
Characterization and Prospect of Irradiated Chitosan as Nano
Complex Material to Deliver MicroRNA in Cancer Therapy
Firasti Agung Nugrahening Sumadi
1
*, Dian Pribadi Perkasa
2
, Tirta Wardana
3
, Ronny Martien
4
and
Sofia Mubarika Harjana
5
1
Pharmacy Department, University of Muhammadiyah Malang, Jalan Bendungan Sutami 188 A, Malang, East Java,
Indonesia 65145
2
Center for Application of Isotopes and Radiation, National Nuclear Energy Agency, Jalan Kuningan Barat, Mampang
Prapatan, Jakarta, Indonesia 12710
3
Department of Medicine, Universitas Jendral Soedirman, Jalan Dokter Medika, Purwokerto, Central Java, Indonesia
53122
4
Faculty of Pharmacy, Universitas Gadjah Mada, Jalan Sekip Utara, Senolowo, Sleman, Yogyakarta, Indonesia 55281
5
Department of Histology and Cell Biology, Faculty of Medicine, Universitas Gadjah Mada, Jalan Farmako, Senolowo,
Sleman, Yogyakarta, Indonesia
.
55281
Keywords: Irradiated Chitosan; Nano complex; MicroRNA
Abstract: Chitosan is odorless white powder derived from the partial deacetylation of chitin which is a polysaccharide
consisting of glucosamine and N-acetylglucosamine. Chitosan is commercially available in several types
and has molecular weights that vary between 10,000 and 1,000,000. Chitosan has positively charged basic
chain that can easily form nano complex with nucleic acid in this case including negatively charged
microRNA. MicroRNA (miRNA) has a large role in the regulation of cancer signaling tissue so that a
therapeutic approach is needed to restore the balance of dysregulated miRNA. The nature of microRNA
which is very susceptible to enzyme degradation requires a special system so that it is competent to deliver
microRNA into the cytoplasm. One of the factors that influence the efficiency of transfection of chitosan
nano complex with a nucleic acid to body cells is molecular weight. In this research, the chitosan molecular
weight reduction method was carried out to increase nano complex delivery using gamma-ray irradiation.
Furthermore, characterization was carried out to determine the irradiated chitosan molecular weight using
intrinsic viscosity then proceed with FTIR analysis to determine changes in chemical structure and applied
further by using it in nano complex formulations with microRNA 155-p, a microRNA that experienced
downregulation in ovarian cancer thus requiring mimic therapy. Results showed a decrease in chitosan
molecular weight after being irradiated from 110,188 dalton to 15,209 dalton while FTIR spectra showed a
break of the 1-4 glycoside bonds which was equivalent to the severance of the main chain of
polysaccharides. Electrophoresis results showed that irradiated chitosan was able to form nano complex
with 155-5p microRNA but transfection was not able to deliver 155-5p microRNA into the SKOV3 ovarian
cancer cells.
1 INTRODUCTION
Chitosan in the form of odorless white powder
derived from the partial deacetylation of chitin
which is a polysaccharide consisting of glucosamine
and N-acetylglucosamine. Chitosan is commercially
available in several types and has molecular weights
that vary between 10,000 and 1,000,000. The
general function of chitosan is as a coating agent,
disintegrant, film-forming agent, mucoadhesive,
tablet binder, and viscosity-enhancing agent.
Chitosan has been processed into several dosage
forms including gels, films, beads, microspheres,
tablets, and coatings for liposomes (Rowe, Sheskey,
Owen, & American Pharmacists Association, 2009).
Chitosan is a polymer that has a positive charge
that strong enough to form nano-sized complexes
with nucleic acids that have opposite charges,
Sumadi, F., Perkasa, D., Wardana, T., Martien, R. and Harjana, S.
Characterization and Prospect of Irradiated Chitosan as Nano Complex Material to Deliver MicroRNA in Cancer Therapy.
DOI: 10.5220/0009127001910196
In Proceedings of the 2nd Health Science International Conference (HSIC 2019), pages 191-196
ISBN: 978-989-758-462-6
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
191

including miRNA. MiRNA can experience
upregulation and downregulation in various cancer
cases (Kinose et al., 2014). MiRNA-based therapies
such as mimic miRNA (artificial miRNA) are used
to restore the function of miRNA that is lost due to
its low expression (Tyagi et al., 2016). Chitosan-
miRNA nanoparticles can increase cellular uptake
by binding to negatively charged cell membranes
and protecting miRNA from endogenous nuclear
digestion (Chen et al., 2014).
There are several factors that affect the
transfection efficiency of chitosan nanoparticles with
nucleic acids such as molecular weight, degree of
deacetylation, n/p ratio, nucleic acid concentration,
nucleic acid dose, pH of transfection media, serum
content, stability of nucleic acid and chitosan
complexes, toxicity of chitosan vector, chitosan
modification to facilitate transfection, and the type
of cell that is transfected. The smaller molecular
weight of chitosan will produce smaller chitosan-
nucleic acid complexation, but chitosan with a larger
molecular weight can bind plasmids more efficiently
(Raftery et al., 2013)
The gamma irradiation factor (γ) used in this
study to reduce the molecular weight of chitosan
also includes methods for the process of sterilizing
pharmaceutical products (Desai & Park, 2006).
However, irradiation will affect the performance of
the drug delivery system so characterization is
needed to determine its properties. Determination of
molecular weight is conducted to prove the
irradiation process can break chemical bonds
between chitosan polymers so that the molecular
weight becomes smaller.
2 METHODS
Irradiated chitosan characterization
Medical grade Chitosan (PT Biotech Surindo) was
dissolved became 1% chitosan solution using 100%
acetic acid as much as 100 ml. Chitosan 1% solution
is then irradiated with gamma-rays at a dose of 5
kGy. Then the radiation results are diluted into
0.01%, 0.05%, 0.1%, 0.15%. and 0.2% chitosan
solution. Irradiated chitosan characterization is done
by calculating intrinsic viscosity and FTIR test.
Intrinsic viscosity is then included in the sakurada
mark-houwink equation ɳ = KMα to obtain
molecular weights with K = 9.66x10-5 (dm3 / g) and
α = 0.742. FTIR testing was carried out with IR-
Prestigo - ZI Shimadzu serial A210048-02492 and
measured using the % transmittance measurement
model, resolution = 2, apolization = hap genzel, total
af.scan = 20, measurement distance = 400-4000 (cm-
1 ) (Sionkowska et al., 2013).
Chitosan nanoparticle
0,05% chitosan solution was prepared using acetate
buffer pH 5. Nanoparticles were prepared by mixing
500 ul mimic miRNA 155-5p 2 µM with 500 μl
0.05% irradiated chitosan (Martien, 2009).
Irradiated chitosan nanoparticle and transfection
40,000 SKOV3 ovarian cancer cell cultures (got
from the KALBE Stem Cell and Cancer Institute)
were planted on a 24-well plate that had been coated
with a borosilicate glass cover. Cells were
transfected with nanoparticles with miRNA that had
been labeled with FAM (green fluorescence) at 4
and 48 hours. Cell nuclei were stained with DAPI
blue fluorescence (4 ', 6-diamidino-2-phenylindole)
(Chen et al., 2014; Ji et al., 2009)
3 RESULTS AND DISCUSSION
Irradiated chitosan characterization
High molecular weight chitosan is widely used in
several biological applications. However, in many
cases, the application of this polysaccharide is
hampered due to high molecular weight which
causes low solubility in water-based media
(Czechowska-Biskup et al., 2005; Minagawa et al.,
2007). Some specific applications use chitosan
degradation products that are considered more
useful. Some degradation methods that can be used
are enzymatic, chemical or radiation. Degradation
using radiation was chosen because it is simple and
very environmentally friendly because it does not
require initiator and by product .
Irradiated chitosan is assumed to undergo cutting
off the main polysaccharide group so that it
produces a smaller molecular weight compared to
non-irradiated chitosan. Determination of molecular
weight is conducted by finding the average
molecular weight of viscosity. The results of the
relative viscosity obtained using the Ostwald
viscosity method. The result then processed to
determine the reduction viscosity and inherent
viscosity (table 1).
HSIC 2019 - The Health Science International Conference
192
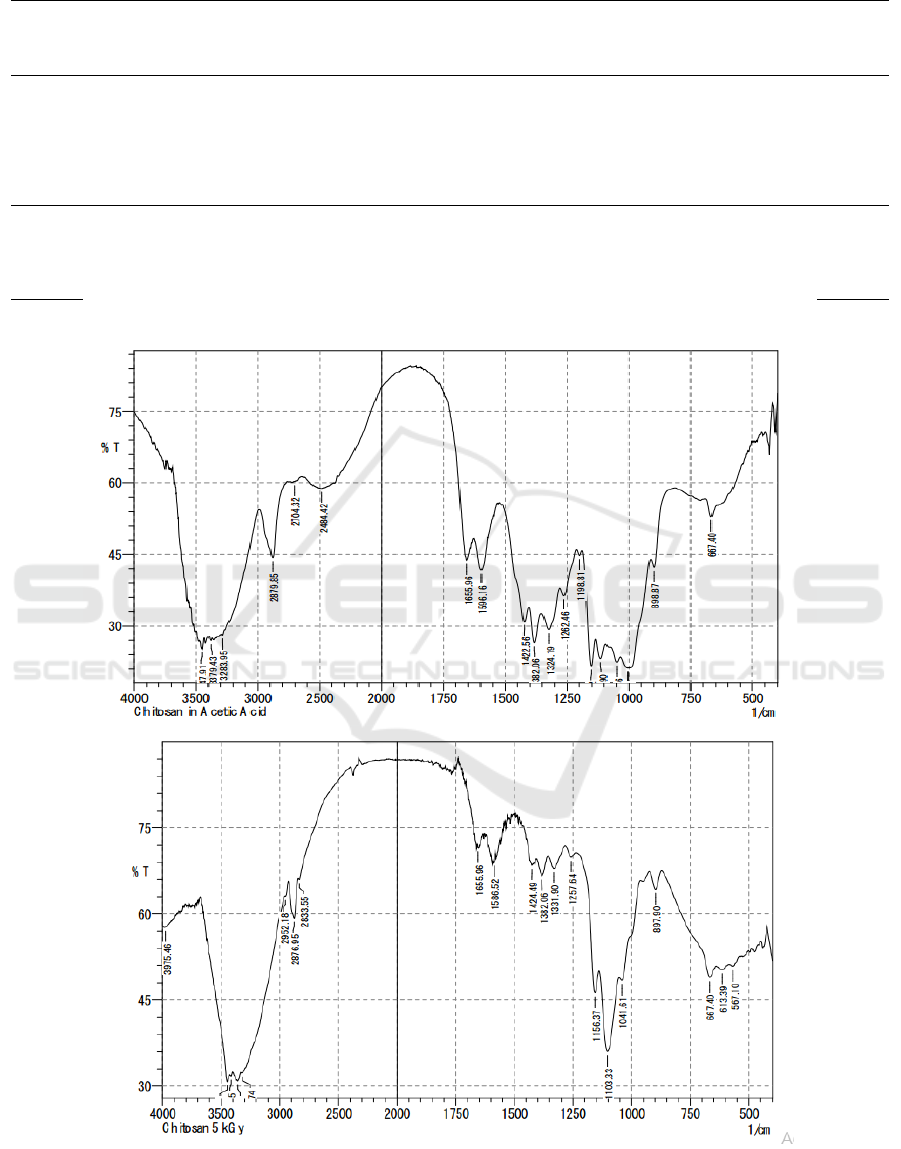
Table 1: Reduction viscosity and inherent viscosity of non-irradiated chitosan and 5kGy irradiated chitosan.
Concen
tratiom
(g/dL)
Flow time of
sample+solvent
(t) sec
Flow
time of
solvent
(to)
ɳr = t/to
ɳsp = ɳr
– 1
Reduction
viscosity,
ɳ
red
= ɳsp
/ c
[ɳ
red
]
ln ɳr
Inherent
viscosity,
ɳ
inh
=ln ɳr/c
5kGy
Irradiated
chitosan
0.0001
30.47111
29.166
1.044748
1.044748
10447.48
10447.48
0.043775
437.7541078
0.0005
30.19
29.166
1.035109
0.035109
70.21875
70.21875
0.034507
69.01419285
0.001
31.052
29.166
1.064664
0.064664
64.66434
64.66434
0.06266
62.65957125
0.0015
31.4
29.166
1.076596
0.076596
51.06402
51.06402
0.073804
49.20283042
0.002
32.06
29.166
1.099225
0.099225
49.61256
49.61256
0.094605
47.30274995
0.0015
30.16
29.166
1.034081
0.034081
22.72052
22.72052
0.033513
22.34193057
0.002
30.92
29.166
1.060139
0.060139
30.06926
30.06926
0.0584
29.19978821
Non-
irradited
chitosan
0.0001
31.66
29.166
1.085511
0.085511
855.1053
855.1053
0.08205
820.5040716
0.0005
35.966
29.166
1.233148
0.233148
466.2964
466.2964
0.20957
419.1408009
0.001
44.66
29.166
1.531235
0.531235
531.235
531.235
0.426075
426.0745991
0.0015
54.408
29.166
1.86546
0.86546
576.9732
576.9732
0.623508
415.6717031
0.002
64.782
29.166
2.221148
1.221148
610.574
610.574
0.798024
399.0120699
Figure 1: FTIR result of (a) non-irradiated chitosan and (b) 5kGy irradiated chitosan.
a
b
Characterization and Prospect of Irradiated Chitosan as Nano Complex Material to Deliver MicroRNA in Cancer Therapy
193
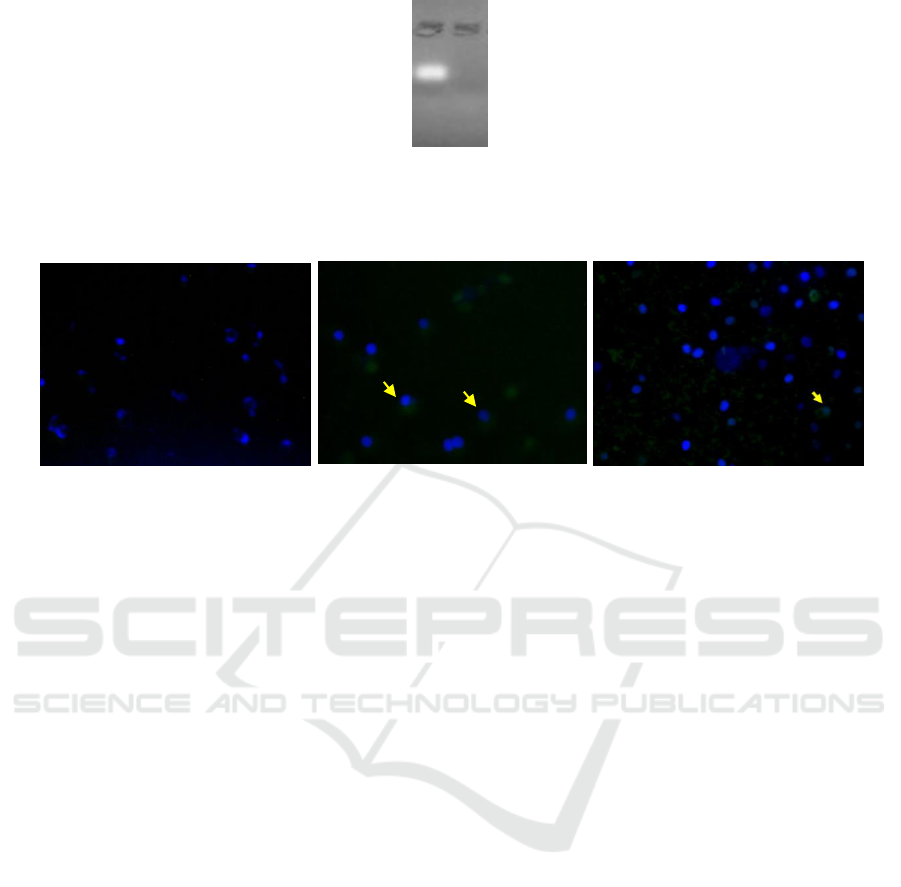
Figure 2: Agarose Electrophoresis Inhibition Test (a) naked miRNA-155 5p (b) Nanoparticle Chitosan irradiated-mimic
miRNA 155-5p
Figure 3: Transfection result of irradiated chitosan-miRNA nanoparticle to SKOV3 cell culture. (a) naked miRNA;
(b) irradiated chitosan-miRNA nanoparticle after 4 hours transfection; and (c) irradiated chitosan-miRNA nanoparticle after
48 hours transfection.
The intercept results from reduction viscosity
and inherent viscosity is intrinsic viscosity (ɳ) which
is then calculated in the Mark-Houwink Sakurada
equation ɳ = KM
α
with the constant for chitosan K =
9.66x10-5 (dm
3
/ g) and
α
= 0.742 to obtain average
molecular weight. The result shows the average
molecular weight of non-irradiated chitosan is
110,188 Dalton while the average molecular weight
for 5 kGy irradiated chitosan is 15,209 Dalton.
FTIR method is used to identify changes in
chemical groups that occur in irradiated chitosan.
From the spectra, we obtained a strong peak
absorption in 1650 cm
-1
area which shows the
presence of carbonyl groups (fig 1a and 1b). There
are also OH groups illustrated with peaks in the
2800 cm
-1
region. The ester group (C-O) is also
found by absorption in the 1100 and 1198 cm
-1
regions. Meanwhile, phenol alcohol groups were
seen from wide absorption in the area of 3300-3400
cm
-1
. The ether group (C-O) can be seen from the
absorption in the area of 1100 to 1300 cm
-1
. The
amino group is seen from a double peak in an area of
about 3400 cm
-1
(Silverstein et al., 2005). The
significance of irradiated and non-irradiated chitosan
spectra can be seen from the decrease in peak
absorption in the region of 1100-1300 cm
-1
.
Meanwhile, the uptake in the area of 1650 cm
-1
shows both maintain their aliphatic structure. The
results of the FTIR (Fourier Transform Infra-Red)
spectrophotometer between irradiated chitosan and
nonradiated chitosan showed similar peaks.
Identification of chitosan irradiation spectra that
is identical at the characteristic peaks shows that
there are no new chemical groups formed by gamma
irradiation. It can be said that gamma irradiation
does not induce cross-binding processes between
chitosan molecules. Although high energy gamma
radiation can react with chitosan groups that form
free radical groups, these high-energy groups cannot
easily interact because chitosan is at a solid level
(Desai & Park, 2006). The absorption of ionizing
radiation agents causes the localization of radical
elements in the carbon atoms C1 and C4, thus
breaking the glycoside bonds 1-4 which is
equivalent to breaking the main chain of the
polysaccharide. This can be seen clearly from the
reduction in irradiation chitosan peak spectra at 1100
cm
-1
to 1300 cm
-1
(fig 1a and 1b) compared to
irradiated chitosan which is a picture of the presence
of C-O groups (Rosiak et al., 1992). This scheme is
compatible with the polysaccharide degradation
scheme which causes a decrease in molecular weight
in chitosan irradiation.
a b
a
b
c
HSIC 2019 - The Health Science International Conference
194

Chitosan nanoparticle and efficacy of miRNA
transfection
The MiR-155-5p expression is known to
experience downregulation in advanced stages of
ovarian cancer compared to early stages and
followed by upregulation of HIF1α mRNA
expression (Chasanah et al., 2016). Mimic-miRNA
is given to ovarian cancer cells to improve the
dysregulation of miR-155 5p on SKOV3.
The results of the agarose electrophoresis
inhibition test showed that mimic-miR 155-5p
trapped in irradiated chitosan (fig.2) to form
nanoparticles so that there is no free miRNA band.
This shows that nano complex can be formed
between mimic-miR-155-5p with irradiated chitosan
(Kaban et al., 2017; Martien, 2009; Wu et al., 2016).
The nanoparticle uptake test in SKOV3 cell
culture was carried out by transfecting mimic-
miRNA 155-5p nanoparticles for 2 different period,
4 hours (fig. 3b) and 48 hours (fig. 3c). The
irradiated chitosan-mimic miRNA 155-5p
nanoparticle transfection test was conducted by
observing whether there was an accumulation of
mimic-miRNA 155-5p green fluorescence around
the SKOV3 cell nucleus that had been stained with
blue dye DAPI. The nanoparticle formula showed
that at least 155-5p mimic-miRNA entered the cell
at the 4
th
hour of transfection and decreased at the
48
th
hour.
Particle size and charge from the surface of the
nanoparticles play an important role in the uptake of
nanoparticles to the cell and the efficiency of the
transfection system (Wu et al., 2016). Chitosan
irradiation could reduce the surface charge of the
particles due to amino group termination during the
radiation process. The results of FTIR showed the
bias of the absorption of amino groups showed in the
region 3300-3400 cm
-1
. This shows that during the
irradiation process there was an interruption of the
NH
3
+
group which caused the chitosan to lose its
positive charge.
4 CONCLUSIONS
5kGy gamma-rays irradiated chitosan showed a
reduction in molecular weight so that the viscosity
of chitosan solution was decrease than non-
irradiated chitosan. Irradiation indicates a break in
the main polysaccharides chain of chitosan. mimic-
miRNA 155-5p nanoparticle formulation with
irradiated chitosan showed good results in the
electrophoresis test but not in transfection test.
Irradiated chitosan nanoparticles were not able to
bring mimic-miRNA into the cell. This is possible
because the irradiation process cuts off the amino
group which makes the chitosan charge more
negative and is unable to carry mimic-miRNA 155-
5p through the positively charged cell membrane.
ACKNOWLEDGMENTS
The author thanks SCI KALBE, Center for
Application of Isotopes and Radiation, National
Nuclear Energy Agency, Indonesia and also LPPT
UGM as the opportunity to conduct the research.
REFERENCES
Chasanah, S. N., Fitriawan, A. S., Pukan, F. K., Kartika,
A. I., Oktriani, R., Trirahmanto, A., … Haryana, S. M.
(2016). The expression of miRNA-155 and hypoxia
inducible factor alpha (HIF1A) mRNA in the early
and advanced stages of ovarian cancer patients blood
plasma. Journal of Thee Medical Sciences (Berkala
Ilmu Kedokteran), 48(04 (Suplement)), 22–22.
https://doi.org/10.19106/JMedScieSup004804201620
Chen, Y., Wang, S.-X., Mu, R., Luo, X., Liu, Z.-S., Liang,
B., … Li, T. (2014). Dysregulation of the MiR-324-
5p-CUEDC2 Axis Leads to Macrophage Dysfunction
and Is Associated with Colon Cancer. Cell Reports,
7(6), 1982–1993.
https://doi.org/10.1016/j.celrep.2014.05.007
Czechowska-Biskup, R., Rokita, B., Ulanski, P., &
Rosiak, J. M. (2005). Radiation-induced and
sonochemical degradation of chitosan as a way to
increase its fat-binding capacity. Nuclear Instruments
and Methods in Physics Research Section B: Beam
Interactions with Materials and Atoms, 236(1–4),
383–390. https://doi.org/10.1016/j.nimb.2005.04.002
Desai, K. G., & Park, H. J. (2006). Study of Gamma-
Irradiation Effects on Chitosan Microparticles. Drug
Delivery, 13(1), 39–50.
https://doi.org/10.1080/10717540500309123
Kaban, K., Salva, E., & Akbuga, J. (2017). In Vitro Dose
Studies on Chitosan Nanoplexes for microRNA
Delivery in Breast Cancer Cells. Nucleic Acid
Therapeutics, 27(1), 45–55.
https://doi.org/10.1089/nat.2016.0633
Kinose, Y., Sawada, K., Nakamura, K., & Kimura, T.
(2014). The Role of MicroRNAs in Ovarian Cancer.
BioMed Research International, 2014, 1–11.
https://doi.org/10.1155/2014/249393
Martien, R. (2009). Oral Delivery of Nucleic Acid Drugs.
In A. Bernkop-Schnürch (Ed.), Oral Delivery of
Macromolecular Drugs (pp. 223–236).
https://doi.org/10.1007/978-1-4419-0200-9_12
Minagawa, T., Okamura, Y., Shigemasa, Y., Minami, S.,
& Okamoto, Y. (2007). Effects of molecular weight
Characterization and Prospect of Irradiated Chitosan as Nano Complex Material to Deliver MicroRNA in Cancer Therapy
195

and deacetylation degree of chitin/chitosan on wound
healing. Carbohydrate Polymers, 67(4), 640–644.
https://doi.org/10.1016/j.carbpol.2006.07.007
Raftery, R., O’Brien, F., & Cryan, S.-A. (2013). Chitosan
for Gene Delivery and Orthopedic Tissue Engineering
Applications. Molecules, 18(5), 5611–5647.
https://doi.org/10.3390/molecules18055611
Rosiak, J., Ulanski, P., Dutkiewicz, J., & Judkiewicz, L.
(1992). Radiation Sterilization of Chitosan Sealant for
Vascular Prosthesis. 159 No. 1, 87–96.
Sionkowska, A., Płanecka, A., Lewandowska, K.,
Kaczmarek, B., & Szarszewska, P. (2013).
INFLUENCE OF UV-IRRADIATION ON
MOLECULAR WEIGHT OF CHITOSAN. 18, 21–28.
Tyagi, N., Arora, S., Deshmukh, S. K., Singh, S.,
Marimuthu, S., & Singh, A. P. (2016). Exploiting
Nanotechnology for the Development of MicroRNA-
Based Cancer Therapeutics. Journal of Biomedical
Nanotechnology, 12(1), 28–42.
https://doi.org/10.1166/jbn.2016.2172
Wu, G., Feng, C., Hui, G., Wang, Z., Tan, J., Luo, L., …
Chen, X. (2016). Improving the osteogenesis of rat
mesenchymal stem cells by chitosan-based-microRNA
nanoparticles. Carbohydrate Polymers, 138, 49–58.
https://doi.org/10.1016/j.carbpol.2015.11.044
HSIC 2019 - The Health Science International Conference
196
