
The Symptoms-based Algorithm for Early Detection of Systolic Heart
Failure
Krishna Ari Nugraha
1,2
, M. Rizki Fadlan
1,2
, Dea Arie Kurniawan
1,2
, Liemena Harold Adrian
1,2
,
Faris Wahyu Nugroho
1,2
, Puspa Lestari
1,2
, Seprian Widasmara
1,2
, Anita Surya Santoso
1,2
and Mohammad Saifur Rohman
1,2
1
Department of Cardiology and Vascular Medicine, Faculty of Medicine, Brawijaya University – Dr. Saiful Anwar General
Hospital, Malang, East Java, Indonesia
2
Brawijaya Cardiovascular Research Center, Brawijaya University, Malang, East Java, Indonesia
{krishnaari22, naldaf, deaariekurniawan, liemenaharold, fariswahyunugroho, pusparyath, seprian.w,
Keywords: Heart Failure, Algorithm, Self-assessment.
Abstract: Heart failure is one of the global health problem priorities and is largely caused by late recognition of the
symptoms. Early detection is paramount to diagnosing heart failure; thus, a simplified algorithm is required.
The objective is to examine the accuracy of a symptoms-based algorithm for early detection of systolic heart
failure. We developed a symptom-based algorithm, compared to typical echocardiography examination. The
algorithm model in this study consisted of four symptoms with the highest association to systolic heart failure.
To evaluate outcomes in a larger population, we performed the derivation phase to assess the sensitivity and
specificity of this algorithm. The derivation phase was tested on 477 heart failure patients. All symptoms in
the algorithm—dyspnoea on exertion (DOE), paroxysmal nocturnal dyspnoea (PND), orthopnoea and leg
oedema—occur significantly more often among patients with systolic heart failure, compared to those with
diastolic heart failure (p < 0.05). The algorithm obtained an area under the curve and gave a sensitivity of
83.9% and a specificity of 81.1%. The symptom-based algorithm provides good outcomes for early detection
of systolic heart failure and are feasible to be developed into a self-assessment application for heart failure
patients with reduced ejection fraction.
1 BACKGROUND
Heart failure is one of the global health problem
priorities because of the high morbidity and mortality
among sufferers (Ponikowski et al., 2014; Tripoliti et
al., 2017; Ponikowski et al., 2016). Currently, at least
26 million people in the world are living with heart
failure. In Indonesia, based on data from Riset
Kesehatan Dasar (Riskesdas, 2018), the prevalence of
heart failure is 0.3%. This number will continue to
grow, along with the increasing prevalence of heart
failure risk factors, such as diabetes and hypertension.
Heart failure also contributes to increasing the burden
of national healthcare costs every year.
Although it has such a large impact on the health
burden in society, awareness of heart failure is poor.
Thus, numerous premature deaths occur, even though
most types of heart failure are preventable and a
healthy lifestyle can further reduce risk. The
incidence of premature deaths could be prevented if
people have an understanding of how to recognize
symptoms and seek immediate medical attention,
even after heart failure has developed (Ponikowski et
al., 2014; Conrad et al., 2018; Devroey and Van
Casteren, 2011).
There is little development in improving the
progression of heart failure severity, which is largely
due to non-effective approaches for early detection of
heart failure in testing interventions. Lifestyle and
pharmacologic interventions may be effectively
developed by analysing the early detection of heart
failure (Devroey and Van Casteren, 2011; Roberts et
al., 2015). Unfortunately, heart failure is a clinically
complex and heterogeneous disease that is
challenging to detect in routine care due to the
diversity of alternative explanations for symptoms. A
simple algorithm is required to help the general
population be able to early detect heart failure based
38
Nugraha, K., Fadlan, M., Kurniawan, D., Adrian, L., Nugroho, F., Lestari, P., Widasmara, S., Santoso, A. and Rohman, M.
The Symptoms-based Algorithm for Early Detection of Systolic Heart Failure.
DOI: 10.5220/0009388300380041
In Proceedings of the 4th Annual International Conference and Exhibition on Indonesian Medical Education and Research Institute (The 4th ICE on IMERI 2019), pages 38-41
ISBN: 978-989-758-433-6
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
on symptoms by using smartphone applications (Van
Riet et al., 2016).
2 METHOD
The data for this study were collected from the Saiful
Anwar Hospital Heart Failure Registry between 2016
and 2019. All patients hospitalized with the diagnosis
of heart failure were eligible for this study. We
included a patient with documented heart failure who
had an echocardiography examination performed
during hospitalization. All study participants were
given informed consent.
This study used a data survey of patients,
including history, physical examination, 12-lead
ECG and chest X-ray at the time of the initial patient
examination. Patients received an echocardiography
examination. The quantitative two-dimensional
(Biplane or Simpson) method was to assess the left
ventricular ejection fraction (LVEF) of the patients in
the study. Patients with LVEF ≤40% were
categorized as systolic dysfunction, while those who
had LVEF >40% were classified as diastolic
dysfunction, and preserved EF further was grouped as
diastolic heart failure group.
The investigators were
blinded to the echocardiography results, and they
reviewed all medical records pertaining to the patient
after completion of the clinical evaluation described
above.
To determine the symptoms with the highest
association to the diagnosis of systolic heart failure,
we performed a backward stepwise logistic
regression, with the diagnosis of heart failure based
on LVEF data as a dependent variable. The
symptoms with the highest association that were
found to be significant were dyspnoea on exertion
(DOE), paroxysmal nocturnal dyspnoea (PND),
orthopnoea and leg oedema. We built and validated
an algorithm model on our prior study from the basis
of these four symptoms with the highest association
to systolic heart failure. To evaluate outcomes in a
larger population, we performed the derivation
phase to assess the sensitivity and specificity of this
algorithm.
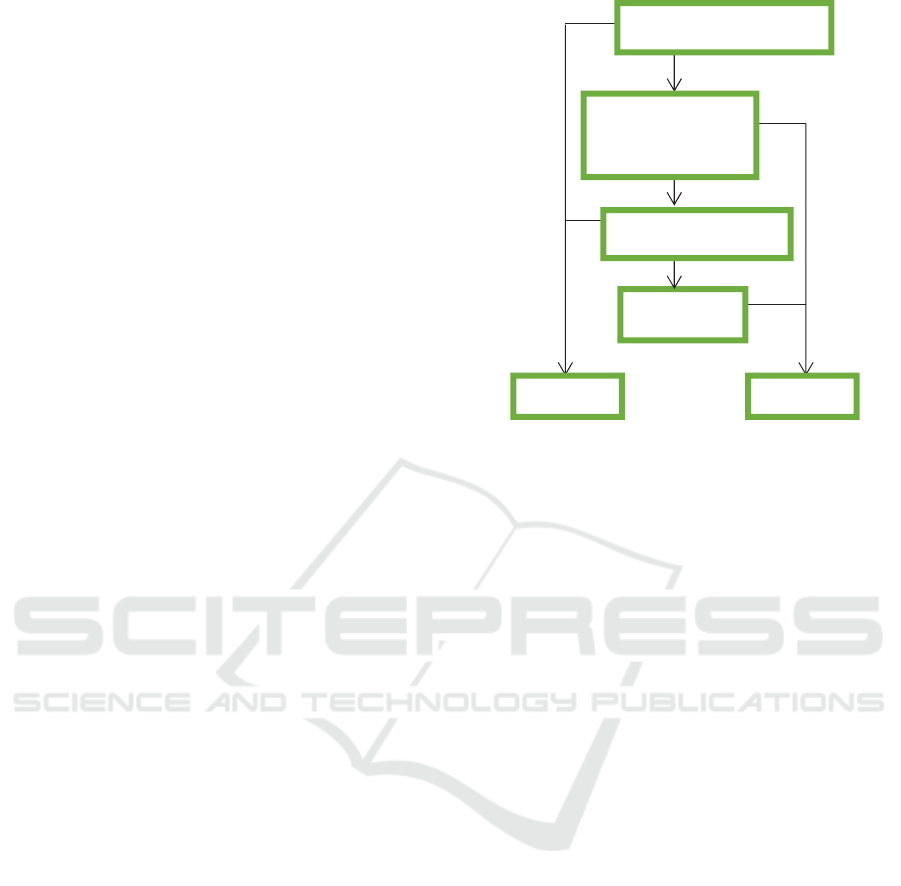
Figure 1: Symptom-based algorithm for systolic heart
failure.
3 RESULTS
This study was the derivation phase of our prior
study. We tested the algorithm on 477 heart failure
patients derived from the Saiful Anwar Hospital
Heart Failure Registry between 2016 and 2019. In this
study, 284 patients were male and there was no
significant difference in mean age between the two
groups. The average age of the systolic heart failure
group was 56.05 years (SD = 11.8), and 56.59 years
(SD = 13.2, P = 0.02) was the average age of those
with diastolic heart failure (table 1).
Traditional risk factors associated with systolic
HF analysed in this study include diabetes mellitus
type II, a history of coronary artery disease (CAD),
impaired renal function and the presence of atrial
fibrillation. Statistically, the frequency of the four risk
factors analysed in the two study groups was not
significantly different (p > 0.05). In this study,
patients with type II DM and atrial fibrillation were
more prevalent in the systolic heart failure
population, whereas CAD and kidney function
disorders were more prevalent in patients with
diastolic heart failure.
Algorithms that have been created based on tests
in the previous validation phase include four
symptoms: dyspnoea on exertion (DOE), paroxysmal
nocturnal dyspnoea (PND), orthopnoea and leg
oedema. This algorithm was then tested in the study
population in the derivation phase, and it appears that
patients with systolic heart failure had experienced all
yes
yes
yes
yes
no
no
no
Orthopnoea
Diastolic Systolic
Paroxysmal
nocturnal
dyspnoea
Dyspnoea on exertion
Bilateral leg edema
The Symptoms-based Algorithm for Early Detection of Systolic Heart Failure
39

four symptoms more often than others with systolic
heart failure, compared to those with diastolic heart
failure (p < 0.05).
Table 1: Patient characteristics according to HF
classification (n = 477).
Demographic
and clinical
features
HF classification P
value
Systolic HF Diastolic HF
Men/ women 131/61 153/132 0.02
Age (years) 56.05(±11.8) 56.59(±13.2) 0.65
DM (%) 33.5 30.5 0.36
Known CAD
(%)
27.7 33.1 0.21
Renal
dysfunction
(%)
1 3.2 0.12
AFib (%) 18.3 16.4 0.64
Symptoms
(%)
DOE 93.8 47 0,00
PND 88 8.1 0,00
Orthopnea 68.2 46 0,00
Leg edema 43.8 26 0,00
Figure 2: ROC of the derivation phase.
As the final result from the receiver operating
curve analysis obtained area under the curve (AUC)
0.895 (95% CI, 0.867-0.922) the value obtained is
better than the results of the previous validation
phase. This algorithm provides a sensitivity of 83.9%
and a specificity of 81.1%.
4 DISCUSSION
Heart failure is one of the main cardiovascular
diseases in the world and has also become a major
concern in developing countries. The outreach of the
health system and the lack of awareness in the
population of cardiovascular risk factors results in
treatment delays, which ultimately increases
mortality. As shown in Devroey and Van Casteren's
study (2011), heart failure is one of the problems in
primary health care that requires careful history-
taking and physical examination.
Unfortunately, the symptoms of heart failure are
very diverse, so additional modalities are needed for
diagnosis. The algorithm to diagnose heart failure
according to ESC guidelines in a non-acute setting is
based on the previous clinical history of the patient,
the presenting symptoms, physical examination and
resting ECG. If all elements are normal, heart failure
is impossibly found in the patients. Plasma
Natriuretic Peptides should be measured when one
element is detected as being abnormal. This
measurement provides the opportunity for doctors to
detect those patients who need an echocardiography.
Clinical symptoms, such as dyspnoea on exertion
(DOE), paroxysmal nocturnal dyspnoea (PND),
orthopnoea and leg oedema, are arranged in an
algorithm, giving AUC 0.895 (95% CI, 0.867-0.922).
This is consistent with Devroey and Van Casteren's
study that DOE and leg oedema are heart failure
predictors with good sensitivity and specificity. The
combination of peripheral oedema, breathlessness on
exercise and pulmonary rales had good specificity to
detect heart failure, but low sensitivity.
This study attempted to create a simpler approach
with the symptom-based diagnostic tool so that
patients are able to do an accurately self-assessment
and then quickly seek medical advice. This symptom-
based algorithm was developed in an effort to
facilitate the common population in recognizing heart
failure symptoms that they may have experienced
before.
With high sensitivity and specificity, this
algorithm is expected to be able to reduce the
diagnostic delay that has happened thus far. We
expect that the heart failure morbidity and mortality
rate could be reduced in the future. This algorithm is
also expected to be used as a simple tool to help
medical practitioners in the early detection of systolic
heart failure. For further research, this algorithm
model can be tested in the general population,
especially for primary healthcare patients, as a first-
line screening tool before using more advanced
diagnostic modalities.
5 CONCLUSIONS
The symptom-based algorithm provides good
outcomes for early detection of systolic heart failure.
The 4th ICE on IMERI 2019 - The annual International Conference and Exhibition on Indonesian Medical Education and Research Institute
40

With the massive development of smartphone-based
technology, this algorithm is feasible to be developed
into a self-assessment application for heart failure
patients with reduced ejection fraction.
REFERENCES
Conrad, N., Judge, A., Tran, J. K., Mohseni, H., Hedgecott,
D., Perez-Crespillo, A., … Rahimi, K. (2018).
Temporal trends and patterns in heart failure incidence:
A population-based study of 4 million individuals.
The Lancet, 391(10120), P572–P580. http://doi.org/
10.1016/S0140-6736(17)32520-5
Devroey, D., and Van Casteren, V. (2011). Signs for early
diagnosis of heart failure in primary health care.
Vascular Health and Risk Management, 2011(7), 591–
596. http://doi.org/10.2147/VHRM.S24476.
Ponikowski, P., Anker, S. D., Al Habib, K. F., Cowie, M.
R., Force, T. L., Hu, S., … Filippatos, G. (2014). Heart
failure: Preventing disease and death worldwide. ESC
Heart Failure, 1(1), 4–25. http://doi.org/10.1002/
ehf2.12005.
Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H.,
Cleland, J. G. F., Coats, A. J. S., … van der Meer, P.
(2016). 2016 ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure: The Task
Force for the diagnosis and treatment of acute and
chronic heart failure of the European Society of
Cardiology (ESC). European Heart Journal, 37(27),
2129–2200. http://doi.org/10.1093/eurheartj/ehw128
Roberts, E. J., Ludman, A. R., Dworzynski, K. J.,
Al-Mohammad, A. undefined, Cowie, M. undefined,
McMurray, J. undefined, and Mant, J. undefined.
(2015). The diagnostic accuracy of the natriuretic
peptides in heart failure: Systematic review and
diagnostic meta-analysis in the acute care setting.
BMJ, 350 (mar04 22), h910. http://doi.org/10.1136/
bmj.h910.
Tripoliti, E. E., Papadopoulos, T. G., Karanasiou, G. S.,
Naka, K. K., and Fotiadis, D. I. (2017).
Heart Failure: Diagnosis, Severity Estimation and
Prediction of Adverse Events Through Machine
Learning Techniques. Computational and Structural
Biotechnology Journal, 15, 26–47. http://doi.org/
10.1016/j.csbj.2016.11.001
van Riet, E. E., Hies, A. W., Limburg, A., Landman, M. A.,
Kemperman, H., and Rutten, F. H. (2016). Extended
prediction rule to optimise early detection of heart
failure in older persons with non-acute shortness of
breath: A cross-sectional study. BMJ Open, 6(2),
e008225. http://doi.org/10.1136/bmjopen-2015-008225.
The Symptoms-based Algorithm for Early Detection of Systolic Heart Failure
41
