
Forward Head Posture Examination and its Association to Lung
Expiratory Function in Chronic Obstructive Pulmonary Disease
(COPD) Patient: A Case Series
Siti Chandra Widjanantie
1
, and Kevin Triangto
2
1
Department of Physical Medicine and Rehabilitation Persahabatan Hospital, Jakarta, Indonesia
2
Department of Physical Medicine and Rehabilitation, Dr Cipto Mangunkusumo Hospital,
University of Indonesia, Jakarta, Indonesia
Keywords: Respiratory Disorder, Forward Head Posture, Lung Expiratory Function, Chronic Obstructive Pulmonary
Disease
Abstract: The expiratory function of the lung could be easily measured by using the peak flow meter, and is recorded
as a peak flow rate (PFR). This function has been known to be effectively correlated with mucus clearance
and effective cough. Other than general muscle weakness, COPD patients generally have altered body
structures to the chronic hyperventilation condition. Structural adaptations include thoracic kyphosis with
forward head posture (FHP). This study aimed to quantify the severity of FHP and observe its impacts on
PFR in COPD patients. We recruited a small cohort of COPD patients in the outpatient clinic of the Medical
Rehabilitation Department, Persahabatan Hospital, Jakarta. The peak flow meter will be used to measure
PFR, while FHP will be measured as occiput to wall distance, measured in centimeters. Additional records
such as submaximal exercise testing, peak cough flow (PCF) and COPD Assessment Test (CAT) score will
be obtained as well. An independent T-test will be performed on the data to obtain the difference of PFR
among severity grades of FHP. In this study, eight patients acquired, they were all above the age of 60,
classified as the geriatric population. We obtained underweight median Body Mass Index (BMI) 18.29
kg/m2 (15.05-22.04), COPD GOLD A to C, limited chest expansion, and median CAT score of 14 (4-30).
This study also exhibited a median OWD of 8.10 cm (6.80-9.30), PFR 227.50 ml (70-400), and PCF 255 ml
(180-410). These results showed that postural changes could simply be measured and may have an impact
on respiratory biomechanics, which deems comprehensive COPD care.
1 INTRODUCTION
It is a common knowledge that Chronic Obstructive
Pulmonary Disease (COPD) patients are very prone
to anatomical changes, owing to malnutrition and
ongoing hypercarbia, reducing effective muscular
metabolism. (Wada et al., 2016) In the rehabilitation
setting, a medical diagnosis of COPD will then lead
to several functional diagnoses such as general
muscle weakness, systemic endurance disorder,
postural imbalance, and airway clearance disorder.
Therefore nowadays, many interventions are then
focused more comprehensively to address these
matters.
Widjanantie, S. and Triangto, K.
Forward Head Posture Examination and its Association with Lung Expiratory Function in Chronic Obstructive Pulmonary Disease (COPD) Patient: A Case Series.
DOI: 10.5220/0009088602310237
In Proceedings of the 11th National Congress and the 18th Annual Scientific Meeting of Indonesian Physical Medicine and Rehabilitation Association (KONAS XI and PIT XVIII PERDOSRI
2019), pages 231-237
ISBN: 978-989-758-409-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
231
Among the known goals, postural correction
seems to be simple, yet remained to be a goal which
is rather difficult to maintain in COPD. Previous
studies had shown how thoracal kyphotic structures
are prevalent in COPD, and this phenomenon is
generally coupled with forward head posture (FHP).
(Kim et al., 2012) These anatomical changes are
then expected to impact respiration and additional
respiratory muscle functions as described in the
"Body Function" by the Comprehensive ICF core set
of COPD. Additionally, it will result in recruitments
of accessory inspiratory muscles, which in turn
increases energy expenditure for the tidal breathing
process. Previous studies had revealed how the FHP
will result in an expansion of the upper thorax, and
the indrawing of the lower thorax. As it turned out,
the immobility of the lower thorax could bring about
several drawbacks, such as reduction of diaphragm
excursion, resulting in ineffective breathing pattern.
(Koseki et al., 2019)
It is interesting to know that diaphragm itself lies
in the abdominal cavity, and thus movement of
abdominal muscles surely would affect its effective
moment arm. In that particular case, the central
nervous system modulation will counteract the
impulses required for effective abdominal muscle
contraction, without disturbing the rhythmicity of
diaphragmatic contraction. Several studies had
shown that straight posture with mild FHP would
correlate to better breathing mechanics, owing to the
ideal thoracic cage shape, leading to ease of
respiratory muscle recruitment, and effective
moment arm to exhibit good length-tension
relationship. (Mesquita Montes et al., 2017)
Several animal studies had shown that mucus
movement correlates positively with peak expiratory
flow, and the concept has been utilized in
personalizing the human airway clearance technique.
(McIlwaine et al., 2017; Mahajan et al., 2019) The
expiratory action itself would recruit abdominal
muscles during labored breathing, and it was shown
that several of these core muscles have dual roles in
both keeping the effective intrathoracic pressure, as
well as stable erect posture; thus these biomechanics
are disturbed in the presence of FHP. (Mesquita
Montes et al., 2017)
With all the questions that arise regarding the
impact of FHP on respiratory processes, we
established our research question into: is the severity
of FHP associated with pulmonary expiratory
function test values in COPD patients? In response
to the question, we hypothesize that patients with
severe FHP will have a worse expiratory function, as
FHP will cause a restrictive disorder that overlaps
with the obstruction in COPD patients, resulting in
an overall reduction in pulmonary function. It is our
general aim to exhibit the importance of postural
screening, especially in specific patient groups such
as COPD.
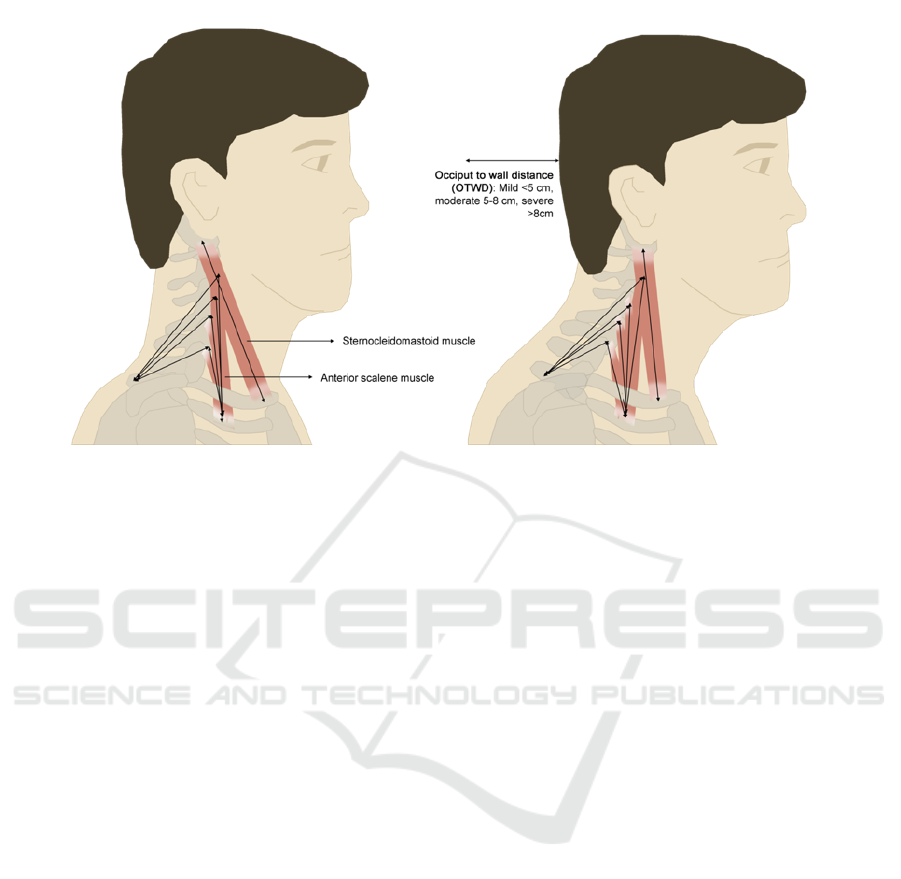
Figure 1: Forward Head Posture, changes in cervical muscles with Occiput to Wall Distance.
KONAS XI and PIT XVIII PERDOSRI 2019 - The 11th National Congress and The 18th Annual Scientific Meeting of Indonesian Physical
Medicine and Rehabilitation Association
232
2 METHODS
A case series observation was performed on COPD
patients who routinely checks up in the Medical
Rehabilitation outpatient clinic of Persahabatan
General Hospital, East Jakarta.
Through consecutive sampling, all patients ≥18
years old with COPD who could ambulate
independently, living in the community and
clinically stable over a month were recruited.
Adhering to prior study on posture, exclusion
criteria include previous thoracic or abdominal
surgery in the past one year, recurrent
musculoskeletal injury on the upper extremity,
previous mastectomy, and severe musculoskeletal,
neurological, or cardiovascular disorders. (Morais,
Cruz, and Marques, 2016) Additionally, we exclude
patients with a tracheostomy tube or oropharyngeal
disorders, which disallows patient to perform
adequate mouth seal on the mouthpiece, to preserve
optimal peak flow meter examination.
A comprehensive physical examination was
performed by two physiatrists and discussion will be
performed when there is any disagreement regarding
FHP severity. This study had recorded individual
FHP values which will be measured from occiput to
wall distance in centimeters, by using standardized
ruler and goniometer (Figure 2). FHP values below 5
cm were taken as mild, between 5 to 8 cm as
moderate, and finally above 8 cm is considered
severe.
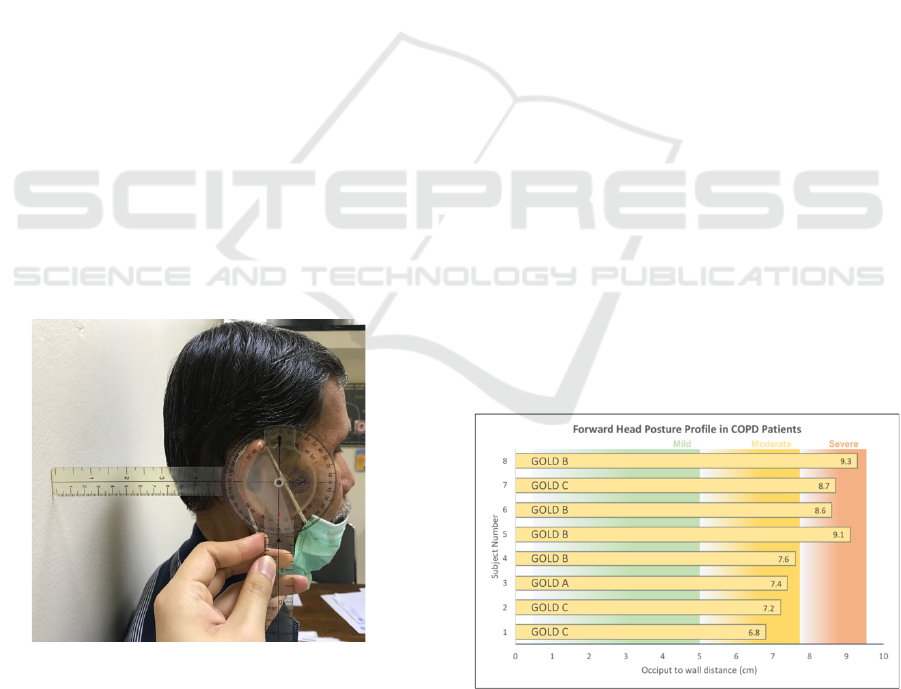
Figure 2: Measurement of occiput to wall distance.
A different examiner, blinded to the FHP
severity group, measured expiratory indices by using
a peak flow meter. Both peak flow rate (PFR), and
peak cough flow (PCF) values were recorded as lung
expiratory function values. Other physical
examinations include measurement of
anthropometry, vital signs, chest expansion, COPD
assessment test (CAT), and submaximal exercise
testing with the 6-minute walking test were all done
to provide better discussion.
Each data of FHP and COPD profile are then
presented and compared to each other, before
grouping them each according to their FHP severity.
The data are presented in a table form for better
comparison between FHP severity subgroups, and
charts were utilized when applicable. Statistical
comparison between FHP severity groups was done
with Mann Whitney U-test after identifying the
normality of the samples, this includes a comparison
between PFR & PCF. Graphical presentations of the
data were also constructed to provide ease in
comparison. All statistical tests will be considered
significant when P is <0.05. with a power of 80%.
The tests will be performed using SPSS (Statistical
Package for the Social Sciences) for Macintosh ver.
20.0.
3 RESULTS
This study had obtained a total of 8 samples, with 4
being admitted to the moderate FHP group and 4 in
the severe FHP group. Classification of the FHP
profile in each of the COPD subjects was shown in
Figure 2 below. It could be inferred that subject 1-4
has less than 8 cm occiput to wall distance, and thus
classified in the moderate subgroup, whereas subject
5-8 are classified in the severe subgroup owing to 8
cm and above occiput to the wall distance value.
Figure 3: Forward head posture severity as measured by
occiput to wall distance in the study subjects.
Forward Head Posture Examination and its Association with Lung Expiratory Function in Chronic Obstructive Pulmonary Disease (COPD)
Patient: A Case Series
233
The descriptive values for each of these groups
are shown in Table 1. It could be seen that in
general, all the data are similar in both groups
(p>0.05). The age group of the sample is all above
60 years old, putting them in the geriatric
population. BMI values, although indifferent
between the groups, moderate FHP seemed to be in
the underweight category, whereas severe FHP
ranges from underweight to normal. The GOLD
classification ranges from A to C in moderate FHP
and B to C in severe FHP. Chest expansion seemed
to be similar in values in both groups, however, it
could be seen that the upper chest is lower in severe
FHP, as well as middle chest, and no difference
could be found on the lower chest. CAT score is
generally lower in moderate FHP. The patient's
subjective symptoms as rated by Borg scale, are high
in the effort for both groups. Oxygen saturation
doesn't differ between groups, but both are lower
than 99%. Submaximal exercise testing values
revealed that severe FHP is seemingly better in
cardiorespiratory endurance as compared to
moderate FHP.
Table 1: Descriptive findings of the study subjects.
Moderate FHP
(n=4)
Severe FHP
(n=4)
p
a
Age
(years)
74.50
(61-
76)
75.50
(70-
84)
0.48
6
BMI
(kg/m
2
)
16.87
(15.05
-
18.95)
20.96
(15.59
-
22.04)
0.20
0
Chest Expansion (cm)
Upper
3.50
(3-4)
3
(2-3)
0.20
0
Middle
4
(2-
4.50)
3.75
(3-4)
0.68
6
Lower
3
(2-
3.50)
3
(2-
3.50)
1.00
0
CAT
Score
9.50
(4-30)
16.50
(9-29)
0.48
6
Borg Scale
Effort
10
(7-11)
9
(9-11)
0.88
6
Dyspne
a
1
(0-2)
0.75
(0-2)
0.88
6
Fatigue
1
(0-2)
1.25
(0-2)
1.00
0
Oxygen
Saturati
on (%)
98
(93-
99)
97.50
(96-
98)
0.68
6
6 Min
Walk
Distanc
e (m)
327.7
5
(216-
453.60
)
368.5
0
(345-
390.30
)
0.48
6
%
56.08
(37.48
64.99
(57.81
0.68
Predicte
d
-
77.44)
-
69.22)
6
METs
3.89
(3.20-
4.66)
4.03
(3.60-
4.57)
1.00
0
Speed
(m/s)
0.91
(0.60-
1.26)
1.02
(0.96-
1.08)
0.48
6
*All values are expressed in Median (Min-Max) unless
stated
a
ll statistical tests were done by using the Mann-Whitney
U Test
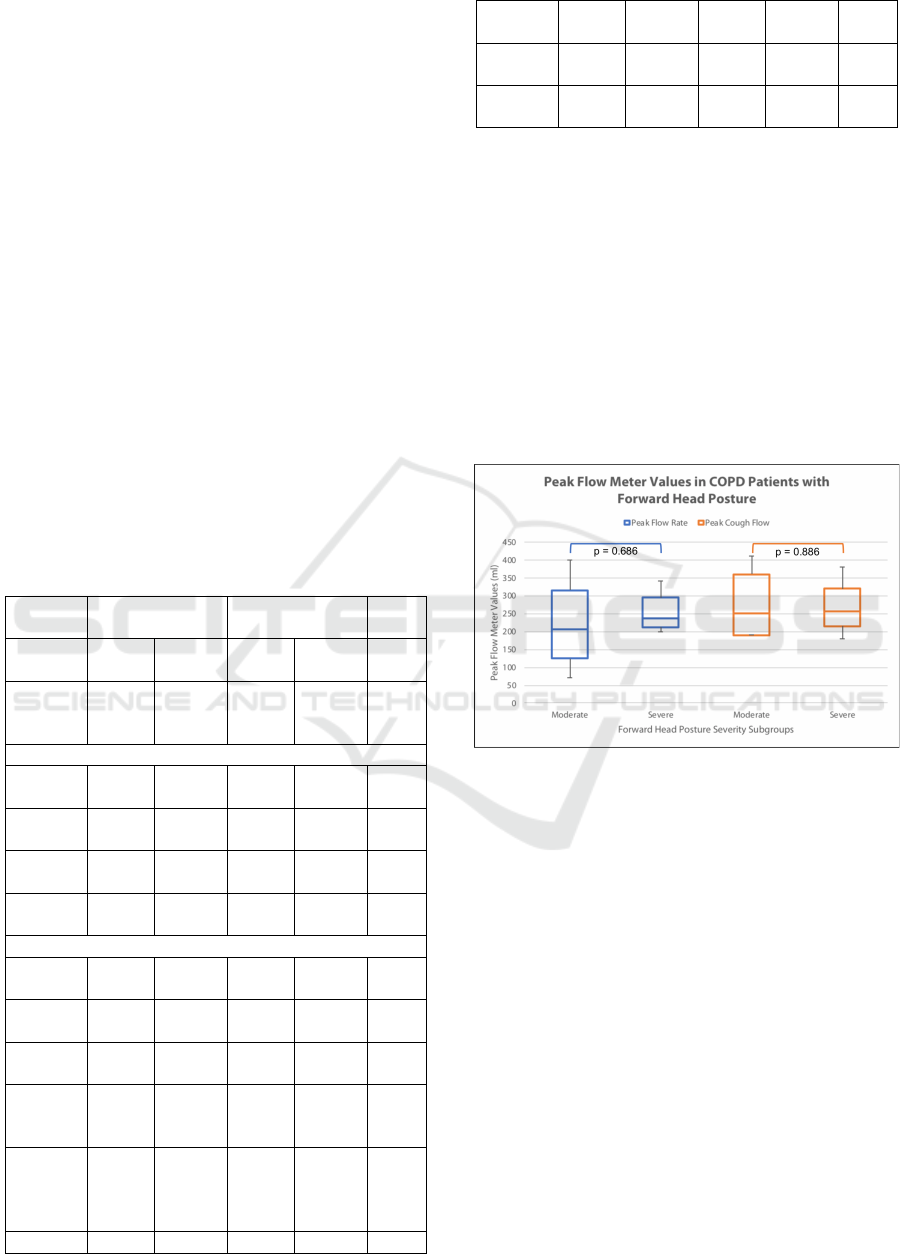
The main findings of this study could be seen
from the box and whisker plot depicted in Figure 3.
It could be seen briefly that both PFR and PCF has a
wider range for moderate FHP, while severe FHP
has narrower range values. PFR could be seen higher
by about 50 ml in severe subgroups, while PCF is
more or less similar in the median with a value of
250 ml. Despite these differences, none of these
differences have reached statistical significance.
Figure 4: Comparison of expiratory indices as measured
by a peak flow meter in the subjects, stratified by forward
head posture severity (Mann-Whitney U Test).
4 DISCUSSIONS
All the results of this study had shown how the
severity of FHP may not associate directly with the
reduction of expiratory, or submaximal exercise
testing values. It should also be acknowledged that
this study only measured COPD severity through
GOLD classification, without having any clear
temporal description as to when or how long has the
subject suffered from COPD. This becomes
weakness of this study, that spirometry values,
length of COPD and lifestyle were not measured, but
may be related to the severity of FHP. However, the
individual case comparison between the subjects
could draw out a conclusion that FHP could be
measured and classified, despite not directly
associated with COPD severity. Additionally, the
KONAS XI and PIT XVIII PERDOSRI 2019 - The 11th National Congress and The 18th Annual Scientific Meeting of Indonesian Physical
Medicine and Rehabilitation Association
234

study also showed measurable differences within the
physical examination findings, which then deems
further discussion.
As the definition goes, COPD is known to be a
progressive respiratory disease, which then accounts
for both progressive body structure and functional
decline. (Bach and Altschuler, 2010) Secondary
postural changes that occur due to lung
hyperinflation and higher demand in work of
breathing, will eventually happen, although no study
has investigated the clear timings of its occurrence
in the course of COPD. (Morais, Cruz, and Marques,
2016) Studies had mentioned that the increase of
thoracic kyphosis angle is the most prevalent among
structural changes, ranging from 3% to 62% as
compared to healthy controls. (Lee et al., 2017)
Additionally, moderate COPD is observed to have
higher shoulder elevation as compared to controls.
(Lee et al., 2017) These coupling of these two
changes will result in an overall alteration of the
upper body musculoskeletal structure and thus
resulted in FHP in COPD patients.
FHP itself is well known to result in other
changes in the thoracic cage structure, hence even
affecting much lower segments other than the
cervical vertebra itself. FHP will disrupt the natural
sagittal curves of the spine, thus adaptively
modifying the thoracic vertebrae, these changes
would then affect all the muscular attachments of the
diaphragm as the primary inspiratory muscle.
(Tortora and Derrickson, 2012; Koseki et al., 2019)
On the other hand, since the thoracic cage dimension
is altered, surely intercostal muscle length-tension
relationship is disturbed, this would further cause
ineffective tidal inspiration in the patients. It was
also shown that upper thorax will be more expanded
in FHP, whereas lower thorax will be less mobile,
impairing chest expansion. (Koseki et al., 2019)
Although the current study had already accounted
for these changes, it is still possible that the severe
group had adapted to the condition longer, and have
been treated for a longer time. It is also another
challenge to identify COPD in the earliest stage, as
there's often delay in diagnosis due to reluctance and
shame to have a medical consultation due to
smoking habits. (Jagana, Bartter, and Joshi, 2015;
Jonsdottir and Ingadottir, 2018) Simultaneously,
Indonesia has a rising number of young smokers
since the age of 10, which prevalence rises from
7.2% in 2013, up to 9.1% in 2018. (Balitbangkes,
2018) This has not been correlated directly with the
incidence of COPD but should be an alarm sign
towards respiratory health awareness especially in a
developing country.
Even when there are no significant differences
between them, this study also exhibited severe FHP
to have higher BMI as compared to the moderate
subgroup (median 20.96 kg/m2 vs 16.87 kg/m2
respectively), which may also be explained by
longer treatment time. Another study had shown that
BMI is related to COPD subtype, thus cachexia
(BMI <21 kg/m2) only appear in emphysematous
COPD. On the other hand, obesity (BMI >30 kg/m2)
is then related to bronchitic COPD. (Voica et al.,
2016) Unfortunately, almost all patients in this study
had obtained patients were in the low BMI group,
therefore no further analysis could be made on BMI.
Future studies could then be focused on this and
observing the prevalence of FHP in the higher BMI
group.
Several studies had exhibited lung function tests
in normal subjects with FHP, their main findings
include a reduction in diaphragm contraction (with
lower mobility of lower ribcage) and rib elevation.
The anatomic changes would eventually lead to a
reduction in pulmonary function test (spirometry)
values such as vital capacity (VC), forced vital
capacity (FVC), forced expiratory volume in 1
second (FEV1), PFR, and even sniff nasal
inspiratory pressure (SNIP). (Dimitriadis et al.,
2014; Kim, Cha, and Choi, 2017; Kang, Jeong, and
Choi, 2018; Beyer et al., 2019; Koseki et al., 2019)
One control comparison study with neck pain
subjects, presumably due to upper cross syndrome
(severe FHP with muscular manifestations of
weakness and tightness) had also shown normal or
increased values in FEV1/FVC within observed
subjects. Therefore the summation of all these
findings concluded that FHP results in restrictive
lung disorder, which would manifest in a mixed lung
disorder when it occurs in a COPD patient.
(Dimitriadis et al., 2014) Further study
recommendations would require spirometry
measurements to be compared in between FHP
severity, as that would be the next step after
examining PFR values to identify the changes in
both obstructive and restrictive lung disorder
through FEV1/FVC.(Ranu, Wilde and Madden,
2011)
All in all, the thoracic kyphosis and core
muscular weakness, which is caused by both FHP
and COPD, would surely lead to postural control
disorder. One of the ultimate manifestations of these
simultaneous anatomic changes could be described
as a balance disorder in COPD. A systematic review
had shown how they are mainly affected by the loss
of muscle strength and are generally associated with
low physical activity, independence, and functional
Forward Head Posture Examination and its Association with Lung Expiratory Function in Chronic Obstructive Pulmonary Disease (COPD)
Patient: A Case Series
235

capacity level. The disorder itself expands beyond
the vertebra, with a study that shows how the latency
time of Achilles and patellar tendon reflex is longer
in COPD patients. The nerve damage, in this case,
could then be caused by secondary impairment
following peripheral muscle weakness in COPD. In
times where continuous oxygen supply is required,
studies had shown that postural control, balance, and
gait speed is worst in this group. This probably is
caused by the inability of the body to recruit
effective muscles and at the same time unable to
extract oxygen to provide adequate muscular
metabolism. (Lee et al., 2017) Despite there are no
significant differences in the gait speed in our
sample, this may be caused by longer treatment time
in the severe FHP group, and thus gait speed or
balance should be initially examined as the patient is
diagnosed as COPD.
Among the progressive secondary
musculoskeletal changes, FHP could be seen to be
one of the earliest changes in COPD, owing to the
change of thoracic cage following hyperventilation.
It is then advised that further studies would be able
to show how FHP to correlate to other functions,
such as postural control, balance, and gait. Recently,
these neuromusculoskeletal manifestations have
commonly been made into achievable
comprehensive goals in COPD patients. (Morais,
Cruz, and Marques, 2016)
5 CONCLUSIONS
This study had shown that FHP could be measured
and its severity is suggestively associated with
expiratory function in COPD patients. The
impairments of COPD, mainly hyperventilation, will
alter the thoracic kyphotic angle, rises shoulder
elevation, finally resulting in FHP. Several studies
had shown that FHP will exhibit a restrictive lung
disorder, which when coupled with COPD, result in
a mixed lung disorder, thus worsening the clinical
condition.
Longer duration of the COPD presence itself will
result in other functional disorders, as such will
reduce physical activity level. Musculoskeletal
disorders then must not be underestimated, simple
changes such as a postural correction in the cervical
vertebra would be able to impact respiratory
function, owing to a better length-tension
relationship of the respiratory muscles.
Aside from requiring more samples to discuss
the association, a further recommendation would
require a temporal description of both the COPD and
the treatment, to see the changes within the group, as
well as their respective improvements.
REFERENCES
Bach, J. A., and Altschuler, E.,2010. ‘Rehabilitation of the
Patient with Respiratory Dysfunction', in Frontera, W.
R. et al. (eds) DeLisa's Physical Medicine &
Rehabilitation: Principles and Practice. 5th ed.
Philadelphia (PA): Lippincott Williams & Wilkins, pp.
1099–1124.
Balitbangkes, 2018. ‘Hasil Utama RISKESDAS 2018’,
Kementrian Kesehatan Republik Indonesia.
Beyer, B. et al., 2019. ‘Respiratory Physiology &
Neurobiology Relationship between costovertebral
joint kinematics and lung volume in supine humans',
Respiratory Physiology & Neurobiology. Elsevier
B.V., 232(2016), pp. 57–65. DOI:
10.1016/j.resp.2016.07.003.
Dimitriadis, Z. et al., 2014. ‘Pulmonary Function of
Patients with Chronic Neck Pain : A Spirometry
Study', pp. 543–549. DOI: 10.4187/respcare.01828.
Jagana, R., Bartter, T. and Joshi, M., 2015. ‘Delay in
diagnosis of chronic obstructive pulmonary disease:
reasons and solutions.', Current opinion in pulmonary
medicine. The United States, 21(2), pp. 121–126.
DOI: 10.1097/MCP.0000000000000133.
Jonsdottir, H. and Ingadottir, T. S., 2018. ‘Reluctance of
patients with chronic obstructive pulmonary disease in
its early stages and their families to participate in a
partnership-based self-management trial: A search for
an explanation.', Chronic respiratory disease. England,
15(3), pp. 315–322. DOI:
10.1177/1479972317743758.
Kang, J.-I., Jeong, D.-K. and Choi, H., 2018. ‘Correlation
between pulmonary functions and respiratory muscle
activity in patients with forward head posture.', Journal
of physical therapy science. Japan, 30(1), pp. 132–
135. DOI: 10.1589/jpts.30.132.
Kim, K.-S. et al., 2012. ‘Effects of breathing maneuver
and sitting posture on muscle activity in inspiratory
accessory muscles in patients with chronic obstructive
pulmonary disease.', Multidisciplinary respiratory
medicine. England, 7(1), p. 9. DOI: 10.1186/2049-
6958-7-9.
Kim, M., Cha, Y. and Choi, J.,2017. ‘Correlation between
forward head posture, respiratory functions, and
respiratory accessory muscles in young adults', 30, pp.
711–715. DOI: 10.3233/BMR-140253.
Koseki, T. et al., 2019. ‘Effect of forward head posture on
thoracic shape and respiratory function.', Journal of
physical therapy science. Japan, 31(1), pp. 63–68.
DOI: 10.1589/jpts.31.63.
Lee, A. L. et al., 2017. ‘Systematic Review of Postural
Assessment in Individuals With Obstructive
Respiratory Conditions: MEASUREMENT AND
CLINICAL ASSOCIATIONS.', Journal of
cardiopulmonary rehabilitation and prevention. The
KONAS XI and PIT XVIII PERDOSRI 2019 - The 11th National Congress and The 18th Annual Scientific Meeting of Indonesian Physical
Medicine and Rehabilitation Association
236

United States, 37(2), pp. 90–102. DOI:
10.1097/HCR.0000000000000207.
Mahajan, M. et al., 2019. ‘Time-controlled adaptive
ventilation (TCAV) accelerates simulated mucus
clearance via increased expiratory flow rate.’,
Intensive care medicine experimental. Germany, 7(1),
p. 27. DOI: 10.1186/s40635-019-0250-5.
McIlwaine, M. et al., 2017. ‘Personalising airway
clearance in chronic lung disease.', European
respiratory review : an official journal of the European
Respiratory Society. England, 26(143). DOI:
10.1183/16000617.0086-2016.
Mesquita Montes, A. et al., 2017. ‘Abdominal muscle
activity during breathing in different postural sets in
healthy subjects.', Journal of bodywork and
movement therapies. The United States, 21(2), pp.
354–361. DOI: 10.1016/j.jbmt.2016.09.004.
Morais, N., Cruz, J. and Marques, A., 2016. ‘Posture and
mobility of the upper body quadrant and pulmonary
function in COPD: an exploratory study.', Brazilian
journal of physical therapy. Brazil, 20(4), pp. 345–
354. DOI: 10.1590/bjpt-rbf.2014.0162.
Ranu, H., Wilde, M., and Madden, B., 2011. ‘Pulmonary
function tests.’, The Ulster medical journal. Northern
Ireland, 80(2), pp. 84–90.
Tortora, G. J., and Derrickson, B., 2012. ‘Muscles of the
thorax that assist in breathing', in Tortora, G. B., and
Derrickson, B. (eds) Principles of Anatomy &
Physiology. 13th ed. Danvers (MA): John Wiley &
Sons Inc, pp. 393–5.
Voica, A. S. et al., 2016. ‘Chronic obstructive pulmonary
disease phenotypes and balance impairment.',
International journal of chronic obstructive pulmonary
disease. New Zealand, 11, pp. 919–925. DOI:
10.2147/COPD.S101128.
Wada, J. T. et al., 2016. ‘Effects of aerobic training
combined with respiratory muscle stretching on the
functional exercise capacity and thoracoabdominal
kinematics in patients with COPD: a randomized and
controlled trial.', International journal of chronic
obstructive pulmonary disease. New Zealand, 11, pp.
2691–2700. DOI: 10.2147/COPD.S114548.
Forward Head Posture Examination and its Association with Lung Expiratory Function in Chronic Obstructive Pulmonary Disease (COPD)
Patient: A Case Series
237
