Biology Department, Faculty of Mathematics and Natural Sciences, University of Brawijaya Jalan Veteran Malang, East
Java, Indonesia
Keywords: Naja sputatrix, acidimetric assay, dorsal color, snake venom phospholipase A
2
, venom storage.
Abstract: Naja sputatrix (Javan spitting cobra) is one of medically important snake species in Indonesia which have
various dorsal scales color. This research purposes to examine the differences of venom general profile and
its phospholipase A
2
(svPLA
2
) activity from some N. sputatrix with different dorsal scales colors, and to
examine the activity of N. sputatrix svPLA
2
in different storage conditions. A total of 6 N. sputatrix from
East Java with various dorsal scales color were milked. Venom storage was performed at -80, 4 and 37°C in
a maximum period of 14 days. Venom profile and phospholipase A
2
activity were examined through 15%
SDS-PAGE and acidimetric method using egg yolk substrate respectively. Statistical analyses were
performed to evaluate svPLA
2
activity in every dorsal color and storage condition. Few protein bands range
in 16 – 22 kDa are only found in the venom of the certain dorsal color snake. Protein bands at 37°C were
found to have the lightest intensity among other groups. The svPLA
2
activity of brown dorsal N. sputatrix is
found as the highest activity. An interaction between the storage temperature factor and period factor has
effects on post-storage svPLA
2
activity. Storage in 37°C effects on svPLA
2
activity declining compared to
the control group and other experimental groups.
1 INTRODUCTION
Elapidae, Viperidae, and Colubridae snakes can
produce venom as their secreted product which is
useful in foraging activity and survival mechanism
(Vitt & Caldwell, 2009; Warrel, 2010). Venomous
snakes are present around the world. Thus, the
conflict between human and venomous snake
becomes a global health problem. The total of the
conflicts around the world, in 2008, reaches the
number of 421.000 – 1.841.000 envenomation cases
per year (Kasturiratne et al., 2008). Snakebite
problem does not get enough attention and is
included in Neglected Tropical Disease (NTD) since
2009 (Gutierrez et al., 2013; Williams et al., 2019).
Tropical and subtropical area, including Indonesia,
are susceptible to snakebite problem (Hijaz et al.,
2018; Megawati, 2014; Safitrih et al., 2016; Pratama
& Oktafany, 2017).
Naja sputatrix is only one of various venomous
snakes in Indonesia that is considered medically
important. N. sputatrix is classified to Catagory I
venomous snake because of its high venom and its
habitat preference that is near to human (Warrel,
2010). N. sputatrix in Indonesia can naturally be
found in Java, one of the most populated islands in
Indonesia, also in Lombok, Sumbawa, Padar, Rinca,
Komodo, Flores, Adonara, Lomblen and Alor
Islands. This snake is a terrestrial organism that
often found in rice fields and swamps near
residential areas (Iskandar, et al., 2012). N. sputatrix
has a total body length of 1,5 meters with a wide
head and an elongated hood. The dorsal scales color
of this species is varied. N. sputatrix in West Java
have blackish gray dorsal scales color, while those
from East Java and Islands of Southeast Nusa (Nusa
Tenggara) have silver to brown color (Das, 2010).
The scales color of this snake is possibly a result of
an adaptation process. The snakes with darker scales
live in rain forest with high relative humidity, while
the snakes with lighter scales color live in dry soil
habitat (Kurniawan, et al., 2017).
Kurniawan, N., Chrestella, D., Hapsari, D. and , F.
The Differences of Electrophoretic Profile and Snake Venom Phospholipase A2 (svPLA2) Activity from the Venom of Javan Spitting Cobra, Naja sputatrix, based on Body Scales Color and
Storage Condition.
DOI: 10.5220/0009587600190028
In Proceedings of the 6th International Conference on Advanced Molecular Bioscience and Biomedical Engineering (ICAMBBE 2019) - Bio-Prospecting Natural Biological Compounds for
Seeds Vaccine and Drug Discovery, pages 19-28
ISBN: 978-989-758-483-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
19

The venom of N. sputatrix is a dangerous
mixture solution for humans containing many
protein and non-protein components. It generally
contains major and minor components: three-finger
toxins, cytotoxin, short-chain-α-neurotoxin, long-
chain-α-neurotoxin, muscarinic toxin-like protein,
snake venom metalloproteinase, snake venom serine
protease, phospholipase A
2
, Kunitz-type serine
protease inhibitor, cobra venom factor,
phosphodiesterase, nucleotidase, L-amino acid
oxidase, nerve growth factor, acetylcholinesterase,
and many more (Tan et al., 2017). Phospholipase A
2
(PLA
2
), a major component of the venom, is an
enzyme that hydrolyzes glycerophospholipid
(Sunagar et al., 2015). This enzyme has been
extensively explored because of the stable structure
(Vija et al., 2009; Kang et al., 2011). Many
physiological and pathological effects are caused by
svPLA
2
: presynaptic neurotoxin, edema, necrosis
also hemolysis (Sunagar et al., 2015; Doley & Kini,
2009; Asad et al., 2014). Some PLA
2
antidote
potentials are also estimated useful to handle or
alleviate snakebite effects (Xiao et al., 2017).
The abundance of each component in venom is
varied in every single snake, even in the same
species with differences in the locality. The
composition of the venom can be affected by
geographic condition, habitat, season variation, diet
preference, the age of the snake, also sexual
dimorphism (Tan et al., 2015; Sarhan et al., 2017).
Other than the factors described in these studies, the
profile of venom from different dorsal scale colors
was not researched well yet. This makes the factor
of dorsal scales color is needed to be considered. It
would be very important in research to use fresh-
milked snake venom in wildlife to get a holistic
description of snake venom. Considering the
importance, the storage of the venom before it
arrives in the research center is very important to
note. The previous study put forward the results that
svPLA
2
and the venom of Crotalus molossus
molossus were generally stable in various
temperature storage for 7 days. The study however
told that the results might be generalized for other
snakes, but further researches are still required to
conduct (Munekiyo & Mackessy, 1998). This
research purposes to examine whether the
differences of venom profile and enzymatic activity,
which is represented by svPLA
2
as a major
component of the venom, of some N. sputatrix with
different dorsal scales colors are present or not.
Besides, this research also purposes to evaluate the
activity of N. sputatrix svPLA
2
in different storage
conditions. The activity of svPLA
2
was evaluated
after the crude venom was stored at various
temperatures for 14 days.
2 MATERIALS AND METHODS
2.1 Sample Preparation
The examination of Naja sputatrix venom profile
and svPLA
2
activity were carried out to examine the
difference or similarity among different dorsal scales
color snakes, also different storage condition. The
samples used in this research comprises in total of 6
East Javan N. sputatrix individuals. Black dorsal
scales color snakes were collected from Malang, the
brown dorsal scales snakes from Jombang, and
yellow dorsal scales snakes from Bangil. The snakes
used in this research have a total length of 1,2 – 1,5
meters. Venom milking was conducted after the
snakes 3 days fasting. Venom milking was done in a
beaker glass which covered with parafilm. N.
sputatrix venom from each dorsal color scales was
pooled separately. These samples were used to
examine whether dorsal scales color would affect the
venom profile and svPLA
2
activity or not.
Meanwhile, snake venom solution from black dorsal
scales snakes was pooled together and aliquoted into
some different storage condition groups to evaluate
the effect of storage temperature in 7, 9 and 14 days.
All fresh-milked venom were centrifuged at 4000
rpm for 5 minutes at 4°C. The supernatant was
stored for further analysis. Storage temperature used
to evaluate the effect of dorsal scales color was -
80°C, while 37, 4 and -80 °C were used to store
snake venom sample that would be evaluated under
different storage condition. Three times replication
was used at any data collection.
2.2 Protein Concentration
The whole crude protein and the svPLA
2
examinations were done in Molecular Biology
Laboratory of Life Science Central Laboratory and
Institut Biosains, University of Brawijaya,
Indonesia. The protein concentration of the venom
solution was measured by spectrophotometry
principle, based on the absorbance value of the
sample in wavelength 280 nm by using the
NanoDrop instrument. The protein concentration
data were used to equalize sample for further assays,
both visualization by SDS-PAGE or svPLA
2
activity
assay.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
20
2.3 Snake Venom Electrophoresis
(SDS-PAGE)
Naja sputatrix crude venom solutions were subjected
to Sodium Dodecyl Sulfate Polyacrylamide Gel
Electrophoresis. The preparation of the venom
solution comprises the addition of reducing buffer
followed by incubation at 100°C for 15 minutes. 10-
20 µl venom solution containing 23 µg protein was
loaded to each gel well. The standard marker used
was from Jena Bioscience BlueEye Prestained
Marker 10-245 kDa. Electrophoresis was conducted
at separating gel 15% and stacking gel 3% with a
constant volt of 120 V. CBB R-250 staining process
was used to visualize the protein separation results of
the venom solution.
2.4 Phospholipase A
2
Assay
Phospholipase A
2
assay was conducted based on the
acidimetric method. Phospholipid substrate
suspension was prepared from chicken egg yolk,
CaCl2 18 mM and sodium deoxycholate 8,1 mM
1:1:1 (v/v/v). The material used was mixed well and
adjusted to pH 8,0 by using NaOH 1 M. One
hundred microlitres venom solution containing 50
µg protein was mixed into 15 mL substrate
suspension. The decrease of 1 pH unit between 5 –
65 s was considered equal to the release of 133 µmol
fatty acids (Tan & Tan, 1998).
2.5 Statistical Analysis
The results of the Naja sputatrix svPLA
2
activity
were analyzed statistically. We performed a
Kolmogorov-Smirnov test, Lavene statistic, One
Way ANOVA, and Games-Howell test to evaluate
the svPLA
2
activity in each dorsal scales color
snake. To evaluate whether interactions among
storage factors were happening or not, we performed
univariate analysis and the test of between factors
interactions. Further investigation was done by
Tukey test to compare the results between the
control group and other experimental groups.
3 RESULT AND DISCUSSION
3.1 N. sputatrix Venom Electrophoretic
Profile
The evaluation of N. sputatrix venom electrophoretic
profile based on the dorsal scales color shows that
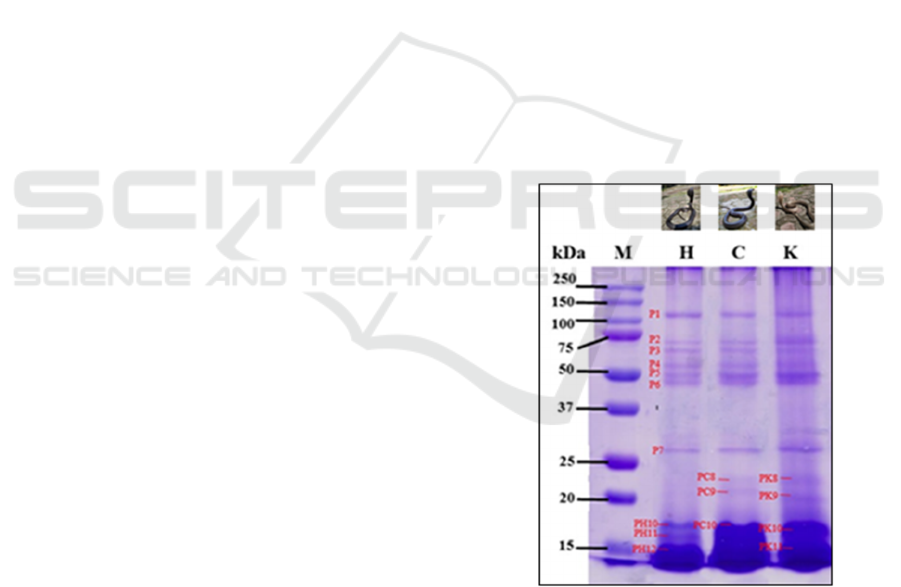
the venom proteins range 16 – 134 kDa. There are
seven protein bands, labeled as P1 until P7. The P1
bands have similar molecular weights and
characteristics among the three kinds of venom. The
same conditions are also found on P2 until P7.
Those bands have molecular weight on 26, 54, 60,
65, 80, 92, 134 kDa (Figure 1, P1 – P7). However,
the venom from yellow dorsal scales N. sputatrix has
a higher intensity in the 2nd, 5th, 6th, and 7th bands.
Other than that, the 8th until 11th bands from all
samples used show various molecular weights and
intensity. Venom from black dorsal scales N.
sputatrix does not show both 8th and 9th protein
bands as the other samples show. Venom from
brown dorsal scales snakes observed to have 20 and
22 kDa proteins (Figure 1, PC8 and PC9). Yellow
dorsal scales snakes observed to have 19 and 21 kDa
proteins (Figure 1, PK8 and PK9). Differences are
also observed in the ≤ 20 kDa protein bands. Three
thick protein bands (16 – 17 kDa) are observed in
the venom of black dorsal scales snakes (Figure 1,
PH10, PH11, and PH12); a thick protein band (16 –
18 kDa) in the venom of brown dorsal scales snakes
(Figure 1, PC10); two protein bands (16 – 17 kDa)
in the venom of yellow dorsal scales snakes (Figure
1, PK10, PK11).
M: marker
H: black dorsal scales
C: brown dorsal scales
K: yellow dorsal scales, P1 – P8: protein bands appearing
in three samples
PH: protein band only appearing in black dorsal snakes
PC: protein band only appearing in brown dorsal snakes
PK: protein band only appearing in yellow dorsal snakes
Figure 1: Molecular weight profile of N. sputatrix protein
venom on SDS-PAGE 15%.
The Differences of Electrophoretic Profile and Snake Venom Phospholipase A2 (svPLA2) Activity from the Venom of Javan Spitting Cobra,
Naja sputatrix, based on Body Scales Color and Storage Condition
21
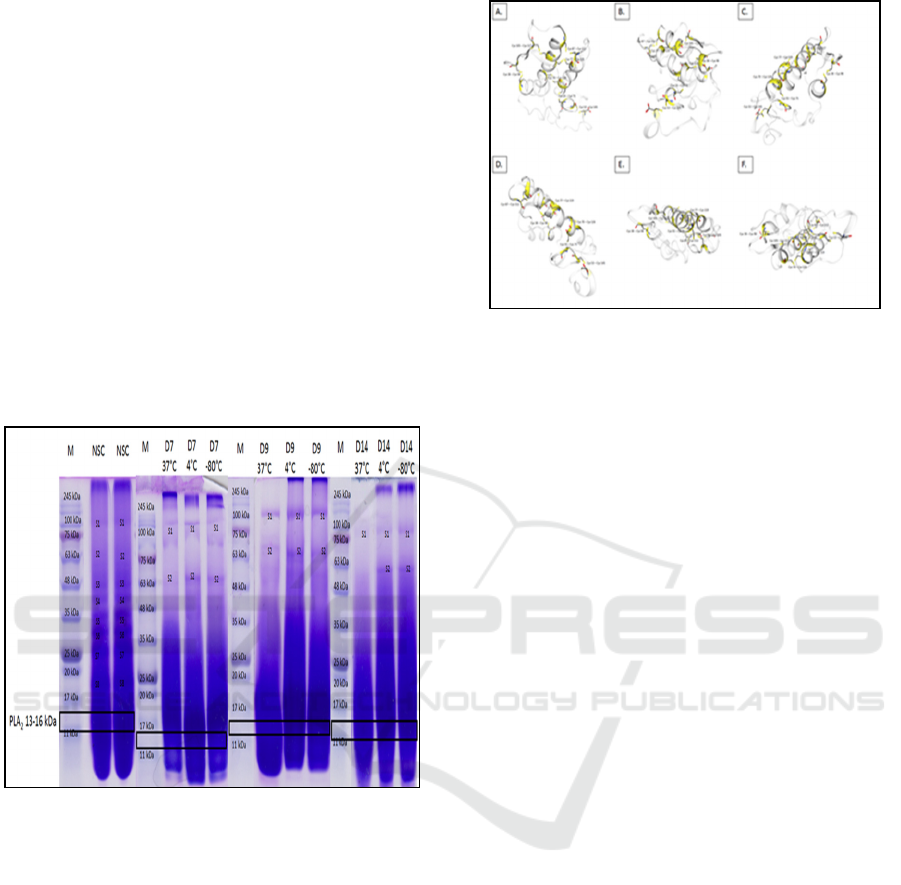
Meanwhile, N. sputatrix venom samples in
which the profile are evaluated under different
storage conditions are mostly composed of proteins
that have a molecular weight of ≤ 35 kDa, which can
be seen as the thick band showed in the crude venom
separation. Separation by SDS-PAGE of N. sputatrix
crude venom does not show a well-separated band
(Figure 2). However, the analysis of control group
results in a better separation of the venom protein.
Few protein bands (19, 27, 30, 34, 39, 44, 64 and
125 kDa) are visualized well in the control group.
Those bands, in contrast, are not visualized well in
the other experimental group, except for the S1 and
S2 bands. Electrophoretic visualization of the stored
N. sputatrix venom solutions shows a lower
intensity, with the lowest intensity is showed in the
experimental groups under 37°C storage
temperature.
NSC: control group
D7: 7 days storage
D9: 9 days storage
D14: 14 days storage.
Figure 2: N. sputatrix venom visualization on 15% SDS-
PAGE in variation of storage condition.
Black outline squares show protein with molecular
weight range on 13 – 16 kDa. N. sputatrix svPLA2
is estimated to be in the range.
Figure 3: Dissulfide bonds in the three-dimentional
structure of N. sputatrix svPLA2 (Q92084).
Dissulfide bonds are represented by yellow parts. A)
Front side, B) Back side, C) Right side, D) Left side,
E) Upper side, F) Under side.
3.2 The Activity of N sputatrix svPLA
2
N. sputatrix svPLA2 from the black dorsal scale
snake performs the lowest activity (144,08
µmoles/minutes/mg) compared to the brown and
yellow dorsal scale color snakes (Figure 4). Statistic
analysis with a confidence level of 95% shows that a
significant difference is present between the svPLA2
activity of the black dorsal scales snakes and the
brown dorsal scales snakes. On the other hand, the
difference of svPLA2 activity in yellow dorsal scales
snakes with both black and brown dorsal color
snakes are not significant (Figure 4). This condition
indicates that the ability to hydrolyze biological cell
phospholipid membrane in brown dorsal snakes
venom is higher than the other venom, and is found
significantly different from the venom of black
dorsal snakes, and not significantly different with
the venom of yellow dorsal snakes. Along with it,
the venom from brown dorsal color might be riskier
to raise various pathophysiological effects in
following the envenomation compared to the two
other snakes.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
22
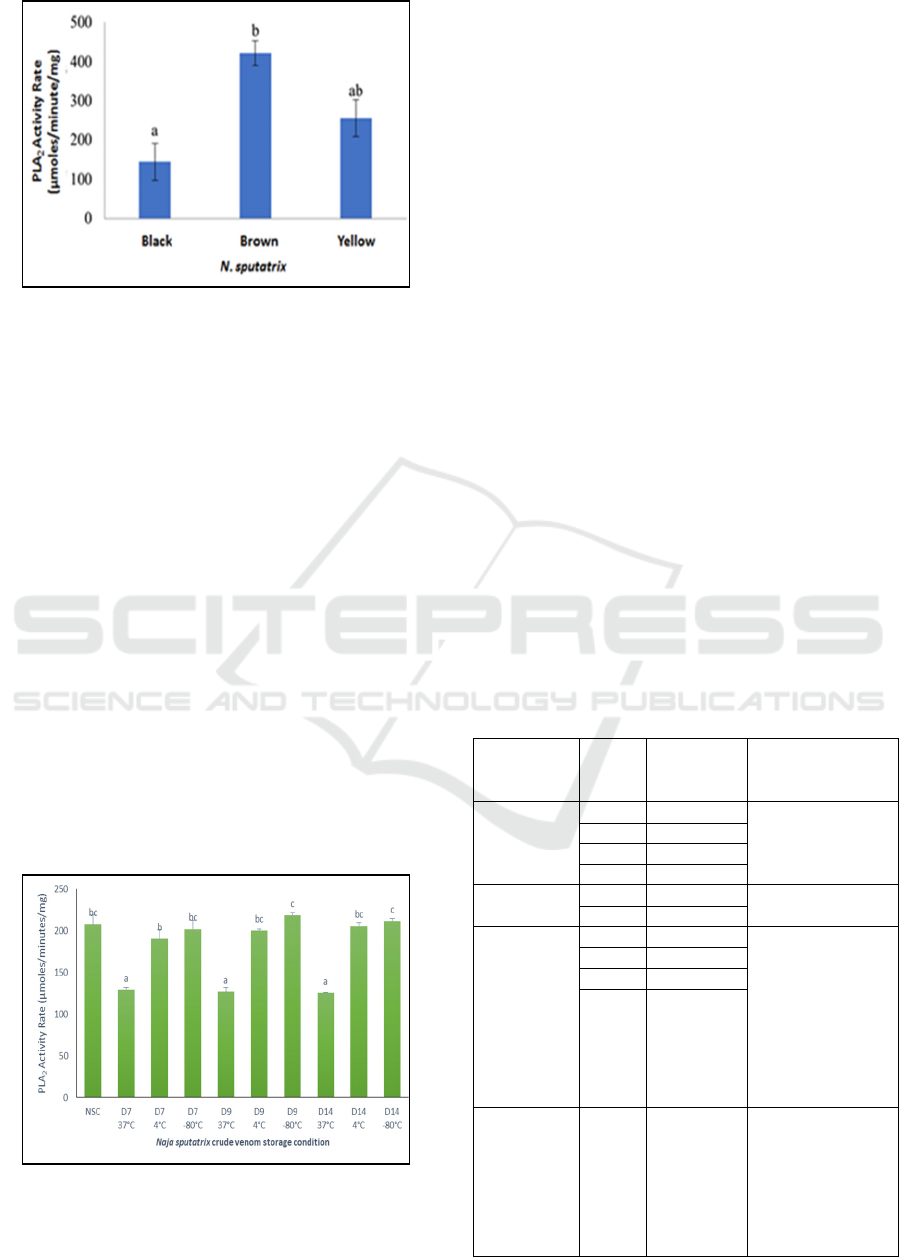
Figure 4: The snake venom phospholipase A2 activity rate
of Javan spitting cobra (N. sputatrix) with different dorsal
scales color. Letter a and b define the statistic notation
among sample groups.
To confirm the stable electrophoretic results of
svPLA2 under different storage conditions, we
performed the svPLA2 activity assay. In this
research, N. sputatrix svPLA2 activity is affected by
the interaction between storage duration and
temperature factors. However, svPLA2 in our
research are estimated stable during the storage at
both 4 and -80°C for two weeks long. There are no
significant differences between svPLA2 activity
from the control group and from the venom
solutions which are kept at 4 and -80°C for 7 - 14.
On the other hand, the activity of svPLA2 that
had been stored at 37°C does not remain stable since
the first 7 days of storage. The N. sputatrix svPLA2
activities in 37°C storage temperature are found
similar in 7, 9 and 14 days storage duration, where
only reach about ¾ of svPLA2 activity in the control
group sample (Figure 5). This may indicate the
damage of svPLA2 native form, which affects its
performance.
Figure 5: Effect of interaction between temperature and
period of time storage on svPLA2 activity rate of N.
sputatrix venom. Letter a, b, c define notation among
sample groups based on statistical data.
All protein bands from the venom of each dorsal
scales color snake (Figure 1) are estimated as four
venom protein families, those are Phospholipase A2
(svPLA
2
), Cysteine-Rich Secretory Protein (CRiSP),
Snake Venom Metalloproteinase (SVMP), and
Nerve Growth Factor (NGF) (Table 1). PLA
2
is
estimated as the protein family from protein bands
16 – 19 kDa. Even though PLA
2
is known as a major
component of venom, the toxic effects caused by
this enzyme are varied. This enzyme also
synergistically works with the other venom
components to support the toxicity potential of cobra
venom (Wong et al., 2017; Tan et al., 2017).
Protein bands 16, 18, 20, 21 and 26 kDa in the
SDS-PAGE results are estimated as CRiSP. This
protein family possesses the inhibition the smooth
muscle contraction through the blockade of cyclic
nucleotide-gated (CNG) and L-type calcium
channels. Protein bands with molecular weight on 22
and 26 kDa can also be estimated as NGF family, a
non-enzyme protein that effects on apoptosis
induction and cytotoxic activity (Tan et al., 2017;
Wong et al., 2017). Few higher molecular weight
venom proteins (80, 65, 60 and 54 kDa) are
estimated as SVMP, which effects on local and
systemic bleeding induction, hemostatic disruption
through the properties of procoagulant or
anticoagulant, inflammation and tissue necrosis
(Sanhajariya et al., 2018).
Table 1: Protein family estimation of the protein bands
appearing in N. sputatrix venom solution 15% SDS-
PAGE.
Protein
family
prediction
Band Molecular
weight
(kDa)
References
SVMP P3 80 Lauridsen et al.,
2017; Shan et al.,
2016; Xu et al.,
2017
P4 65
P5 60
P6 54
NGF P7 26 Xu et al., 2017
PC8 22
CRiSP P7 26 Xu et al., 2017;
Sanhajariya et al.,
2018; Shan et al.,
2016
PK8 21
PK9 20
PH10,
PC10,
PK10,
PH11,
PK11,
PH12,
16 - 18
PLA
2
PK9,
PH10,
PC10,
PK10,
PH11,
PK11,
PH12
16-19 Shan et al. 2016;
Xu et al., 2017
The Differences of Electrophoretic Profile and Snake Venom Phospholipase A2 (svPLA2) Activity from the Venom of Javan Spitting Cobra,
Naja sputatrix, based on Body Scales Color and Storage Condition
23

Venom protein visualization in this research is in
accordance to the previous studies. Liu et al. (2018)
conducted research which found that few protein
families are identified on the whole venom of Naja
atra, those are SVMP, Venom Complement C3,
CRiSP, PLA
2
, NGF, and 3FT. Xiao et al. (2017)
found that the venom of Naja naja, Naja
melanoleuca, Naja nigricollis, and Micrurus fluvicus
consist of acetylcholinesterase, SVMP, a serine
protease, CRiSP, PLA
2
, and 3FT. The protein
families found in previous studies are estimated
appearing in the sample used. Even few differences
are found in the separation profile, the differences
are in the same protein families among the three
kinds of venom used. This indicates that the color of
Javan spitting cobra dorsal scales might have effects
in the abundance or characteristics of venom protein
bands. The unclear results lead to the need for
further research with more supportive methods that a
holistic analysis of cobra venom in the consideration
of dorsal color could be done better.
Under different storage condition, the protein
separation through SDS-PAGE of Crotalus molussus
molussus venom showed few variations. The storage
conditions under 4, -20, and -80ºC, in general, did
not affect the visualized protein band. The similar
results also found in previous researches. The
visualization of venom protein through SDS-PAGE
appears to be not affected by the storage temperature
(-80, -20, 4 and so 20ºC) for 1-7 days long (Egen &
Russell, 1984; Munekiyo & Mackessy, 1998). Other
than cold storage, the proteins of snake venom are
found stable in dried storage even until more than 50
years. Few degradations in the protein may happen
but limited to the functionally unimportant peptides
(Jesupret et al., 2014). In contrast, the venom
solution storage under 37ºC caused some changes in
the protein bands. The decrease in intensity,
absence, and appearance of some bands in the
visualization may indicate an autolytic degradation
of some proteins in the venom solution (Munekiyo
& Mackessy, 1998). This also possibly happens in
the N. sputatrix venom solution stored at 37ºC,
which can be observed from the decrease of
intensity in the separation results.
The differences in svPLA
2
activity of N.
sputatrix from Jombang, as the highest activity
among other groups, are possibly an effect of their
habitat and so prey availability. Variation in snake
venom (Casewell et al., 2014) component is an
adaptation to choose prey. These phenomena exist in
both interspecific and intraspecific levels. The
venom system is an important adaptation that
evolved independently in every animal lineage. The
toxins in snake venom are encoded by a few gene
families, in which each gene family can produce
related isoform that had been produced from gene
duplication during the evolution process. Birth and
death model of toxin-gene evolution is often used as
a mechanism that brings out toxin gene paralogue,
with the evidence that natural selection does
facilitate the encoded protein subfunctionalization or
neofunctionalization. This process produces a toxin
complex that synergistically works to cause death in
prey. Venom evolution in the advanced level enables
the changes in prey capture from mechanic
(constricting) to chemical (venom). It plays an
important role in snakes diversification. The
diversity in snake venom is caused by the new toxin
gene recruitment, or the diversification of existing
toxin genes, that happened before and during the
evolution (Xu et al., 2017). The svPLA
2
enzyme is
coded by the ancestor’s physiological gene that
experiences convergent and divergent evolution
several times. PLA2 in snake venom is a single
chain polypeptide consists of 115 – 125 amino acid
residues with a molecular weight of 13 – 15 kDa and
has a high homolog sequence in many cobra species.
However, the pharmacology of svPLA
2
in
envenomation cases are contributed in various way
even the sequence was generally homolog. Naja
melanoleuca, an African cobra, has a very high
PLA
2
activity that reaches 2120,66
µmoles/minutes/mg. Meanwhile, svPLA
2
activity of
Asian cobras range from 864,04 – 1157,56
µmoles/minutes/mg. Asian cobras used in the
research are Naja sputatrix, Naja naja, Naja
kaouthia, Naja atra, Naja sumatrana. Some African
cobras, for example, Naja katiensis, Naja nigricollis,
Naja pallida, Naja mossambica and Naja nubiae
have svPLA
2
activities that do not show many
differences with those in Asian cobras venom. Some
other African cobras, Naja senegalensis, Naja haje,
Naja annulifera, and Naja nivea have very low
svPLA
2
activity (Tan et al., 2019).
A slight difference in venom molecular weight
profile, also with the significant differences in the
svPLA
2
activity which is found in our research could
indicate that some different protein kinds and/or
abundance might exist in different dorsal scales
color snakes, and are related to the habitat of the
snakes. However, the differences are not studied
further because of data limitations. Further studies
with more supportive methods were needed to
confirm these results.
Under different storage conditions, we evidence
that svPLA
2
activity of N. sputatrix venom is
influenced by the interaction of temperature and
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
24

storage time. The activity of svPLA
2
is observed
decreasing significantly under the temperature of
37ºC. These results are not in accordance to the
previous study by Munekiyo and Mackessy (1998).
Munekiyo and Mackessy did research that results in
the stable activity of some enzymes, including
svPLA
2
that had been stored in various
temperatures: -80, -20, 4 and 37°C for 7 days long.
The svPLA
2
enzyme activity, specifically, are
maintained under the storage of 37°C . PLA
2
enzyme is considered as a stable enzyme in various
temperature conditions even in the presence of
proteolysis enzymes because of its small size and
molecular structure (Vija et al., 2009; Kang et al.,
2011). Our examination on a member of N. sputatrix
svPLA
2
family shows that there are 7 dissulfide
bonds in the structure of N. sputatrix svPLA2.
Dissulfide bonds play an important role in
maintaining this molecule stability through
decreasing the protein entropy in the unfolding
condition (Xiao et al., 2017; Fass, 2012).
With the exception in the research conducted by
Munekiyo and Mackessy (1998), Naja naja venom
PLA
2
shows an optimum temperature at 45 – 55°C
after its incubation at 37°C for 60 minutes
(Shashidharamurthy & Kemparaju, 2006). A similar
condition also found in the venom of Ecis ocellatus
and Crotalis durissus terificus (Sallau et al., 2008;
Toyama et al., 2003). In addition, Bothrops asper
svPLA
2
have an optimum temperature at 52°C after
its incubation in the temperature range 6 – 92°C for
30 minutes (Avila et al., 2004). These indicate that
the stable feature of PLA
2
at various temperature are
phenomena performed by svPLA
2
after its
incubation at a various temperature in a relatively
short time, which are not intended to storage
condition.
The crude venom solutions which we stored at
37°C show a visual difference compared to the other
storage condition groups. The solutions are more
turbid and contained some precipitation. The
presence of precipitation is an indication of protein
changes. Wrong storage could lead to the instability
and formation of precipitation. The precipitation
usually is the non-native form of protein which is
irreversible. The aggregate formation that leads to
protein precipitation could occur in some conditions:
changes of pH, freeze-thawing cycles and high-
temperature exposure (including the temperature of
37°C). The aggregate appearance could decrease the
amount of native-form svPLA
2
protein molecule in
the N. sputatrix venom solution, which can be
observed from the decrease of N. sputatrix svPLA
2
rate activity which had been stored in 37°C. The
svPLA
2
that have already aggregated with another
protein would undergo conformation changes that it
could not function on the substrate (Carpenter et al..
2002; Calamai et al., 2005).
Protease activity might be a factor that leads to
the svPLA2 degradation at the 37°C venom solution.
Protease could degrade endogenous inhibitor and/or
svPLA2, which impacts to the decrease of svPLA2
activity. Munekiyo & Mackessy in their study
detected the degradation of endogenous protein
inhibitor in the venom stored for 7 days at 37°C
(Munekyo & Mackessy, 1998). Endogenous protein
inhibitor has a crucial role in maintaining the whole
venom quality. Venom protein in the whole venom
solution form might undergo proteolysis caused by
protease, that impacts on the venom impotent.
Besides, the protease in the venom solution could
also damage the cells that make up the venom gland.
An endogenous inhibitor is found inside the venom
solution and functions to inhibit the protease,
including its activity to damage the svPLA2 (Francis
et al., 1992; Francis & Ivan, 1993).
Another factor that might influence the svPLA2
activity inside the venom solutions which had been
stored at 37ºC is a growth of microbes. Snpake
venom has been known for its antibacterial potential,
however, there is association between bacterial
infection and venomous snakebite cases. Ten years
of research (2001 – 2010) conducted in North
Taiwan pointed that Morganella morganii and
Enterococcus are the most abundant bacteria
identified in the victim's wound culture (Chen et al.,
2011). The snakebite victims in KwaZulu Natal,
South Africa, have an infection of a few kinds of
bacteria, for example, Morganella morganii,
Enterococcus faecalis, Proteus sp., and Salmonella
enterica. The bacteria were collected from necrosis
tissue samples from the victim (Wagener et al.,
2017). The microbes mentioned above can be found
in the intestinal track of human and other warm-
blooded animals (Lee et al., 2009; Dubin & Eric,
2014; Drzewiecka, 2016; ); which is associated to
the storage temperature we performed.
Microbial analysis at oral swab and venom
samples in recent research pointed that microbial
diversity of venomous snake oral cavity is dependent
on the food type and water resource as result from
faeces that might enter the oral cavity or venom
gland. Bacteria from the oral cavity (including
fangs) and venom solution are found to be appearing
in two different clusters, indicates that the venom
gland might be a different ecological niche. The
bacteria community in both the oral cavity and
venom gland was different. The bacteria in the
The Differences of Electrophoretic Profile and Snake Venom Phospholipase A2 (svPLA2) Activity from the Venom of Javan Spitting Cobra,
Naja sputatrix, based on Body Scales Color and Storage Condition
25

venom solution also show that those are viable even
though the venom are air-dried or lyophilized. The
research also pointed out that two new strains for
Enterococcus faecalis appeared as a result of
adaptation to the venom. (Esmaeilishirazifard et al.,
2018).
Protein cold storage at -80°C until 4°C is an easy
method to store protein solution, specifically for a
stable protein in a short time of period range to 4
weeks. The storage at 4°C usually accompanied by
the addition of stabilizer solutions like sucrose,
glycine or glycerol to reduce the protein
concentration. This is important to decrease the
degradation of protein risk as an effect of the kinetic
process inside the protein solution (Carpenter et al.,
2002). Snake venom solution, however, does not
need a stabilizer solution due to its stable profile in
4°C storage. Venom storage at -80°C (or lower)
could also decrease the degradation risk to one year.
A factor that needs to be considered is a freeze-
thawing cycle that might denaturate protein on the
ice surface during freezing or thawing (Cao et al.,
2003), however, research studies also pointed its
stability during freeze-thawing cycles (Egen &
Russell, 1984; Munekiyo & Mackessy, 1998).
4 CONCLUSIONS
To conclude, we evidence differences in protein
bands' abundance and characteristics. Different
dorsal color N. sputatrix. The differences we found
are estimated as a similar protein family. The
svPLA2 activities of these snake venom solutions
also show a significant difference between black and
brown dorsal color. Yet the yellow dorsal color
snakes do not show a significant difference of
svPLA2 activity with both black or brown dorsal
color N. sputatrix. Considering the variation in
storage condition, svPLA2 visualization of Javan
spitting cobra venom in this research were remain
similar, accompanied by the decrease in band
intensity in the 37°C condition. The svPLA2 activity
of N. sputatrix based on the storage condition is
influenced by the factor of storage temperature and
time with a significant different results in the 37°C
storage temperature condition. We suggest the
storage of venom solution in a relatively short time
until 14 days would be performed at 4 or -80°C.
ACKNOWLEDGEMENTS
The present study was funded by Kemenristekdikti -
the Government of the Republic of Indonesia
through the scheme of PDUPT 2019 to Nia
Kurniawan with contract number
330.13/UN10.C10/PN/2019.
REFERENCES
Asad, M. H. H. B., Burr-E-Sabih, T. Yaqab, G. Murtaza,
M. H. Hussain, M. S. Hussain, M. T. Nasir, S. Azhar,
S. A. Khan & I. Hussain. 2014. Phospholipase A
2
:
enzymatic assay for snake venom (Naja naja
karachiensis) with their neutralization by medical
plants of Pakistan. Acta Poloniae Pharmaceutica-
Drug Research. 71 (4): 625 – 630.
Avila, Ramirez, Quevedo B. E., Lopez E., Renjifo J. M.
2004. Purification and partial characterization of
phospholipase A
2
from Bothrops asper (barba
amarilla) snake venom from Chiriguana (Cesar,
Colombia). J. Venom. Anim. Toxins incl. Trop. Dis 10
No. 3.
Calamai, M., Canale C., Relini, A., Stefani, M., Chiti, F.
& Dobson, C. M. 2005. Reversal of protein
aggregation provides evidence for multiple aggregated
states. J. Mol Biol., 346 (2), 603 – 616.
Cao, Enhong, Yahuei Chen, Zhanfeng Cui, Peter R.
Foster. 2003. Effect of freezing and thawing rates on
denaturation of proteins in aqueous solutions.
Biotechnology and Bioengineering Vol 82 No. 6: 684 –
690.
Carpenter, John F., Mark C. Manning, Theodore W.
Randolph. 2002. Long storage of protein. Current
Protocols in Protein Science 4.6.1 – 4.6.6.
Casewell, N. R., S. C. wagstaff, W. Wuster, D. A. N.
Cook, F. M. S. Bolton, S. I. King, D. Pla, L. Sanz, J. J.
Calvete & R. A. Harrison. 2014. Medically important
differences in scake venom composition are dictated
by distinct postgenomic mechanism. PNAS. 111 (25):
9205 – 9210.
Chen, Chun-Ming, Keh-Gong Wu, Chun-Jen Chen,
Chuang-Ming Wang. 2011. Bacterial infection in
association with snakebite: A 10-year experience in a
northern Taiwan medical center. Journal of
Microbiology, Immunology and Infection 44: 456 –
460.
Das, I. 2010. A field guide to the reptiles of South-East
Asia. New Holland Publishers. London.
Doley, R. & R. M. Kini. 2009. Protein complexes in snake
venom. Cell. Mol. Life Sci. 66: 2851 – 2871.
Drzewiecka, Dominika. 2016. Significants and Roles of
Proteus spp. Bacteria in Natural Environments.
Microb Ecol (2016) 72:741-758.
Dubin, Krista & Eric G. Pamer. 2014. Enterococci and
their interaction with the intestinal microbiome.
Microbiol Spectrum 5(6): BAD-0014-2016.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
26

Egen, N. B & Russell F. E. Effects of preparatory
procedures on the venom from a rattlesnake (Crotalus
molossus molossus) as determined by isoelectric
focusing. Toxicon 1984; 22:653 – 657.
Esmaeilishirazifard, E., L. Usher, C. Trim, H. Dense, V.
Sangal, G. H. Tyson, A. Barlow, K. F. Redway, J. D.
Taylor, M. Kremyda-Vlachou, T. D. Loftus, M. M. G.
Lock, K. Wright, A. Dalby, L. A. S. Synder, W.
Wuster, S. Trim, S. A. Moschos. 2018. Microbial
adaptation to venom is common in snake and spiders.
https://doi.org.10.1101/348433. Accessed on 21 April
2019.
Fass, Deborah. 2012. Disulfide bonding in protein
biophysics. Annu. Rev. Biophys 41: 63 – 79.
Francis, B., Seebart C., Kaisser I. I. 1992. Citrate is an
endogenous inhibitor of snake venom enzymes by
metal-ion chelation. Toxicon 30 (10): 1239 – 1246.
Francis, Brian & Ivan I. Kaiser. 1993. Inhibition of
metalloproteinases in Bothrops asper venom by
endogenous peptides. Toxicon 31 (7): 889 – 899.
Gutierrez, Jose M., David A. Warrell, David J. Williams,
Simon Jensen, Nicholas Brown, Juan J. Calvete,
Robert A. Harrison. 2013. The need for full integration
of snakebite envenoming within a global strategy to
combat the neglected tropical diseases: the way
forward. PloS Negl Trop Dis 7(6) e2162.
Hijaz, P. T., Kumar A. C. R., John B. M. 2018. A study on
clinical and laboratory features of pit viper
environment from Central Kerala, India. Int J Adv
Med. 2018(5):644-51.
Iskandar, D., M. Auliya, R. F. Inger, R. Lilley. 2012. Naja
sputatrix. The IUCN Red List of Threatened Species
2012. http://dx.doi.org/10.2305/IUCN.UK.2012-
1.RLTS.T192197A2054180.en. Accessed 15
September 2018.
Jesupret, C., Kate Baumann, Timothy N. W. Jackson,
Syed Abid Ali, Daryl C.. Yang, Laura Greisman,
Larissa Kern, Jessica Steuten, Mahdokht jouiaei,
Dolyce H. W. Low, Sarah Rossi, Nadya Panagides,
Kelly Winter, Vera Ignjatovic, Wayne C. Hodgson,
Kenneth D. Winkel, Paul Monagle, Bryan Grief Fry.
2014. Vintage venoms: Proteomic and
pharmacological stability of snake venpmd stored for
up tp eight decades. Journal of Proteomics XX
JPROT-01672.
Kang, Tse S., Dessislava Georgieva, Nikolay Genov,
Mario T. Murakami, Mau Sinha, Ramasamy P.
Kumar, Punit Kaur, Sanjit Kumar, Sharmistha Dey,
Sujata Sharma, Alice Vrielink, Christian Betzel,
Soichi Takeda, Raghuvir K. Arni, Tej P. Singh dan R.
Manjunatha Kini. 2011. Enzimatic toxins from snake
venom: structural characterization and mecahnism of
catalysis. FEBS Journal 278:4544-4576.
Kasturiratne, A., A. Rajitha Wickremasinghe, Nilanthi de
Silva, N. Kithsiri Gunawardena, Arunasalam
Pathmeswaran, Ranjan Premaratna, Lorenzo Savioli,
David G. Lalloo, H. Janaka de Silva. 2008. The global
burden of snakebite: A literature analysis and
modelling based on regional estimates of envenoming
and deaths. PloS Med. 5(11):e218.
Kurniawan, N., Mulyadiane M. Putri, Ahmad M. Kadafi,
Dea J. Chrestella, Muhammad A. Fauzi, Agung S.
Kurnianto. 2017. Phylogenetics and biogeography of
cobra (squamata: naja) in Java, Sumatra, and other
Asian region. J. Exp. Lide Sci Vol 7:94-101.
Lauridsen, L. P., A. H. Lausten B. Lomonte & J. M.
Gutierrez. 2017. Explorinng the venom of the forest
cobra snake: Toxicovenomics and antivenom profiling
of Naja melanoleuca. Journal of Proteomics. 150: 98
– 108.
Lee, Chun-Yi, Hisiu-Fen Lee, Fang-Liang Huang & Po-
Yen Chen. 2009. Haemorrhagic bullae associated with
a chicken scratch. Annals of Tropicals Paediatrics 29:
309-311.
Liu, C., C. Lin, Y. Hsiao, P. Wang & J. Yu. 2018.
Proteomic characterization of six taiwanese snake
venoms: identification of species-specific proteins and
development of SISCAPA-MRM assay for cobra
venom factor. Journal of Proteomic. 1 – 10.
Megawati, Pramaswari. 2014. Evaluasi penyebab
keracunan serta analisis biaya. Pascasarjana
Universitas Gadjah Mada. Yogyakarta. Tesis.
Munekiyo, Sean M. & Stephen P. Mackessy. 1998. Effects
of temperature and storage conditions on the
electrophoretic, toxic and enzymatic stability of
venom component. Comp. Biochem. Physiol. Vol
119B:119-127.
Pratama, Gilang Yoghi & Oktafany. 2017. Gigitan ular
pada regio manus sinistra. J Medula Unila 7:33-37.
Safitrih, L., Anjar Mahardian Kusuma, Much. Ilham N.
Aji Wibowo. 2016. Angka kejadian dan
penatalaksanaan keracunan di Instalasi Gawat Darurat
RSUD Prof. Dr Margono Soekardji Purwokerto Tahun
2012-2014. Media Litbangkes 26(3):175-180.
Sallau, A. B., Ibrahim M. A., Salihu A., Patrick F. U.
2008. Characterization of phospholipase A
2
(PLA
2
)
from Echis ocellatus venom. African Journal of
Biochemistry Research Vol 2(4):98-101.
Shan, L., J. Gao, Y. Zhang, S. Shen, Y. He, J. Wang, C.
Ma & X. Ji. 2016. Proteomic characterization and
comparison of venoms from two elapid snakes
(Bungarus multicinctus and Naja atra) from China.
Journal of Proteomics. 138: 83 – 94.
Sanhajariya, S., S. B. Duffull & G. K. Ibister. 2018.
Pharmacokinetics of snake venom. Toxin 10 (73): 1 –
21.
Sarhan, M., A. Mostafa, S. E. Elbehiry, A. M. A. Abd el
Reheem & S. A. Saber. 2017. Intersexual variation in
tail length, venom composition, toxicity, and
anticancer activity of Cerastes cerastes (Viperidae).
The Egyptian Journal of Hospital Medicine. 66: 80 –
90.
Shashidharamurty, R. & Kemparaju K. 2006. A
neurotoxic phospholipase A
2
variant: Isolation and
characterization from eastern regional Indian cobra
(Naja naja) venom. Toxicon 47:727-733.
Sunagar, K., T. N. W. Jackson, T. Reeks, B. G. Fry. 2015.
Group I Phospholipase A
2
Enzymes. dalam B. G. Fry
(Ed.). Venomous Reptiles and Their Toxins:
The Differences of Electrophoretic Profile and Snake Venom Phospholipase A2 (svPLA2) Activity from the Venom of Javan Spitting Cobra,
Naja sputatrix, based on Body Scales Color and Storage Condition
27

Evolution, Pathophysiology and Biodiversity. Oxford
University Press. New York.
Tan, Nget-Hong & Tan Tan. 1988. Acidimetric Assay for
Phospholipase A Using Egg Yolk Suspension as
Substrate. Analytical Biochemistry 170:282-288.
Tan, Kae Yi., Choo Hock Tan, Shin Yee Fung, Nget Hong
Tan. 2015. Venomics, lethality and neutralization of
Naja kouthia (monocled cobra) venoms from three
different geographical regions of Southeast Asia.
Journal of Proteomics 120: 105 – 125.
Tan, C. H., K. Y. Wong, N. H. Tan. T. S. Ng & K. Y. Tan.
2019. Distinctive distribution of secretory
phospholipase A22 in the venoms of Afro-Asian cobra
(Subgenus: Naja, Afronaja, Boulengerina and
Uraeus). Toxin. 11 (116): 1 – 12.
Tan, Nget Hong, Kin Yin Wong, Choo Hock Tan. 2017.
Venomics of Naja sputatrix, the Javan spitting cobra:
A short neurotoxin-driven venom needing improved
antivenom neutralization. Journal of Proteomics 157:
18 – 32.
Toyama, Marcos H., Daniela gracia de Oliveira, Luis O. S.
Beriam, Jose Camillo Novello, Lea Rodrigues-
Simioni, Sergio Marangoni. 2003. Structural,
enzymatic and biological properties of new PLA
2
isoform from Crotalus durissus terificus venom.
Toxicon 41:1033-1038.
Vija, H., Mari Samel, Ene Siigur, Anu Aaspollu, Katrin
Trummal, Kulli Tonismagi, Juhan Subbi, Juri Siigur.
2009. Purification, characterization, and cDNA
cloning of acidic platelet aggregation inhibiting
phospholipase A
2
from the snake venom of Vipera
lebetina (Levantine viper). Toxicon 54:429-439.
Vitt, Laurie J. & Janalee P. Caldwell. 2009. Herpetology
3rd edition. Academic Press. Burlington.
Wagener, M., M. Naidoo, C. Aldous. 2017. Wound
infection secondary to snakebite. The South African
Medical Journal 107 No. 4: 315 – 319.
Warrel, David A. 2010. Guidelines for the management of
snake-bites. World Health Organization. New Delhi.
Williams, David J., Mohd Abdul Faiz, Bernadette Abela-
Ridder, Stuart Ainsworth, Tommasco C. Bulfone,
Andrea D. Nickerson, Abdulrazaq G. Habib, Thomas
Junghanss, Hui Wen Fan, Michael Turner, Robert A.
Harrison, David A. Warrell. 2019. Strategy for a
globally coordinated response to priority neglected
tropical disease: Snakebite envenoming. PloS Negl
Trop Dis 13(2): e0007059.
Wong, K. Y., C. H. Tan, K. Y. Tan, N. H. Quraishi & N.
H. Tan. 2017. Elucidating the biogeographical
variation of the venom of Naja naja (spectacled cobra)
from Pakistan through a venom-decomplexing
proteomic study. Journal of Proteomics. 175: 156 –
173.
Xiao, Hui X., Hong Pan, Keren Liao, Mengxue Yang,
Chunhong Huang. 2017. Snake venom PLA
2
, a
promising target for broad spectrum antivenom drug
development. BioMed Research International 2017 ID
6592820.
Xu, N., H. Zhao, Y. Yin, S. Shen, L. Shan, C. Chen, Y.
Zhang, J. Gao, X. Ji. 2017. Combined venomics,
antivenomics and venom gland transcriptome analysis
of the monocled cobra (Naja kaouthia) from Chine.
Journal of Proteomics 159: 19 – 31.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
28
