
In Silico Prediction of High Potential Jararhagin Inhibitor:
Comparison of Batimastat, EDTA and Hydroxytyrosol
Coni A. Kurniasari
1
, Bayu D. Prakoso
1
, Eka D. P. Lestari
1,*
and Nia Kurniawan
1
*
1
Biology Department, Faculty of Mathematic and Natural Science,
University of Brawijaya, Malang, 65145, Indonesia
Keywords: Batimastat, EDTA, envenomation, hydroxytyrosol, inhibitor, jararhagin
Abstract: One example of a group P-III SVMP is jararhagin which originates from a Bothrops jararaca. This study
was conducted to compare the possibility of inhibitors that have the highest effectiveness and exact time of
each compound to inhibit hemorragic effect of SVMP. Inhibition of hemorrhagic activity can be done with
several types of compounds that have been known to be inhibitors for SVMP especially jararhagin (PDB
ID: 1C9G) to bind with integrin
21 (PDB ID: 1AOX). There are batimastat (PubChem ID: 5362422) as
one of peptidomimetic compounds, EDTA (PubChem ID: 6049) as one of zinc chelating agents, and plant
compounds such as hydroxytyrosol (PubChem ID: 82755). The batimastat inhibitory properties from value
of binding energy, found that these inhibitor were more easily bound to jararhagin (-289.0 kcal/mol)
compared to integrin
21 (-277.1 kcal/mol). That inhibitor also more effectively inhibited by bounding to
jararhagin spread in blood vessels after snakebite because of it’s position and more positive binding energy
(-784.1 kcal/mol). However, unfavorable bonds are formed in the interaction between batimastat inhibitors,
jararhagin and integrin
21. In inhibitor EDTA interaction, it was found that this compound also more
easily bound to jararhagin (-227.23 kcal/mol), but this inhibitor are more effectively inhibited by bounding
to integrin
21 because of it’s position and more positive binding energy (-721.57 kcal/mol). In other side
it also has unfavorable bonds. While the interaction of hydroxytyrosol shows that inhibitor are easier to
interact with jararhagin and more effectively acts as a jararhagin inhibitor by being consumed after the body
is exposed to jararhagin (-781.33 kcal/mol) without showing an unfavorable bond. We can conclude that the
natural inhibitors formed in hydroxytyrosol from olive oil are more stable and have highest possibility in
preventing hemorrhagic symptoms due to snake bites that contain jararhagin venom.
1 INTRODUCTION
Envenomation is one of dangerous health problem
because of the death risk. There are 5,5 millions
envenomation cases annually. Snake venom contains
mixture of various proteins or protein families with
different bioactivities and tissue target (Williams
et.al., 2010). The examples of protein families in
snake venom are snake venom metalloproteinase
(SVMP), snake venom serine proteinase (SVSP),
cysteine-rich secretory protein (CRiSP),
phospholipase A
2, phospholipase type B, C-type
lectin-like protein, L-amino acid oxidase (LAAO), 3
finger toxin (3FTx), et cetera (Kunalan et.al., 2018).
SVMP (Snake Venom Metalloproteinase) is one
of protein family in Elapidae and Viperidae snake
venom. Up to 30% Viperidae venom is consist of
SVMP (Silva et.al., 2016).
SVMP is a zinc-dependent hydrolase which has
catalytic zinc ion in the active site. The catalysis
process of this enzyme needs zinc ion (Zn
2+
) as the
mediator, zinc ion is coordinated with 3 side chain
of histidine and water molecule binded with
glutamate residue (Preciado et.al., 2018). SVMP has
the hemorrhagic effect and fibrynogenolytic. It can
cleave A and B chain on fibrynogen. SVMP can
degrade some of extracellular matrix proteins i.e.
collagen IV, laminine, fibronectin, and proteoglycan
perlecan. SVMP can act as the mediator of local
tissue damage, and induce the endothelial cell
hemorrhage as well. The damage of endothelial cell
and basal membrane on blood vessels will helps the
toxic protein spread to the tissue target
(Pithayanukul et.al., 2009). Jararhagin is a member
of SVMP protein family with high hemorrhagic
effect. It is a 52 kDa PIIIb SVMP, the first
Kurniasari, C., Prakoso, B., Lestari, E. and Kurniawan, N.
In Silico Prediction of High Potential Jararhagin Inhibitor: Comparison of Batimastat, EDTA and Hydroxytyrosol.
DOI: 10.5220/0009586800050014
In Proceedings of the 6th International Conference on Advanced Molecular Bioscience and Biomedical Engineering (ICAMBBE 2019) - Bio-Prospecting Natural Biological Compounds for
Seeds Vaccine and Drug Discovery, pages 5-14
ISBN: 978-989-758-483-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
5

metalloproteinase isolated from B. jararaca
(Ferreira et.al., 2018).
The specific treatment of envenomation case is
conducted by antivenom treatment. According to
WHO (2010), antivenom or antivenin is consisted of
pure immunoglobulin fragment from animal plasma
which have been immunized by snake venom.
Antivenom treatment has several disadvantages.
Antivenom only neutralize any of the venoms used
in its production, or from closely related species. It
needs suitable storage condition because of the
sensitive components in antivenom. It is unsuitable
to neutralize the local tissue damage (Preciado et.al.,
2018). Antivenom has high effectivity in
neutralizing systemic effect of a venom, but it has
low effectivity in neutralizing local effect of snake
venom, i.e. effect of SVMP (Romero et.al., 2012).
Enzyme inhibitor has become a potention to treat
local tissue damage effect of SVMP. Some
compounds which are known as inhibitor of SVMP
are peptidomimetic, zinc chelating agent, and
phenolic compound. Peptidomimetic such as
Batimastat and Marimastat; zinc chelating agents
such as EDTA, DTPA, TTD; and phenolic
compounds are known as SVMP enzyme inhibitor
(Preciado et.al., 2018). But there is no knowledge
about which compound is most effective to inhibit
SVMP hemorrhagic activity. The aim of this study is
to compare Batimastat (peptidomimetic), EDTA
(zinc chelating agent), and hydroxytyrosol (phenolic
compound) as the most effective inhibitor of
jararhagin SVMP.
2 MATERIALS AND METHODS
2.1 Protein and Ligand Structure
Preparation
We use the structure of 2 proteins, the snake venom
metalloproteinase (SVMP) jararhagin (PDB ID:
1C9G) and α
2β1 intregin (PDB ID: 1AOX). Protein
structure was downloaded from RCSB PDB
database (https://www.rcsb.org/). We also use
structure of 3 ligands, Bastimastat (PubChem ID:
5362422), EDTA (PubChem ID: 6049), and
Hydroxytyrosol (PubChem ID: 82755). Ligand
structure was downloaded from PubChem database
(https://pubchem.ncbi.nlm.nih.gov/). Preparation of
protein structure was conducted using Discovery
studio 16.1.0 software. Each protein (jararhagin and
α
2β1 intregin) was prepared by deleting the water
and ligand molecule. Protein structures then saved as
PDB format (.pdb). The ligand structure was
prepared using PyRx software. Ligands was
prepared by minimizing free energy and converted
to PDB format.
2.2 Docking and Visualization
The docking of jararhagin and ligand with α2β1
integrin collagen receptor on the cell-surface of
platelet were carried out in two types of conditions
to determine the effectiveness and efficiency of
inhibitors. The first condition is interaction between
jararhagin-ligand complex and α
2β1 integrin. The
second condition is interaction between α
2β1
integrin-ligand complex and jararhagin. Since we
used 3 kind of ligands (Batimastat, EDTA, and
hydroxytyrosol), there are 6 types of interaction
between jararhagin, ligand, and α
2β1 integrin. The
docking was performed using HEX 8.0.0. Docking
results were visualized using Discovery studio
16.1.0.
3 RESULT AND DISCUSSION
3.1 The Interaction of Jarharagin with
Batimastat and Α2β1 Integrin
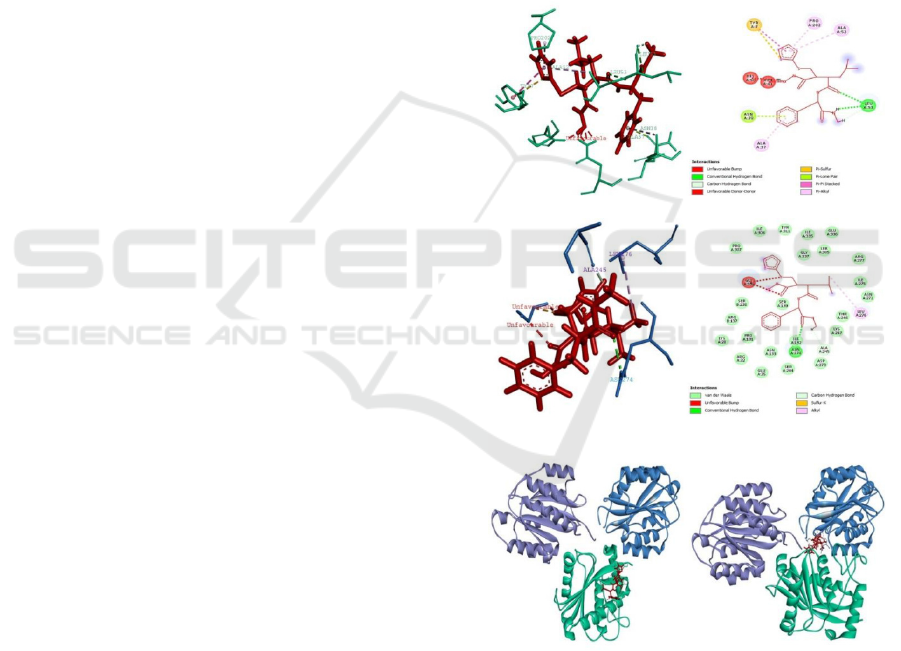
Docking energy between jararhagin (green) and
batimastat (red) ligand is -289.0 kcal/mol. Whereas
if the results of jararhagin-batimastat docking are re-
docked with the integrin receptor α
2β1 (α: blue, β:
violet) , the docking energy decreases with a value
of -784.1 kcal/mol. The interaction between
(jararaghin + batimastat) and integrin α
2β1 are a first
condition that indicated treatment of batimastat after
envenomation. This condition has 10 of favorable
bonds. Two conventional hydrogen bonds that
binding the amino acid residues of Leu53 with atom
H and O on Ligan 1. Then 2 carbon-hydrogen bonds
between Ligand with the amino acid residues Leu53
and Tyr53. One Pi bond with sulfur with amino acid
residue Tyr7. One Pi bond with a lone pair is Asn38.
Three hydrophobic bonds with a Pi type with alkyl
are all three Lig1 with Ala52, Pro202, and Ala37.
One type hydrophobic bond between Pi is amino
acid residue Tyr7. There are also appear 6 pieces
that are unfavorable, which is appear in 2 kind of
amino acid residue like Asn42 and His50.
The interaction between jararhagin and the
docking results of batimastat and integrins α
2β1. The
docking energy of the batimastat and integrin α2β1
is -277.1 kcal/mol. If the result of the docking is
docked again with a fault, the value of the docking
energy becomes -857.5 kcal/mol. The interaction of
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
6
jararhagin and (Batimastat + Integrin α2β1) are
second condition that indicate treatment of
batimastat before envenomation. This condition has
4 favorable binding bonds, each of which has a
different type of bond. The first bond is a
conventional hydrogen bond that binds with amino
acid residue Asn27, then carbon bonds with
hydrogen that binds atom H with amino acid
residues Ala245, alkyl bonds that bind atom C with
amino acid Leu276, sulfur bond with X which binds
atom S with Glys338 amino acid residu. The last,
there are also 3 unfavorable bond that appeat in 2
kinds of amino acid residues like Gly338 and
Asn274. The docking results between jararhagin and
integrin wich are a presumed condition if jararhagin
envenomation occure. produce docking energy of -
841.3 kcal/mol.
The result show that batimastat is easier to
interact with jararhagin than the integrin receptor
α2β1 because the energy used to interact with
jararhagin is smaller than the interaction with the
integrin receptor α2β1. Morover, the docking
condition 2 that is batimastat-intgerine complex
α2β1 interaction with jararhagin has a lower
(negative) docking energy value of -841.3 kcal / mol
compared with the jararhagin-batimastate complex
interaction with the α2β1 integrin the value is higher
(positive) which is -784.1 kcal / mol. So that the
batimastatic-integrin α2β1complex that was
interacted with jararahgin was less effective in
inhibiting the enzyme formation of jararhagin
because batimastate was easier to tie jararhagine
than before binding to the integrin α2β1.Lower
docking energy shows that the bonds between
protein requires more energy to bind, so that it can
be used to inhibit jararhagin for integrin receptors.
In addition, when viewed from a 3-dimensional
structure, it can be seen from the 3-dimensional
interaction that the batimastat ligand is positioned
between jararhagin and the integrin domain α2
(Figure 1F). This is in accordance with the reference
which states that jararhagin will bind to integrins in
the α2 domain then integrin β1 cleavage, even with
low docking energy that allows easy unbonding.
Batimastat inhibits jararhagin which contains ZBG
or zinc binding group by cleavage BaP1 protein
between dermal-epidermal formed from basic
membrane components. High docking energy also
shows that the bonds between molecules are strong
so they are not easily released (Jimenez et.al., 2008).
Integrin-binding motif α
2β1 is located to or within
the hyper-variable region of the cycstein-rich
domain. Part that causes inhibition of platelet
aggregation is the catalytic or proteolytic site that
interacts with the integrin α
2β1 so that it will trigger
signal transduction on platelets (Tanjoni et.al.,
2010).
The atomic interactions between batimastats and
jararhagin interact more than the atomic interactions
between batimastats and integrins α2β1. The
interaction of the jararhagin-batimastat complex
with integrins α2β1 has more hydrophobic and
hydrogen bonds than the complex interactions of
jararhagin-integrin α2β1 with jararhagin.
Conventional hydrogen bonds are stabilizing bonds
in biomolecular structures. Hydrogen bonds occur
between proton donor groups. The donor part is an
electronegative element and the acceptor group is a
free electron pair or phi bond, especially on oxygen
and nitrogen atoms (Horowitz & Trievel, 2012).
Figure 1. Interaction of Jararhagin, Batimastat, and
Integrin
21. A. Ligan interaction between complex
jararhagin-batimastat and integrin
21 (3D). B. Ligan
interaction between complex jararhagin-batimastat and
integrin
21 (2D). C. Ligan interaction between complex
integrin
21 -batimastat and jararhagin (3D). D. Ligan
interaction between complex integrin
21 -batimastat and
jararhagin (2D). E. Interaction complex jararhagin-
batimastat and integrin 21. F. Interaction complex
integrin
21-batimastat and jararhagin.
A
B
C D
E
F
In Silico Prediction of High Potential Jararhagin Inhibitor: Comparison of Batimastat, EDTA and Hydroxytyrosol
7
Table 1. Bonding interactions between the jararhagin batimastat complex and the integrin receptor α2β1.
Name
Distance
Category
Type
From
To
(Å)
Chemistry
Chemistry
A:LEU53:HN -
2,64948Å
Hydrogen
Conventional
H-Donor
H-Acceptor
A:LIG1:O Bond
Hydrogen Bond
A:LIG1:H -
2,89807Å
Hydrogen
Conventional
H-Donor
H-Acceptor
A:LEU53:O
Bond
Hydrogen Bond
A:ALA52:HA -
2,37972Å
Hydrogen
Carbon Hydrogen
H-Donor
H-Acceptor
A:LIG1:O Bond
Bond
A:LIG1:H -
2,13003Å
Hydrogen
Carbon Hydrogen
H-Donor
H-Acceptor
A:LEU53:O Bond
Bond
A:LIG1:S -
4,163Å Other
Pi-Sulfur
Sulfur Pi-Orbitals
A:TYR7
A:ASN38:OD1 -
2,82212Å Other Pi-Lone Pair
Lone Pair
Pi-Orbitals
A:LIG1
A:LIG1 -
4,23106Å
Hydrophobic
Pi-Pi Stacked
Pi-Orbitals
Pi-Orbitals
A:TYR7
A:LIG1 -
5,49552Å
Hydrophobic
Pi-Alkyl
Pi-Orbitals Alkyl
A:ALA52
A:LIG1 -
4,09041Å
Hydrophobic
Pi-Alkyl
Pi-Orbitals Alkyl
A:PRO202
A:LIG1 -
4,39295Å
Hydrophobic
Pi-Alkyl
Pi-Orbitals Alkyl
A:ALA37
A:ASN41:ND2 -
1,84028
Unfavorable Unfavorable Bump Steric
Steric
A:LIG1:O
A:ASN41:ND2 -
1,46595
Unfavorable Unfavorable Bump Steric
Steric
A:LIG1:H
A:ASN41:HD21
1,76436
Unfavorable Unfavorable Bump Steric
Steric
- A:LIG1:O
A:ASN41:HD22
1,45353
Unfavorable Unfavorable Bump Steric
Steric
- A:LIG1:O
A:ASN41:HD22
Unfavorable
Steric;H- Steric;H-
0,646487
Unfavorable
Bump;Unfavorable
- A:LIG1:H
Donor Donor
Donor-Donor
A:HIS50:HD1 -
1,52363
Unfavorable
Unfavorable
H-Donor
H-Donor
A:LIG1:H Donor-Donor
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
8
Table 2. Bonding interactions between the batimastat integrin α2β1 complex and jararhagin.
Name
Distance
(Å)
Category TYPE
From
Chemistry
To
Chemistry
A:ASN274:HD21
- B:LIG1:O
2,1888Å
Hydrogen
Bond
Conventional
Hydrogen Bond
H-Donor
H-
Acceptor
B:LIG1:H -
A:ALA245:O
1,6891Å
Hydrogen
Bond
Carbon
Hydrogen Bond
H-Donor
H-
Acceptor
B:LIG1:S -
A:GLY338:N
2,4186Å Other Sulfur-X Sulfur O,N,S
B:LIG1:C -
A:LEU276
4,5753Å Hydrophobic Alkyl Alkyl Alkyl
A:ASN274:HB1 -
B:LIG1:O
1,67052 Unfavorable
Unfavorable
Bump
Steric Steric
A:GLY338:CA -
B:LIG1:O
2,23482 Unfavorable
Unfavorable
Bump;Carbon
Hydrogen Bond
Steric;H-
Donor
Steric;H-
Acceptor
A:GLY338:HN -
B:LIG1:S
1,83166 Unfavorable
Unfavorable
Bump
Steric Steric
The results of research on hemorrhagic ability by
jararhagin in the lungs and skin with experimental
animals showed that batimastat was able to reduce
hemorrhagic activity in these organs. Other results
showed that jararhagin incubated with batimastat
and inserted into intradermal mice showed that there
was a reduction in hemorrhagic diameter in
experimental animals compared to incubation of
jararhagin with human 2-macroglobulin and normal
serum mouse (Escalante et.al., 2003).
3.2 The Interaction of Jararhagin with
Α2β1 Integrin and EDTA
The first condition is the interaction between
jararhagin-EDTA complex which requires an energy
of -227.23 kcal/mol to bound each other. This
interaction are made by α
2β1 integrin receptor which
is a common target of the inhibition process by
jararhagin. The energy needed from the ligand
complex jararhagin-EDTA to bound with I domains
of α
2 (colored blue) is -733.96 kcal/mol. While the
position of EDTA is area that does not interact with
integrin amino acid residues (jararhagin is green)
(Figure 2E). The second condition is an alternative
interaction between the α
2β1-EDTA complex which
requires higher energy, which is -215.46 kcal/mol to
bound with I domain of α
2. That interaction made
with jararhagin being the natural inhibitor of the
integrin receptor α
2β1. The energy required from the
receptor complex α
2β1-EDTA to bound with the
disintegrin-like domain of jararhagin (green) is -
721.57 kcal/mol (Figure 2F). This condition showed
that EDTA is more easily bound with jararhagin
compared to α
2β1 integrin. In addition, when
jararhagin-EDTA are the ligand complexed it
required lower energy to bound to platelet integrin
surface receptors, compared to the energy needed
when EDTA in the form of receptor complexed with
integrins. While the tendency of bond energy and
bounding-site position between the receptor complex
and jararhagin indirectly indicates that inhibition by
EDTA is more effective by bounding to the integrin
α
2β1 even before the venom spreads in the blood
vessels after the envenomation.
Furthermore, the interaction between the
jararaghin-EDTA ligand complex and
21 integrins
forms 5 kind of favorable bonds (Figure 2F). Four of
them are conventional hydrogen bonds with an
average distance of 2.3 Å to 2.9 Å that can be
classify as strong until medium H-bond. Strong
covalent H-bond have length 2.2 Å - 2.5 Å whereas
moderate mostly electrostatic have length of distance
2.5 Å -3.2 Å (Baey, 2013). The hydrogen bond
bound to asparagine amino acid residues (Asn194),
proline (Pro195), tyrosine (Tyr5), and aspartic acid
(Asp3). Then also formed one carbon hydrogen bond
is formed at A LIG: 1. A hydrogen bond interaction
span a large interval, ranging from tiny energies to
large values when the acceptor is an anion that can
devise interaction stability. Hydrogen bond is
generally also stronger interaction, but still less
stable than van der Waals interaction cause hydrogen
bond have shorter distance (Mingos, 2004). A
hydrogen bond can be called conventional or
classical if it is formed between a partly positively
charged hydrogen atom in proton-donor component
and the lone electronic pair of electronegative
element acting as a proton-accepting component.
This conventional hydrogen bonds forming from
weak to medium energy and accompanied by a
remarkable interpenetration (Bakhmutov, 2008). In
In Silico Prediction of High Potential Jararhagin Inhibitor: Comparison of Batimastat, EDTA and Hydroxytyrosol
9
addition, almost same as the results of interactions
with batimastat inhibitors, the interaction of EDTA
inhibitor ligand complex also forms 4 unfavorable
bonds, that appear namely between ligands in 2 kind
of amino acid residues like proline (Pro4) and lysine
(Lys6). This unfavorable bump interaction bond in
the wrong area that can cause the interaction
unstable. Unfavorable bump are generally formed by
tripled carbon interaction (Karimi & Nalapogaja,
2012).
The interaction of jararhagin with the EDTA-
integrin receptor complex
21 forms 8 kind of
favorable bonds (Figure 2D). The first type of bond
is a conventional hydrogen bond which consists of 5
bonds, namely ligand bonds with serine amino acid
residues (Ser244), alanine (Ala245), lysine (Lys247),
asparagine as a donor and H receptor ( Asn274). The
five bonds show a distance of 1.7 Å to 3 Å. The
second type of bond is carbon hydrogen bonds with 1
bond between ligands with serine amino acid
residues (Ser138) and 2 bonds between amino acids
alanine (Ala245) with bond distances ranging from
1.7 Å to 3.7 Å that can be classify as strong until
weak H-bond. Strong covalent H-bond have length
2.2 Å - 2.5 Å whereas moderate mostly electrostatic
have length of distance 2.5 Å - 3.2 Å, and the weak
electrostatic dispersed have length of distance more
than 3.2 Å (Escalante et.al., 2003). While the last
type of bond is 5 unfavorable bonds, that appear
namely between ligands in 3 amino acid residues like
alanine (Ala245), arginine (Arg243) and tyrosine
(Try 235). This bond shows that the formed
interaction is less stable even though it successfully
inhibits the bounding of jararhagin to the position of
domain I α
2 according to the form of the disturbance
caused by the ability of jararhagin to block integrin
interactions of
21 with collagen by bounding to α2
domain I or by cleavage of
21 [16]. This inhibitor
also proven to be the most effective in vivo and in
vitro method to irreversibly inactivate the proteolytic
activity of jararhagin by remove the active site zinc
and structural calcium molecules from the protein
using EDTA (Gallagher et.al., 2005).
3.3 The Interaction of Jararhagin with
α2β1 Integrin and Hydroxytyrosol
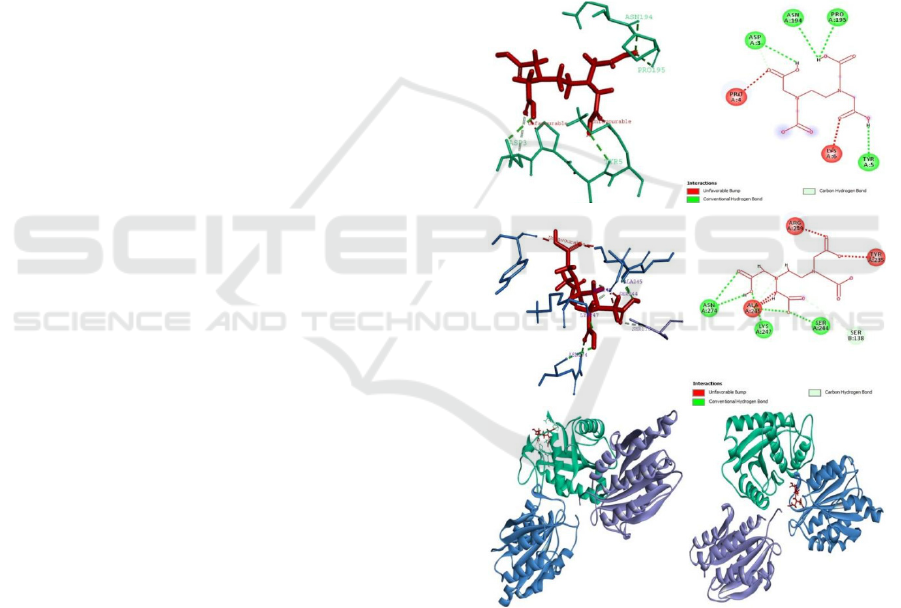
The binding energy of jararhagin-hydroxytyrosol is -
178.3 kcal/mol. The interaction between jararhagin-
hydroxytyrosol and α2β1 integrin needs -781.33
kcal/mol to bind. Jararhagin-hydroxytyrosol is
binding α2β1 integrin on 2-I domain (symbolized
with blue coloration on Figure 3), the inhibitor is not
attached on the interaction site between α2β1
integrin and jararhagin.
On the other hand, the docking in second
condition showed that interaction between
hydroxytyrosol and α2β1 integrin has binding
energy -166.7 kcal/mol. Hydroxytyrosol as ligand
bind at the 2-I domain of α2β1 integrin. The
interaction between α2β1 integrin-hydroxytyrosol
and jararhagin needs -809.3 kcal/mol to bind. The
binding position of hydroxytyrosol is located near to
binding target site of jararhagin generally. The
energy yielded from interaction of α2β1 integrin-
hydroxytyrosol is more than the interaction of
jararhagin-hydroxytyrosol. It is showed that
hydroxytyrosol is easier to bind with jararhagin than
α2β1 integrin after the envenomation.
Figure 2. Interaction of Jararhagin, EDTA, and Integrin
21. A. Ligand interaction between complex jararhagin-
EDTA and integrin
21 (3D). B. Ligand interaction
between complex jararhagin-EDTA and integrin
21
(2D). C. Ligand interaction between complex integrin
21-EDTA and jararhagin (3D). D. Ligand interaction
between complex integrin 21-EDTA and jararhagin
(2D). E. Interaction complex jararhagin-EDTA and
integrin
21. F. Interaction complex integrin 21-EDTA
and jararhagin.
A
F
E
D
C
B
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
10
Table 3. Detail information of H-Bond interaction between jararhagin-EDTA ligands complex with α2β1 integrin.
Name
Distance
Category
Types
From Chemistry To Chemistry
(
Å
)
A:LIG1:H - 2,98453 Hydrogen Bond Conventional
H-Donor
H-Accepto
r
A:ASN194:O
Hydrogen Bond
A:LIG1:H - 2,84653 H
y
dro
g
en Bond Conventional
H-Donor
H-Acce
p
to
r
A:PRO195:O
Hydrogen Bond
A:LIG1:H - 2,81501 Hydrogen Bond Conventional
H-Donor
H-Accepto
r
A:TYR5:O
Hydrogen Bond
A:LIG1:H - 2,57797 H
y
dro
g
en Bond Conventional
H-Donor
H-Acce
p
to
r
A:ASP3:OD1
Hydrogen Bond
A:ASP3:CA -
3,2238
H
y
dro
g
en Bond Carbon H
y
dro
g
en
H-Donor
H-Acce
p
to
r
A:LIG1:O
Bond
A:PRO4:CD -
Unfavorable
Steric;H-
2,09393 Unfavorable Bump;Carbon
Steric;H-Donor
A:LIG1:O Acceptor
Hydrogen Bond
A:PRO4:HD1 -
1,53485 Unfavorable
Unfavorable
Steric Steric
A:LIG1:O Bump
A:LYS6:CG -
2,19754 Unfavorable
Unfavorable
Steric Steric
A:LIG1:O Bump
A:LYS6:CD -
2,19833 Unfavorable
Unfavorable
Steric Steric
A:LIG1:O Bump
Table 4. Detail information of H-Bond interaction between α2β1-EDTA receptors complex with jararhagin.
Name
Distance
Category Types
From Chemistry
To
(
Å
)
Chemistry
A:SER244:HN - 1,85267 Hydrogen Conventional
H-Donor
H-Accepto
r
B:LIG1:O
Bond
Hydrogen Bond
A:ALA245:HN -
2,62916 H
y
dro
g
en Conventional
H-Donor
H-Acce
p
to
r
B:LIG1:O
Bond
Hydrogen Bond
A:LYS247:HN -
1,76284 Hydrogen Conventional
H-Donor
H-Accepto
r
B:LIG1:O
Bond
Hydrogen Bond
A:ASN274:HD21
3,033 H
y
dro
g
en Conventional
H-Donor
H-Acce
p
to
r
- B:LIG1:O
Bond
Hydrogen Bond
B:LIG1:H -
2,45232 H
y
dro
g
en Conventional
H-Donor
H-Acce
p
to
r
A:ASN274:O
Bond
Hydrogen Bond
B:SER138:CB -
3,76774 H
y
dro
g
en
Carbon H
y
dro
g
en
H-Donor
H-Acce
p
to
r
B:LIG1:O
Bond Bond
B:LIG1:H -
2,24188 Hydrogen
Carbon Hydrogen
H-Donor
H-Accepto
r
A:ALA245:O
Bond Bond
B:LIG1:H -
1,73094 Hydrogen
Carbon Hydrogen
H-Donor
H-Accepto
r
A:ALA245:O
Bond Bond
A:TYR235:O -
1,93483 Unfavorable Unfavorable Bump
Steric Steric
B:LIG1:O
A:ARG243:N -
1,99267 Unfavorable Unfavorable Bump
Steric Steric
B:LIG1:O
A:ARG243:HN -
Unfavorable
Steric;H-
1,6825 Unfavorable
Bump;Conventional Steric;H-Donor
B:LIG1:O
Acceptor
Hydrogen Bond
A:ALA245:O -
2,07184 Unfavorable Unfavorable Bump
Steric Steric
B:LIG1:C
B:LIG1:H -
Unfavorable
Steric;H-
1,27798 Unfavorable Bump;Carbon
Steric;H-Donor
A:ALA245:O
Acceptor
Hydrogen Bond
In Silico Prediction of High Potential Jararhagin Inhibitor: Comparison of Batimastat, EDTA and Hydroxytyrosol
11
When α2β1integrin-hydroxytyrosol interacted to
jararhagin, the binding energy is less than energy of
interaction between jararhagin-hydroxytyrosol and
α2β1 integrin. It is showed that hydroxytyrosol as
the inhibitor would inhibit effectively if it is
consumed after envenomation.
Hydroxytyrosol is one of phenolic compound
which has the high level of antioxidant (Obied et.al.,
2012). Interaction of phenolic compound and SVMP
will form the hydrogen bond with three histidine
residue on zinc binding motive area. Thus, zinc ion
will be chelated from SVMP complex. Zinc ion is an
important component of SVMP, because SVMP is
categorized as zinc-dependent hydrolase. When zinc
ion is chelated from SVMP, its enzimatic activity is
inhibited (Pithayanukul et.al., 2009).
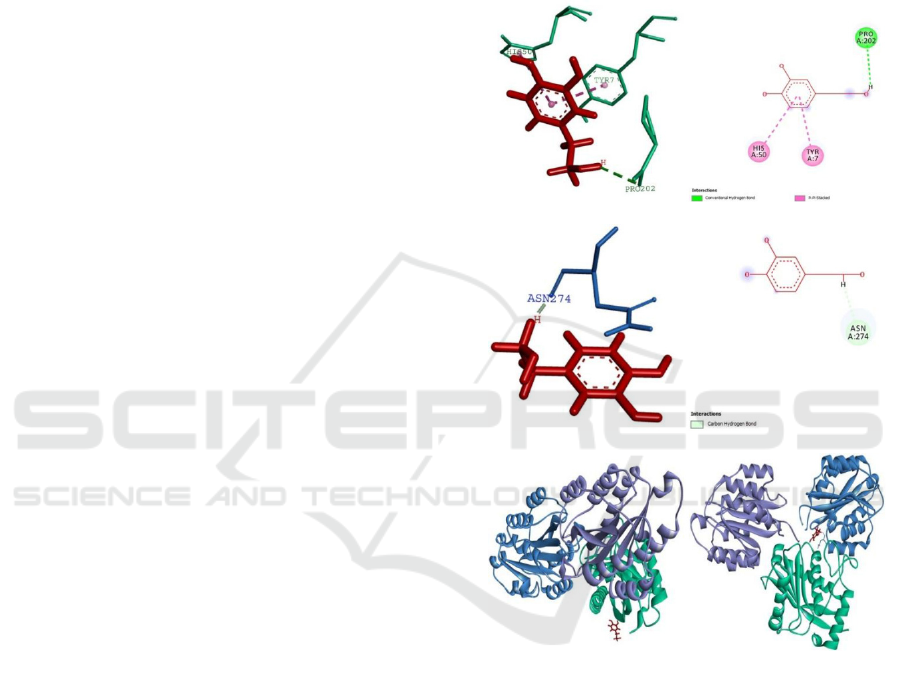
Interaction of jararhagin-hydroxytyrosol and
21 integrin is consist of 1 hydrogen bond and 2
hydrophobic bonds. The first bond is hydrogen bond
which formed from hydrogen atom on ligand to
Pro202 residue as hidrogen receptor. The distance of
this bond is 2,88431. The second bond is
hydrophobic bond (Pi-Pi Stacked) which formed
from Tyr7 residue of jararhagin protein (to an atom
of ligand. The distance of this bond is 4,14095. The
third bond has same type as the second bond, which
formed from His50 residue to an atom of
hydroxytyrosol. The distance of this bond is
4,82085.
Pi-pi (-) stack is a type of non-covalent bond.
That type of bond is formed between two aromatic
ring from different compound. It has acquainted for
its role to stabilize the macromolecular structures
such as nucleic acid, protein, and other material
(Boehr et.al., 2002). Thus, the presence of two Pi-Pi
stacked hydrophobic bond, indicate the strong
interaction between jararhagin-hydroxytyrosol and
21 integrin.
On the other hand, interaction between
21
integrin-hydroxityrosol with jararhagin has only one
hydrogen bond. This bond is formed from hydrogen
atom on ligand with Asn274 residue. The distance of
this bond is 1,90548. Hydrogen bond is the
interaction between hydrogen atom and
electronegative atom group, it has stronger bond
than van der Waals interaction, and weaker than
covalent or ionic bond. Hydrogen bond cosidered to
be the regulator of protein-ligand binding. This bond
can create stronger protein-ligand interaction but
causing absence of net gain binding affinity, but this
bond is also reported to enhance ligand binding
affinity by displacing protein-bound water molecule
to the bulk solvent (Chen et.al., 2016).
Since there is no any unfavorable bond, the
interaction with phenolic compound is better than
other ligand because of the stability. Besides, this
natural inhibitor is more efficient because it include
the metal chelator activity, high level of antioxidant
which can support the cell regeneration, free radical
scavenger, enzyme activity modulator, and
anticancer (Pithayanukul et.al., 2009).
Figure 3. Interaction of Jararhagin, Hydroxytyrosol, and
Integrin
21. A. Ligan interaction between complex
jararhagin-hydroxytyrosol and integrin 21 (3D). B. Ligan
interaction between complex jararhagin-hydroxytyrosol
and integrin
21 (2D). C. Ligan interaction between
complex integrin
21 - hydroxytyrosol and jararhagin
(3D). D. Ligan interaction between complex integrin
21
-hydroxytyrosol and jararhagin (2D). E. Interaction
complex jararhagin-hydroxytyrosol and integrin
21. F.
Interaction complex integrin
21-hydroxytyrosol and
jararhagin.
A
B
C
D
E
F
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
12
Table 5. Interaction between jararhagin, hydroxytyrosol, and 21 integrin.
Name
Distance
Category Types
From
To
(Å)
Chemistry Chemistry
Interaction
A:LIG1:H -
Hydrogen
Conventional
between
2,88433 Hydrogen
H-Donor H-Acceptor
A:PRO202:OXT
Bond
jararhagin-
Bond
hydroxytyrosol
ligands
A:TYR7 -
4,1407
Hydrophobic Pi-Pi Stacked Pi-Orbitals Pi-Orbitals
complex with
A:LIG1
21 integrin
A:HIS50 -
4,8207
Hydrophobic Pi-Pi Stacked Pi-Orbitals Pi-Orbitals
A:LIG1
Interaction
between 21
integrin -
B:LIG1:H -
Hydrogen
Carbon
hydroxytyrosol
1,90548 Hydrogen
H-Donor H-Acceptor
A:ASN274:O
Bond
ligands
Bond
complex with
jararhagin
4 CONCLUSIONS
Inhibition of hemorrhagic activity can be done with
several types of compounds that have been known to
be inhibitors for SVMP especially jararhagin can
done by batimastat (peptidomimetic compounds),
EDTA (zinc-chelating agents), and plant compounds
such as hydroxytyrosol. Based on the results of the
in silico analysis from batimastat, EDTA and
hydroxytyrosol inhibitory properties, found that
these inhibitor were more easily bound to jararhagin
compared to integrin
21. But only two of them are
more effectively inhibited
by bounding to jararhagin
spread in blood vessels after snakebite cases.
However, unfavorable bonds are formed during
interaction between batimastat inhibitors, jararhagin
and integrin
21. These inhibitor are Batimastat and
hydroxytyrosol. Furthermore, in the second inhibitor
EDTA, it was found that this compound more
effective doing inhibition by inhibiting integrin
21.
In other side inhibitor batimastat and EDTA also
has
unfavorable bonds. While the last alternative of
phenolic compounds in the form of hydroxytyrosol
shows that inhibitor are interact with jararhagin and
integrin α
2β1 without showing an unfavorable bond.
From that result we can conclude that the natural
inhibitors formed in hydroxytyrosol from olive oil
are more stable and have highest effectiveness and
efficiency in preventing hemorrhagic symptoms due
to snake bites that contain jararhagin venom.
ACKNOWLEDGEMENTS
The present study was funded by Kemenristekdikti -
the Government of the Republic of Indonesia
through the scheme of PDUPT 2019 to Nia
Kurniawan with contract number
330.13/UN10.C10/PN/2019.
REFERENCES
Williams, D., Gutierrez, J., Harrison, R., Warrell, D.,
White, J., Winkel, K., and Gopalakrishnakone, P.
(2010). The global snake bite initiative: An antidote
for snake bite. Lancet. 375, pp. 89-91.
Kunalan, S., Othman, I., Hassan, S., and Hodgson, W.
(2018). Proteomic Characterization of Two Medically
Important Malaysian Snake Venoms, Calloselasma
rhodostoma (Malayan Pit Viper) and Ophiophagus
hannah (King Cobra). Toxins, 10 (434), pp. 1-36.
Silva, M., Tamires, L., Murilo, V., Caroline, M.,
Fernanda, M., Kelly, C., Fábio, O., Tiago, W., and
José, R. (2016). Interaction between TNF and
BmooMP-Alpha-I, a Zinc Metalloprotease Derived
from Bothrops moojeni Snake Venom, Promotes
Direct Proteolysis of This Cytokine: Molecular
Modeling and Docking at a Glance. Toxins, 8(223),
pp.1-20.
Preciado, L., Pereanez, J., Singam, E., and Comer, J.
(2018). Interactions between Triterpenes and a P-I
Type Snake Venom Metalloproteinase: Molecular
In Silico Prediction of High Potential Jararhagin Inhibitor: Comparison of Batimastat, EDTA and Hydroxytyrosol
13

Simulations and Experiments. Toxins, 10(397), pp. 1-
20.
Pithayanukul, P., Jiraporn, L., and Patchreenart, S. (2009).
Molecular Docking Studies and Anti-Snake Venom
Metalloproteinase Activity of Thai Mango Seed
Kernel Extract. Molecules, 14(1), pp. 3198-3213.
Ferreira, B., Simone, R., Francyelle, B., and Tatiana, C.
(2018). Inflammation, angiogenesis and fibrogenesis
are differentiallymodulated. International Journal of
Biological Macromolecules, 119 (1), pp.1179–1187.
Romero, F., Anna, G., Andrés, E., Rebeca, A., Javier, Q.,
Robson, L., Juan, J., Mavis, M., Renato, M.,
Alexandra, R., José, M., and Enrique, P. (2012).
Identification of New Snake Venom Metalloproteinase
Inhibitors Using Compound Screening and Rational
Peptide Design. Medicinal Chemistry Letters, 3(1), pp.
540−543.
Jimenez, N., Escelante, T., Gutiterrez, J., and Rucavado,
A. (2008). Skin Pathology Induced by Snake Venom
Metalloproteinase: Acute Damage, Revascularization,
and Re-epithelization in a Mouse Ear Model. Journal
of investigative dermatology, (128), pp. 2421-2428.
Tanjoni, I., Evangelista, K., Della-Casa, M., Butera, D.,
Magalhaes, G., Baldo, C., Clissa, P., Fernandes, I., lbe,
J., Moura da Silva, A. (2010). Different regions of the
class P-III snake venom metalloproteinase jararhagin
are involved in binding to α2β1 integrin and collagen.
Toxicon, 55(2010), pp.1093-1099.
Horowitz, S. and Trievel, R. (2012). Carbon-Oxygen
Hydrogen Bonding in Biological Structure and
Function. The journal of biological chemistry,
287(50), pp.41576-41582.
Escalante, T., Javier, N., Ana, M., Moura, S., Alexandra,
R.,. David, G., and Gutierrez, J. (2003). Pulmonary
hemorrhage induced by jararhagin, a metalloproteinase
from Bothrops jararaca snake venom. Toxicology and
Applied Pharmacology, 193, pp.17-28.
Baev, A. (2013). Specific Intermolecular Interactions of
Nitrogenated and Bioorganic Compounds. New York:
Springer Science & Business Media..
Mingos, D. (2004). Supramolecular Assembly Via
Hydrogen Bonds II. New York : Springer Science &
Business Media.
Bakhmutov, V. (2008). Dihydrogen Bond: Principles,
Experiments, and Applications. New York : John
Wiley & Sons.
Karimi, I., and Nalapogaja S. (2012). 11th International
Symposium on Process Systems Engineering. New
Delhi : Elsevier.
Tanjoni, I., Weinlich, R., Della-Casa, M., Clissa, P.,
Saldanha-Gama, R., de Freitas, M., Barja-Fidalgo, C.,
Amarante-Mendes, G., and Moura-da-Silva, A.
(2005). Jararhagin, a snake venom metalloproteinase,
induces a specialized form of apoptosis (anoikis)
selective to endothelial cells. Apoptosis, 10, pp. 851–
861.
Gallagher, P., Yongde B., Solange M., Gavin D., David
R., Gutierrez, J., Teresa, E., Paola, Z., Ana, M.,
Roswitha, N., Cornelia, M., Christopher, M., and Jay,
W. (2005). Role of the snake venom toxin jararhagin
in pro-inflammatory pathogenesis: In vitro and in vivo
gene expression analysis of the effects of the toxin.
Archives of Biochemistry and Biophysics, 441(1), pp.
1–15.
Obied, H., Paul, D., Syed, H., and Rania, I. (2012).
Advances in Molecular Toxicology. Amsterdam :
Elsevier. pp. 195-242.
Boehr, D., Farley, A., Wright, G., and Cox, J. (2002).
Interactions between the Aminoglycoside Antibiotic
Kinase APH(3’)-IIIa and Its Nucleotide Ligands. Cell
Press. Elsevier Inc.
Chen, D., Numan, O., Petri, U., Colin, F., Sara, M., and
Tor, C. (2016). Regulation of protein-ligand binding
affinity by hydrogen bond pairing. Science Advances,
2, pp. 1-6.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
14
