
Electrolyte Membrane Composite from Modified Chitosan-Vanillin
and Zeolite Filler for Direct Methanol Fuel Cell Application
Anjas Badarani S.
1
, Gugus Handika
1
, Mochammad Purwanto
1
, Edi Pramono
2
, Cynthia Linaya R.
2
1
Institut Teknologi Kalimantan
2
Department of Chemistry, Bandung Institute of Technology, Bandung, Indonesia
cynthia@chem.itb.ac.id
Keywords: Fuel cell, Membrane, Chitosan-Vanillin, Zeolite
Abstract: Fossil fuels, which are the main fuels in the world has a negative impact on the environment and must be
replaced immediately with other fuels, DMFC is variant of fuel cell which works as portable to maintain daily
human activity and potential to replace fossil fuels as the main source of energy. DMFC regulates as similar
to an electrochemical cell in which favor of separator referred to as Polymer Electrolyte Membrane (PEM).
PEM shows the main feature, such as hindering the electrons and reactants from trespassing between the
electrodes while acting as a proton conductor. The main parameter of PEM for DMFC are good proton
conductivity and low methanol permeability. Chitosan-Vanillin (CV) was synthesized by reacting chitosan
and vanillin at 1: 2,5 wt ratio and stirred continuously to obtain the product. The membrane then cast by
mixing the CV with some variations of zeolite with the compositions of 1,5%, 3%, and 6% wt towards CV.
The membrane then cast into a petri dish and left it overnight. The resultant membrane then characterized
with FTIR, ATR, molecular weight, water and methanol uptake, ion exchange capacity, ionic conductivity,
and methanol permeability. The optimum membrane result was the 6% wt zeolite in the CV, in which ionic
conductivity reached 0,1 S/cm and 1,266 x 10-3 cm2/s for methanol permeability.
1 INTRODUCTION
Fossil fuels still dominating in the world, especially
in countries that still use transportation, which fossil
fuels as the main fuel. Fossil fuels have a large
negative impact on the environment. Fossil fuels had
82 percent of total global energy sources in 25 years,
although any efforts to reduce still requires a lot of
alternative energy because the percentage is still the
same (Republika, 2013). If fossil fuels still used
continuously, impacts such as acid rain and global
warming will occur. Therefore, the purpose of this
study is to replace the fossil fuels with fuel cells that
produce more energy and fewer emissions
Direct methanol fuel cell (DMFC) is a type of
PEMFC. DMFC provides good power density almost
as high as PEMFC but is safe and capable. One of the
main factors for this DMFC to work is electrolytes.
Electrolytes known as separators as selective barriers
to methanol and H+ ions pass through the membrane.
DMFC will provide more energy and fewer
emissions. Nafion is famous for commercial
electrolytes used in PEMFC because of high
conductivity and excellent chemical stability.
Although the excellent performance of Nafion has
made it a good choice of electrolytes, methanol
permeability still occurs. Such permeability affects
the efficiency of methanol in DMFC. Therefore,
several developments have been made to overcome
this problem.
Chitosan is a biopolymer in crustacean animals.
Chitosan functions as a good biocompatibility
composite and a good polycationic ability to provide
chemical stability. Another feature of chitosan is that
OH and NH2 backbones can be modified, so chitosan
acts as a flexible matrix for any application. In
electrolyte membranes for DMFC applications,
parameters such as proton conductivity and methanol
permeability must be considered. So, to modify
chitosan, several methods are offered, such as
modifying chitosan, whether inside and outside the
matrix. This research was conducted by combining
these methods. Vanillin is used for reagent polymers
and inorganic silica materials such as zeolites as
modifications outside the matrix. Both of these
ingredients enhance chitosan as a DMFC electrolyte,
94
S., A., Handika, G., Purwanto, M., Pramono, E. and Linaya R., C.
Electrolyte Membrane Composite from Modified Chitosan-Vanillin and Zeolite Filler for Direct Methanol Fuel Cell Application.
DOI: 10.5220/0009406100940099
In Proceedings of the 1st International Conference on Industrial Technology (ICONIT 2019), pages 94-99
ISBN: 978-989-758-434-3
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

which is suitable in terms of proton 2 experimental
methods. Zeolite was chosen because it has good
results, specifically decreased water uptake,
decreased methanol uptake, and reduced methanol
permeability. Whereas, vanillin was chosen as an
anti-bacterial.
2 MATERIALS
Dried shrimp shells of Penaeus monodon as chitosan
sources. Vanillin powder purchased from PT Subur
Kimia Jaya. Zeolite purchased from PT Bratacho
Chemica. Sodium hydroxide (NaOH), hydrochloric
acid (HCl), methanol (MeOH), acetic acid, hydrogen
peroxide (H2O2), sulfuric acid (H2SO4), toluene,
ethanol (EtOH), phenolphthalein indicator, and
sodium chloride in the pure analytical grade were
purchased from Merck.
2.1 Chitosan Preparation
Chitosan preparation has three steps deproteinized,
demineralized, and deacetylated. The first step is
deproteinized, shrimp shells that have been prepared
ground into a powder and then stir it at 60-70C in
NaOH 3,5% wt solution by the composition 1:10 (gr
powder/mL NaOH) for about 2 hours. The shells then
filtered from the solution, washed it by demineralized
water (aqua dest), and dried at 100C for about 4
hours. The shells then demineralized in HCl solution
at 60-70C by the composition 1:10 (gr powder/mL
HCl) for about 2 hours. The shells turned into chitin
this time and underwent the same procedure to get
dried chitin. Chitin then deacetylated in NaOH 50%
wt solution by the composition 1:10 (gr powder/mL
NaOH) for about 1 hour at 90-100C. Chitin was
degraded into chitosan, and lastly, the same procedure
is applied to get dried chitosan.
The dried chitosan underwent modification by
vanillin. Chitosan first diluted by 1% of acetic acid
(2% w/v ratio) and vanillin diluted by absolute
ethanol (1:2 ratio). Both diluted chitosan and vanillin
then mixed in one container with 1:2,5 of chitosan:
vanillin ratio for about a day at 35. The resultant
solution then filtered by using a vacuum filter flask to
get the chitosan powder. The product itself has a
brown-yellowish.
2.2 Membrane Preparation
The electrolyte membrane was prepared using the sol-
gel process. Initially, the experiments were performed
by preparing various concentrations (1,5%, 3%, and
6% wt zeolite) chitosan membrane. Chitosan and
desire filler were dissolved in acetic acid 2% v/v and
then stirred at room temperature for 24 h until the
solution formed a gel. The solution was placed into a
petri dish for 24 h. After the thin film was formed, the
membrane then poured with NaOH solution to faster
the peeling. The resultant membrane then washed and
dried at room temperature.
2.3 Chitosan and Chitosan-Vanillin
Characterization
Membrane characterization was used Fourier
Transport Infra-Red (FTIR) as instrumental analysis.
Chitin and chitosan functional groups was
identification by Fourier Transport Infra-Red (FTIR).
Samples are directly exposed to electromagnetic
radiation to obtain absorbance and then correlate
between absorbance and functional groups.
2.4 Water and Methanol Uptake
Water uptake and methanol uptake determine the
ability of the membrane absorbs the solution. The
membrane was weighed before soaked with methanol
or water for 12 hours. Then wet membranes are
weighed and can be applied to the following formula:
Uptake (%) = ((W
wet
–W
dry
)/W
dry
)x100% (3)
Where : W
wet
= Mass Membrane after immersion
aaaaaaaaaaaaaaaa(gram)
W
dry
= Mass Membrane before immersion
aaaaaaaaaaaaaaaa(gram)
2.5 Methanol Permeability
Methanol permeability to determine membrane
performance for methanol crossover. The membrane
is cut to the size of 1.6 X 1.6 cm and placed into the
diffusion cell compartment. Compartment A contains
1M methanol, and compartment B contains water.
Methanol concentration was measured using samples
in compartment B every 30 minutes for 2 hours.
Methanol concentration was measured using a
pycnometer and corrected with a calibration curve.
The sampled concentrations were regressed to get its
slope. Methanol permeability formula as follows :
P = [ΔCB/Δt] * [L*VB/(A*CA)] (4)
Where :
P = methanol permeability (cm2/s)
ΔCB/Δt = slope determined by function of time
(mol/L.s)
Electrolyte Membrane Composite from Modified Chitosan-Vanillin and Zeolite Filler for Direct Methanol Fuel Cell Application
95
L = membrane thickness (cm)
VB = volume of water in compartment (cm3)
A = membrane surface area (cm2)
CA = methanol concentration in compartment
(mol/L)
2.6 Ion Exchange Capacity (IEC)
IEC is used to determine the movement of protons in
membrane. Membrane is immersed into HCL for 1
hour as protonate. Then the membrane was immersed
in NaCl as a second protonate for 24 hours.
membrane has been titrated using 0.01 M NaOH with
phenoptalein indicator as a titer after protonation.
Titration data can be used in the following formula:
IEC (meq) = [V*C/m] (5)
Where :
V = Volume of titer used to netralize the NaCl
solution (mL)
C = NaOH concentration (M)
m = Dry weight of the membrane (gram)
2.7 Proton Conductivity
Proton conductivity is the main parameter to find out
membrane quality to delivering protons. Zdata could
be found from the LCR meter. Zview will be
implemented Zdata to get the Rbulk value. Then
Rbulk can be used into the formula :
Ϭ = L/(R*A) (6)
Where :
Ϭ = Conductivity (S/cm))
R = Bulk resistance (ohm)
L = Membrane thickness (cm)
A = Area of the membrane (cm2).
3 RESULTS AND DISCUSSION
3.1 Membrane Structure
3.1.1 Chitosan-Vanillin Structure
FTIR assessments were to determine the group
functionality of chitosan and chitosan-vanilline.
Numerous wavelengths were caught in the FTIR
spectrum. There is numerous vibration of functional
groups as informed in the table below:
Table 1: Functional group vibrations of chitosan
Functional Group Absorbance Spectrum
-O-H 3550-3100
-N-H (COCH3) 1680-1630
-N-H 1650-1580
-C-H 3000-2850
-C-N 1250-1020
-C=O 1650
-C-O (Eter) 1150-1085
(Beuchamp,1981)
Figure 1: Infrared Spectra Chitosan and Chitosan-Vanillin
Deacetylation by compare amide absorption
against water absorption. As a result, the best DD
obtained of 92%. Later, the substitution of imine
groups' appearance in CV was determined in
wavenumber 1690-1640 cm-1. As depicted in figure
1, the –C=N group was in 1666,65484 cm-1, which
indicating CV was successfully synthesized. As
explained by Pramono (2014), FTIR analysis of
vanillin chitosan showed peaks in the area of 1641
cm-1, including the formation of an imine (C = N) or
schif base bond.
3.1.2 Chitosan-Vanillin Filler Zeolite
Structure
The filler membrane was also be examined to
determine the substitution of functional groups
between CV and zeolite. When the chitosan
membrane is modified with zeolite, the adhesion
strength between the polymer and zeolite particles
will increase and reducing the void space on the
membrane (Fitri, 2016).
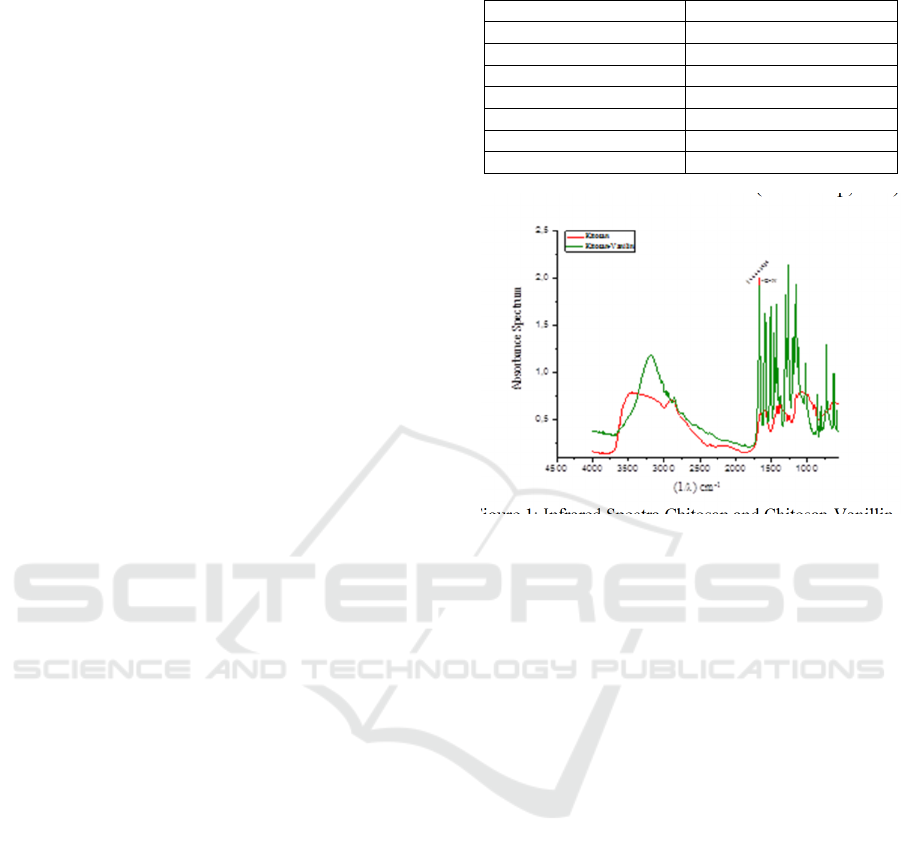
From figure 2, chitosan-vanillin successfully
binds to the zeolite. It is proved by the vibrations peak
at range 1100-980 cm-1 dan 1000-500 cm-1. This
vibration is functional group Al-O and Si-O, which is
chitosan-vanillin filler zeolite bonding comparable
with Saikia (2010). But the highest vibrations peak at
1,5% zeolite because mixing didn’t go properly.
ICONIT 2019 - International Conference on Industrial Technology
96
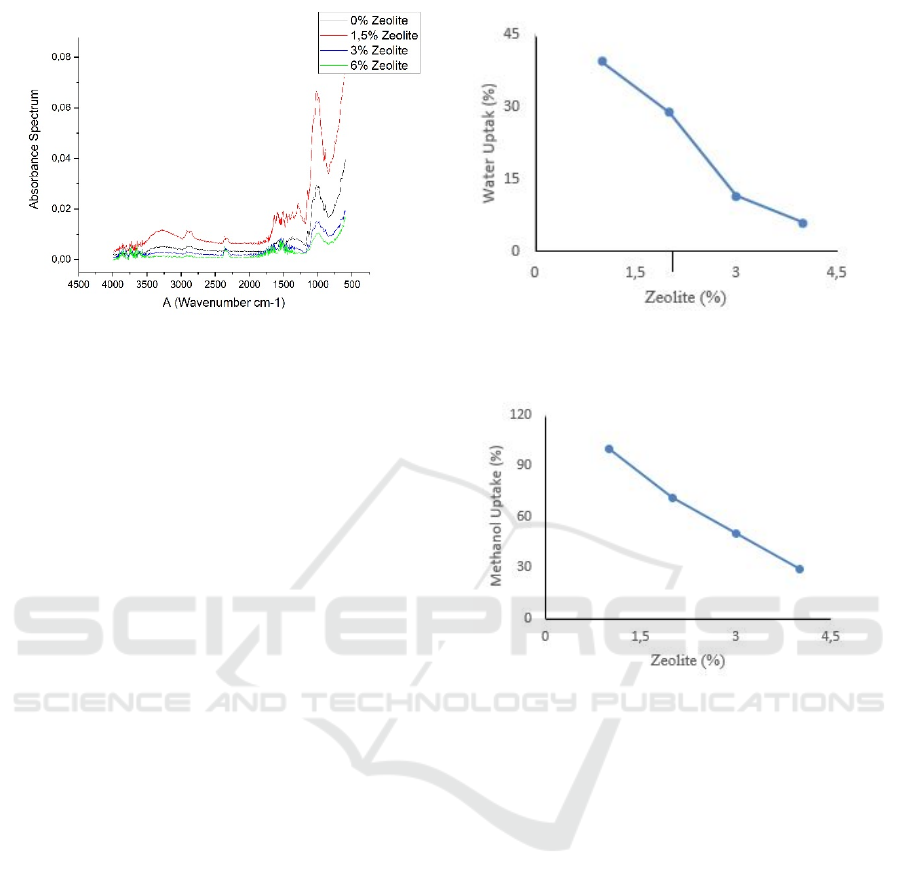
Figure 2: Infrared Spectra Chitosan-Vanillin Filler Zeolite
3.2 Water and Methanol Uptake
Chitosan generally has hydrophilic and hydrophobic
properties. Evidenced by the measurement of water
contact angel membrane with range 100-115
prominent in hydrophobic properties but has
hydrophilic properties also. Water contact angles
below 90 and hydrophobic 90-180 (Dwivedi,
2017).Water molecules in chitosan increase the
mobility of ions, but higher intensity water can be
damaging membrane structure. Therefore a water
uptake measurement is necessary to determine the
results of the addition zeolite filler to the chitosan
membrane because zeolite has hydrophobic
properties. Methanol uptake measurements to
determine the selectivity of the membrane against
methanol as the main fuel in the direct methanol fuel
cell because higher methanol uptake can affect the
chemical reaction of the direct methanol fuel cell. As
depicted from the figure below, the water uptake and
methanol uptake of chitosan were determined.
Figure 3 showed water uptake decreased, and new
molecular bonds decrease the membrane's ability to
absorb water molecules from the solvent (Wang,
2010). Zeolite filler has silica molecules that increase
hydrophobic properties. Further, in figure 4, methanol
uptake decreased. It has two possibilities, methanol
structure similar to the fenolic group, so it has a high
affinity (Antony, 2019) or methanol density smaller
than water molecules. This makes it easy for
methanol molecules to pass through the membrane
based on particle size.
Figure 3: Water Uptake
Figure 4: Methanol Uptake
3.3 Ion Exchange Capacity
Ion exchange capacity was conducted to determine
the delivery of ions on the membrane. Therefore, an
ion exchange capacity measurements are performed
to compare ion delivery each variable of zeolite.
Figure 5 showed the chitosan-vanillin membrane
with zeolite filler increase ion exchange capacity at
concentration 3% with a value ion exchange capacity
is 1,1935 milliequivalent and then decreased at a
concentration of 6% of zeolite. Chitosan-vanillin
filler zeolite bond makes new proton pathways.
However, the silica content of zeolite reduced water
molecules causes a lack of proton transport — vehicle
mechanism which one requires water molecules to
formed H3O+ for delivering a proton (Wang 2008).
Electrolyte Membrane Composite from Modified Chitosan-Vanillin and Zeolite Filler for Direct Methanol Fuel Cell Application
97
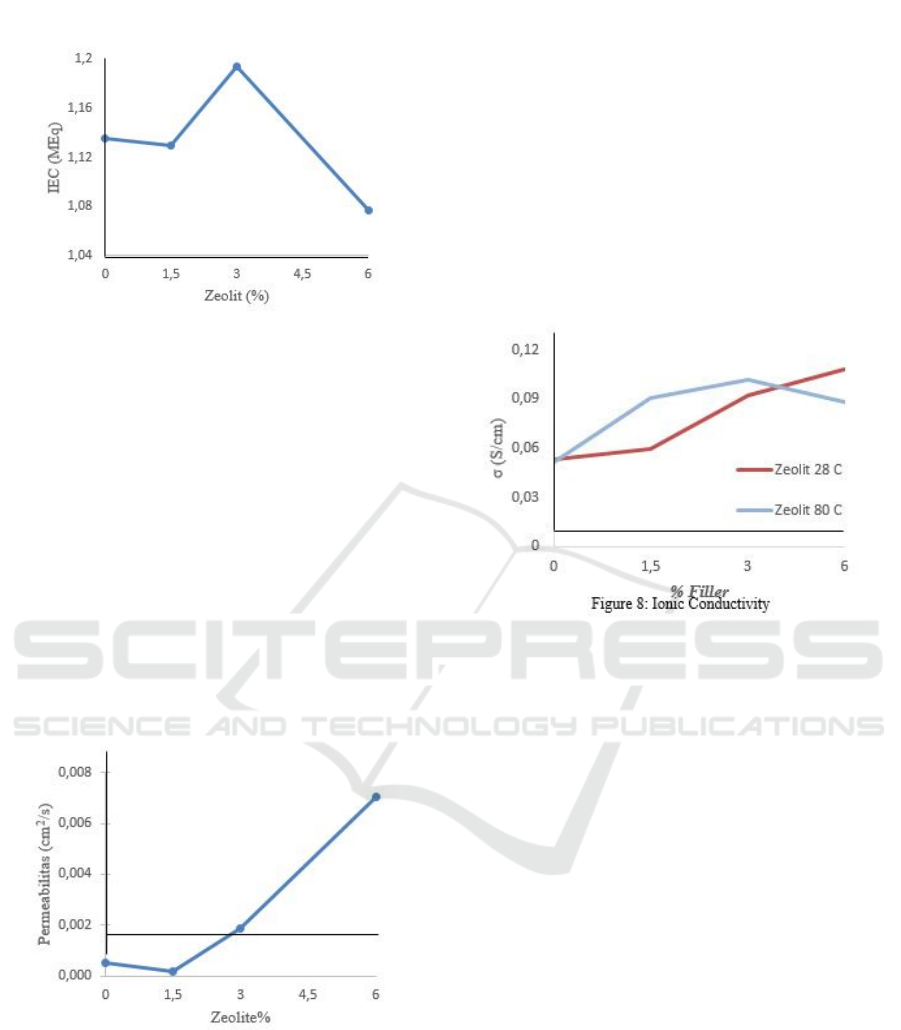
Figure 5: Ion Exchange Capacity
3.4 Methanol Permeability
This membrane will be applied for direct methanol
fuel cells that methanol as the main fuel. The low
permeability of methanol generates high fuel
efficiency and reduce fuel loss. And methanol
permeability is expected to be low and does not
interfere with membrane performance.
Methanol permeability test can be linked with
methanol uptake. The results were not enough to
outrun the Nafion's. From figure 6, the best result was
only 1,875, E-04 cm2/s by CV-Z 3%. Comparable
with Wang (2010), high zeolite content can be
affected by zeolite molecules because it has a low
affinity for each other enlarges membrane pores.
Figure 6: Methanol Permeability
Such a membrane could absorb the amount of
methanol that would make this membrane-less
efficient. Also, from IR spectra result was also
affecting the result. These results concluded that the
addition of silica content was one method to reduce
the permeability of the membrane. Despite this
successfully reducing methanol permeability, the
optimization should be conducted for later research.
3.5 Ionic Conductivity
This membrane will be applied to direct methanol
fuel cells, which are used as methanol to produce
electrical energy. The membrane functions as a
conduit of H+, which will then react with O2 and
produce H2O. So, the proton conductivity test is
needed to determine the ability of the membrane to
deliver H+.
Figure 7: Ionic Conductivity
As shown in figure 7, at 28C ionic conductivity
increased along with zeolite concentration additional.
Zeolites provide hydroxyl groups as pathways proton
jumping mechanism. Higher temperature causes the
polymer chain to fluctuate, causing a movement to the
electrolyte group (Salman, 2018). Especially
hydroxyl and phenolic groups in the membrane
matrix. This movement causes the transferring proton
at 80C is better than 28C. However, at 80C,
with a 6% zeolite concentration, ion conductivity was
decreased because high temperature is the main
problem for water content at the membrane. Proton
delivery in-vehicle mechanism requires water
molecules to delivers protons. This phenomenon was
previously known by the measurement of ion
exchange capacity. It has a connection to the delivery
of protons.
4 CONCLUSIONS
Chitosan-Vanillin with zeolite membrane for fuel cell
application was prepared with the addition of zeolite
content. The best composition for this research for
water uptake at CV-Z 1.5% with 28.834%, methanol
uptake at CV-Z 6% with 29.03226%, ion exchange
ICONIT 2019 - International Conference on Industrial Technology
98

capacity at CV-Z 3% with 1.1935 MEq, proton
conductivity at KV-Z 6% with 0.088474546 S / cm,
and permeability of 3% KV-Z methanol with 1,875 X
10-4 cm2 / S. The best composition in this research
are methanol uptake and water uptake because the
parameter decreases the point near 30% as the best
composition. But needs optimization, especially in
methanol permeability, because it is still higher than
Nafion, which is one essential parameter for the fuel
cell.
ACKNOWLEDGMENTS
The authors gratefully acknowledge for LPPM
Kalimantan Institute of Technology for supporting
this research. We also regard the Bandung Institute of
Technology for funding and facilities provided.
REFERENCES
Antony,R., Arun, T., dan Theodore, D. M. S. (2019), A
Review On Applications of Chitosan-Based Schiff
Bases, International Journal of Biological
Macromolecules vol. 159, hal. 615-633.
Beauchamp, J.L., dan Wight, C.A. (1981), Infrared Spectra
of Gas-Phase Ions and Their Use inElucidating
Reaction Mechanisms. Identification of C7H7-
Structural Isomers by Multiphoton Electron
Detachment Using a Low-Power Infrared Laser,
Journal of American Chemical Society vol.21, hal.
6499-6501.
Dwivedi C , Pandey I, Pandey H, et al., Electrospun
Nanofibrous Scaffold as a Potential Carrier of
Antimicrobial Therapeutics for Diabetic Wound
Healing and Tissue Regeneration, Elsevier, Chapter 9
Fitri Kurnia S, W. Nurul, (2016), Penambahan Polieteriida
pada Membran Komposit kitosan/zeolit-A untuk
meningkatkan kinerja membran PEMFC, Fakultas
Kimia dan Ilmu Pengetahuan Alam, Institut Teknologi
Sepuluh November.
Komi, Daniel, E.A., dan Hambiln, M.R. (2016), Chitin and
Chitosan: Production and Application of Versatile
Biomedical Nanomaterials, Int J Adv Res (Indore), vol.
4, issue 3, hal. 411–427.
Pramono, E, Prabowo, A, Purnawan, C. (2012), Pembuatan
Dan Karakterisasi Kitosan Vanilin Sebagai Membran
Polimer Elektrolit, Jurnal penelitian kimia, vol. 8, no. 1,
hal. 70-78.
Pramono, E., Purnawan, C., Hidayat, Y., Wulansari, J., dan
Wahyuningsih, S. (2014), Komposit Kitosan
Vanilin/Polistirena Tersulfonasi Sebagai Membran
Polimer Elektrolit: Kapasitas Tukar Kation, Derajat
Pengembangan Dan Sifat Termal, ALCHEMY jurnal
penelitian kimia, vol. 10, no. 2, hal.116-129.
Purwanto, Atmaja et.al, (2016), Biopolymer-based
electrolyte membranes from chitosan incorporated with
montmorillonite-crosslinked GPTMS for direct
methanol fuel cell, Journal RSC Advances, 2314-2315.
Republika. (2013). IEA: Penggunaan Bahan Bakar Fosil
Global Tetap Dominan . Dikutip 8 Agustus 2019 dari
Republika.c.id:https://www.republika.co.id/berita/eko
nomi/bisnis-global/13/11/14/mw8f2s-iea-penggunaan-
bahan-bakar-fosil-global-tetap-dominan
Saikia, G. Parthasarathy, (2010), Fourier Transform
Infrared Spectroscopic Characterization of Kaolinite
from Assam and Meghalaya, Journal Scientidic
Research, 206-210.
Salman, Y. A. K., Abdullah, O.G., Rawad R. H., dan
Shujahadeen B. A. (2018), Conductivity and Electrical
Properties of Chitosan -Methylcellulose Blend
Biopolymer Electrolyte Incorporated with Lithium
Tetrafluoroborate, International Journal
Electrochemical Science., vol. 13, hal. 3185 – 3199.
Se-Kwon, (2011), Chitin, Chitosan, Oligosaccharides and
Their Derivatives, CRC Press.
Wang Y, Jiang Z, Li H, (2010), Chitosan membranes filled
by GPTMS-modified zeolite beta particle with low
methanol permeability for DMFC, Elsevier, 278-285.
Wang Y, Yang D, Zheng X, Yi Z, Li ., (2008), Zeolite beta-
filled chitosan membrane with low methanol
permeability for direct methanol fuel cell, Elsevier,
454-463.
Electrolyte Membrane Composite from Modified Chitosan-Vanillin and Zeolite Filler for Direct Methanol Fuel Cell Application
99
