
Thermal and Morphological Properties of Polyvinyl Alcohol-based
Hydrogel Containing Microcrystal Cellulose
Riski Aulia Hasibuan
1
, Diana Adnanda Nasution
2
and Basuki Wirjosentono
2*
1
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Jl. Bioteknologi
No. 1, Medan 20155, Indonesia
Keywords: Polyvinylalcohol, Microcrystal Cellulose, Acrylicacid, Interpenetrating-hydrogels.
Abstract: In this work, the PVA-based hydrogels containing various loading of microcrystal cellulose (MCC: 0; 0.2;
0.4; 0.6 and 0.8) g were prepared in a bench-scale reflux-reactor using water as a solvent in an optimized
condition. Other constituents added, i.e. acrylic acid (AA) and N’N-Methylene bisacrylamide (MBA) as
comonomers as well as ammonium persulphate ((NH4)2S2O8) as initiator was keeping their composition
ratio constant. Products of the interpenetrating-hydrogels were cast in plastic mold and cooled, and then
characterized. First of all, their absorption properties and were measured using swelling test to know
optimum conditions and the next will be characterized with FTIR, DSC, and SEM. Results showed that the
optimum composition ratio of PVA/AA/MCC/MBA/APS = 0.4/4/0.6/0.06/0.2 enhanced the water
absorption. FTIR analysis of the film specimen after exhaustive extraction in n-hexane still contained stable
AA-carbonyl (C=O) absorption peak at 1713 cm
-1
of hydrogel 1 and 1707 cm
-1
of hydrogel 4. The thermal
properties of the optimized composition of the hydrogel 1 showed its stable decomposition temperature
(thermal stability of 482.28
o
C). Morphological properties of the interpenetrating-hydrogel micro composites
also showed finely distributed of the micro filler, which is responsible for its improved mechanical and
thermal properties.
1 INTRODUCTION
Technological progress continues to increase. To
improve research in the field of polymers, new
technologies such as biopolymers are needed.
Biopolymers are one of the materials produced by
modifying a polymer. Modifications can be made by
combining polymers that function to improve the
properties of these polymers such as absorption,
elasticity, and mechanical strength that are
biodegradable, biocompatible, and non-toxic. This
modification can also affect the solubility of
polymers which can dissolve in water to be insoluble
like hydrogels (Sinha, 2018)).
Hydrogels are hydrophilic polymers with three-
dimensional structures that have cross bonds
(Ahmed, 2015). The three-dimensional structure of
the hydrogel formed through crosslinking makes the
hydrogel capable of absorbing and releasing water
reversibly (Ambrosio, Demitri, & Sannino, 2011).
The ability of a hydrogel to absorb water thousands
of times from its dry weight is influenced by a group
of hydrophilic functions found in three-dimensional
structures. This hydrophilic functional group can
hold large amounts of water (Ahmed, 2015, Maitra
& Shukla, 2014). The hydrophilic functional groups
contained in hydrogels such as carboxyl (-COOH),
hydroxyl (-OH), and amide (-CONH
2
) (Ha et al.,
2018). Hydrogels have several very important
properties, which are able to expand well in water,
insoluble in water (Burdick & Stevens, 2005),
softness, elasticity, and flexibility. Because of these
properties, hydrogels can be used in various
applications for food, agriculture, industry, medical,
medicine, and cosmetics (Kiatkamjornwong, 2007,
Anamica & P. P. Pande, 2017).
At present, a lot of research has been done
regarding the manufacture of hydrogels. Making
hydrogels that use polysaccharides such as starch,
chitosan, xanthan, and cellulose can be done through
polymerization reactions. The use of chitosan
polysaccharides with acrylic monomers is very
312
Aulia Hasibuan, R., Nasution, D. and Wirjosentono, B.
Thermal and Morphological Properties of Polyvinyl Alcohol-based Hydrogel Containing Microcrystal Cellulose.
DOI: 10.5220/0008932403120318
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 312-318
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

efficient in making hydrogels (Mahdavinia,
Zohuriaan-Mehr, & Pourjavadi, 2004). Initially, the
hydrogel was made from cellulose and polyvinyl
alcohol which have a hydrophilic group and have a
high affinity for water. This type of hydrogel has
several disadvantages including absorption capacity
is relatively small, less stable to changes in pH,
temperature and physical properties that are not
good (Swantomo, Megasari, & Saptaaji, 2008).
The manufacture of polyvinyl alcohol (PVA) and
polyacrylic acid (PAA) hydrogels with xanthan gum
polysaccharides which can be applied as drug
delivery control through cross-linked polymerization
using N’N-methylene bisacrylamide (MBA) as a
crosslinking agent and ammonium persulfate (APS)
as the initiator. The best water absorption results are
shown in hydrogels containing low MBA, namely
0.010 g (Bhattacharya et al., 2012).
Polyvinyl alcohol (PVA) is one of the most
promising synthetic polymers for the development of
biomaterials (Teodorescu, Bercea, & Morariu, 2018).
PVA is a water-soluble polymer, easily degraded
(Chiellini, Corti, D’Antone, & Solaro, 2003), has high
tensile strength and flexibility, elastic, non-toxic, and
biocompatible polymer. So it can be used in many
applications make PVA a hydrogel which is widely
used in the textile, adhesive, food, medicine, paper,
packaging, and cosmetics industries (Peresin et al.,
2010, Vieira et al., 2009).
Manufacture of semi-IPN hydrogels based on
MCC from cellulose pulp for biomedical applications.
MCC was dissolved in PEG / NaOH solvents with
acrylic acid monomers, crosslink N’ N-
methylenbisacrylamide by free radical
polymerization. Increased crosslink concentration
causes a decrease in water absorption. The increasing
number of crosslinks will affect the polymer chain,
thereby reducing the incoming water and causing the
chain to become stiff (Bajpai & Swarnkar, 2014).
Manufacturer hydrogels from Polyacrylic acid (PAA)
with micro-crystal cellulose amplifier (MCC) carried
out through acrylic acid polymerization using UV
light. Addition of 1% MCC (1% of the weight of
acrylic acid) caused an increase in water absorption
capacity in the hydrogel of 122 g water / g hydrogel
(from 427 to 549 g water / g hydrogel). The water
capacity of the hydrogel matrix network increases
because the formed hydrogels have a porous structure
and have increased surface area in the hydrogels (Ni,
Wen, & Liu, 2015).
The addition of cellulose has many advantages,
including from plants with available widely (Anah &
Astrini, 2015), cheap, and not toxic. Also, the
addition of micro-sized cellulose such as
microcrystalline cellulose to the polymer matrix can
increase the physical strength of the formed
hydrogels (Spagnol et al., 2012). MCC is an
available widely commercial material (Brinchi,
Cotana, Fortunati, & Kenny, 2013) which can be
used as fillers, additives, cosmetics, tablets, and food
products (Ioelovich & Leykin, 2006).
Based on the description above the researchers
were interested in research on the thermal and
morphological properties of a polyvinyl alcohol-based
hydrogel containing microcrystalline cellulose (MCC)
using N'N-Methylene bisacrylamide as a crosslinking
agent and ammonium persulfate as initiator.
2 MATERIALS AND METHODS
2.1 Materials
Commercial Cellulose Microcrystalline (Avicel PH
101 50 μm), Acrylic Acid (Merck Schuchardt from
Germany), Polyvinyl Alcohol, as initiator has used
Ammonium Persulfate (≥ 98% from China), N'N-
Methylene bisacrylamide (Sigma Aldrich) was used
as a crosslinking agent.
2.2 Preparation of Hydrogel
Microcrystalline cellulose was heated with 25 mL
aquadest at 60
o
C and stirred for 30 minutes until
homogeneous. Polyvinyl alcohol is heated at 85
o
C
added to microcrystalline cellulose and stirred. 0.06 g
Ammonium Persulfate is added to the polymer
mixture at a temperature of 60-70
o
C and stirred for 30
minutes. 4 g of acrylic acid which has been added
with 10 mL of aquadest and has been neutralized with
NaOH 17.5% until pH = 5 is added to the polymer
mixture. 0.2 g of N'N-Methylene bisacrylamide was
added to the mixture and stirred. The reaction was
finished at 70
o
C after the reaction has been running
for 30 minutes. The product is released in hot
conditions. The hydrogel was washed with water then
the hydrogel is dried in the oven at 60
o
C. After the dry
hydrogel is inserted into the desiccator. MCC and
PVA variations can be seen in Table 1.
Table 1: Variation of MCC and PVA.
MCC (g)
PVA (g)
0.0
1.0
0.2
0.8
0.4
0.6
0.6
0.4
0.8
0.2
Thermal and Morphological Properties of Polyvinyl Alcohol-based Hydrogel Containing Microcrystal Cellulose
313

2.3 Swelling Test
Three or four replicas of each dried hydrogel were
swollen in deionized water at room temperature for
3 days to achieve equilibrium swelling. The degree
of swelling of hydrogels were measured after 5 min,
10 min, 20 min, 0.5 h, 1.5 h, 1 day, 2 days and 3
days. Completely three replicas were measured, the
standard deviations were marked with error bars in
the swelling profile charts. The degree of swelling
was calculated as the following Yang (2012)
equation:
Degree of swelling = [(Wet weight – Dry
weight) / Dry weight] ×100%
(1)
2.4 Functional Group Analysis
Detection of functional group, was analyzed by a
Perkin Elmer-Fourier transform infrared and
transmission was measured in the range of 4000–600
cm
-1
2.5 Morphological Analysis
Changes in the surface morphology of the hydrogel
were analyzed by scanning electron microscopy
(SEM). The micrographs of samples were taken
using a Hitachi TM3000 scanning electron
microscopy (SEM).
2.6 Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) was
performed using a DSC-60 Plus Shimadzu
containing nitrogen gas and its flow rate was 30
mL/min. Hydrogel samples and Al
2
O
3
comparison
materials were weighed and heated at room
temperature to 600
o
C at a heating rate of 15
o
C /
minute.
3 RESULTS AND DISCUSSION
3.1 Preparation of Hydrogel
The manufacture of polyvinyl alcohol-based
hydrogels containing microcrystalline cellulose with
acrylic acid monomer, N’N-Methylene
bisacrylamide as a crosslinking agent and
ammonium persulfate as initiator. Polyvinyl alcohol
and cellulose microcrystal were varied to produce
five hydrogels can be seen in Figure 1.
Figure 1: Hydrogel with varied microcrystal cellulose.
3.2 Swelling Test
Swelling test is done by determining the percent
swelling ratio. The percentage of the hydrogel
swelling ratio can be seen in Figure 1. Based on
Figure 1. It can be seen that at 5 and 10 minutes.
Hydrogel 3 has the highest percent swelling. At 20
minutes the hydrogel 5 has the highest percent
swelling. The highest swelling percentage at 30 and
90 minutes was shown in hydrogel 1. On the first,
second, and third days hydrogel 4 had the highest
percent swelling. It can be assumed that the hydrogel
4 has the highest percent swelling of around 3245%.
Water absorption from hydrogel 4 has increased.
This is because the addition of the amount of MCC
0.6 g to the hydrogel 4 affects the percent swelling
ratio in the hydrogel. The addition of MCC will
increase the percent swelling ratio but the addition
of 0.8 g MCC percent swelling ratio decreases which
occurs in hydrogel 5. Water absorption will decrease
too.
The addition of 0.2 g MCC of Hydrogel 2 and
0.4 g MCC of hydrogel 3 had lower percent swelling
compared to hydrogel 1 without the addition of
MCC.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
314
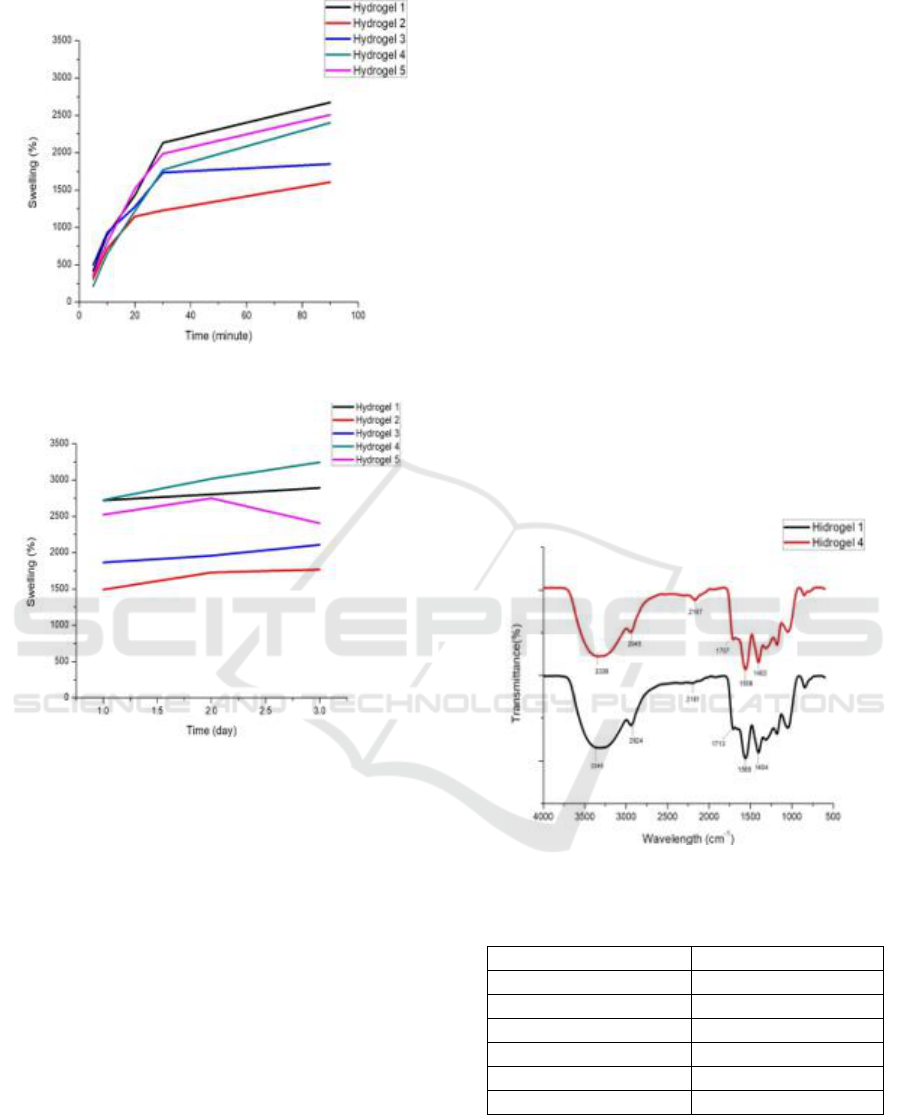
Figure 2: Degree of swelling (minutes).
Figure 3: Degree of swelling (days).
3.3 Analysis of FTIR
Functional group analysis using FTIR is a qualitative
analysis used to interpretation absorption peaks from
the infrared spectrum. This analysis can show
various areas of hydrogel absorption that are
produced so that the resulting functional group
changes can be produced. Data analysis of
functional groups using FTIR is presented in
graphical form in Figure 4 and Table 2.
The FTIR spectrum of hydrogel 1 and hydrogel 4
produced showed several absorption peaks is
hydrogel 1 absorption at 3340, 2924, 2181, 1713,
1565, and 1404 cm
-1
, and hydrogel 4 absorbed at
3336, 2945, 2167, 1707, 1558, and 1402 cm
-1
.
The FTIR spectrum of hydrogels 1 and 4 is a
wide absorption peak at 3340 and 3336 cm
-1
wavenumbers which shows the presence of vibrating
O-H groups from microcrystalline cellulose,
polyvinyl alcohol, and acrylic acid so the absorption
appear wide (Sunardi, Irwan, Nurjannah, &
Istikowati, 2013). The absorption peak at 2924 and
2945 cm
-1
on hydrogel 1 and hydrogel 4 showed the
presence of C-H stretching. Also, there is an
absorption peak at wave number 2181 and 2167 cm
-1
which shows CH
2
in crosslinks (Saragih, Tamrin,
Marpongahtun, Nasution, & Abdillah, 2018).
The presence of a functional group C = O of
acrylic acid at the absorption peak was 1713 and
1707 cm
-1
. Curved absorption sharp peaks at
wavenumber 1565, 1404 cm
-1
from hydrogel 1 and
absorption peaks at wavenumber 1558 and 1402
cm
-1
from hydrogel 4 showed symmetrical and
asymmetrical stretches of carboxylic anions (COO-)
(Bajpai & Swarnkar, 2014).
The results of FTIR data obtained can be
concluded that there was not significant change in
functional groups on hydrogel 1 and hydrogel 4.
This is because hydrogels only occur physical
interactions on hydrogen bonds between O-H
functional groups of MCC, carboxylates, and
polyvinyl alcohol.
Figure 4: FTIR spectra of Hydrogel 1 and Hydrogel 4.
Table 2: Functional group of hydrogel.
Wavelength (cm
-1
)
Functional Groups
3340 and 3336
O-H
2924 and 2945
C-H
2181 and 2167
CH2
1713 and 1707
C=O
1565 and 1558
COO-
1404 and 1402
COO-
3.4 Differential Scanning Calorimetry
The DSC of the hydrogel is shown in Figure 5. The
DSC thermogram of Hydrogel 1 obtained 4 peaks.
At the first peak, there was an endothermic reaction
Thermal and Morphological Properties of Polyvinyl Alcohol-based Hydrogel Containing Microcrystal Cellulose
315
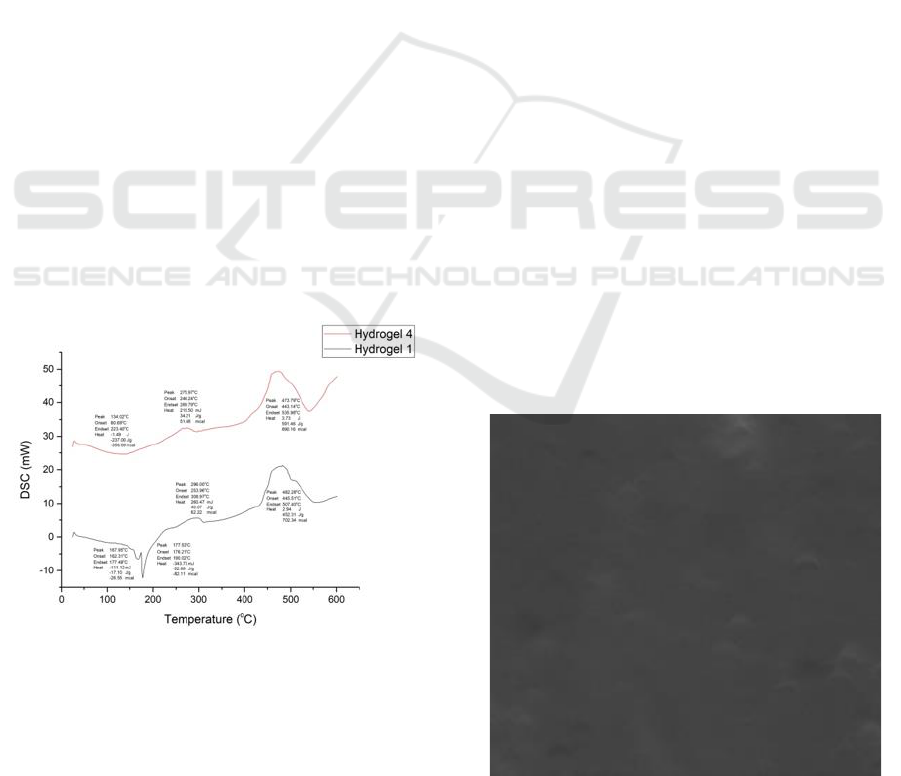
from a temperature of 162.31
o
C to a temperature of
177.48
o
C which required the energy of -111.12 mJ
or equivalent to -26.55 mcal. The second peak of
Hydrogel 1 has an endothermic reaction from a
temperature of 176.21 to a temperature of 190.02
o
C
which requires the energy of -343.73 mJ or
equivalent to -82.11 mcal. Hydrogel 1 experienced
melt at 167.95
o
C and the perfect melt occurred at
177.53
o
C. At the third peak, an exothermic reaction
occurs. Hydrogel 1 began to increase in temperature
from 253.96
o
C to a temperature of 308.97
o
C which
released the energy of 260.47 mJ or equivalent to
62.22 mcal. At this peak of hydrogel 1 is degraded at
296
o
C where the tissue arranged in the hydrogel
begins to weaken the bonds with each other. At the
fourth peak, an exothermic reaction occurs.
Hydrogel 1 experienced an increase in temperature
from 445.51
o
C to 507.40
o
C which released the
energy of 2.94 J or equivalent to 702.34 mcal. At
this time the three-dimensional network structure on
hydrogel 1 experienced decomposition. It means the
composition of hydrogel has burned to be ash and
water vapor. We can see the fourth peak in figure 4.
Hydrogel 1 has good thermal stability occurs at a
temperature of 482.28
o
C.
The DSC thermogram from Hydrogel 4 obtained
3 peaks. At the first peak, there was an endothermic
reaction from a temperature of 60.69
o
C to a
temperature of 223.40
o
C which required the energy
of -1.49 J or equivalent to -356.69 mcal. Hydrogel 4
experienced melt at 134.02
o
C. The second Hydrogel
4 peak exothermic reaction occurs.
Figure 5: Thermogram of Hydrogel 1 and Hydrogel 4.
Hydrogel 4 began to experience an increase in
temperature from 246.24 to 289.79
o
C which released
the energy of 215.50 mJ or equivalent to 51.48 mcal.
At this peak of hydrogel 4 are degraded at a
temperature of 275.97
o
C where the tissue composed
of hydrogels begins to experience weakening of each
other bonds. At the third peak, an exothermic
reaction occurs. Hydrogel 4 experienced an increase
in temperature from 443.14 to 535.98
o
C which
released the energy of 3.73 J or equivalent to 890.16
mcal. At this time the three-dimensional network
structure on hydrogel 4 has experience
decomposition. It means the composition of
hydrogel has burned to be ash and water vapor. We
can see the third peak in Figure 5. The thermal
stability of hydrogel 4 occurred at 473.79
o
C. From
the picture above it can be seen that the thermal
stability in hydrogel 1 is greater than the thermal
stability of the hydrogel 4. Thermal stability
decreases with the addition of 0.6 g MCC.
3.5 Scanning Electron Microscopy
The results of the surface morphology analysis of
Hydrogel 1 without the addition of MCC, Hydrogel 4
with the addition of 0.6 g MCC, and Hydrogel 5 with
the addition of 0.8 g MCC. These results provide
information relating to the surface of the hydrogel
with homogeneity. The surface morphology of
hydrogels was analyzed with 500x magnification.
SEM micrographs of the surface of hydrogel 1
without the addition of MCC in Figure 6 show a
homogeneous, smooth, flat surface without pores. It
can be concluded that PVA has been distributed to
all hydrogel 1 networks.
Figure 7 surface of the hydrogel 4 with the
addition of 0.6 g MCC shows a slightly coarse
surface, and a surface that looks deep and dark so it
looks slightly porous. MCC spreads well in
hydrogels. Higher absorption occurs due to the
irregular shape of the hydrogels and pores (Saragih
et al., 2018).
Figure 6: SEM micrographs of Hydrogel 1 without using
MCC.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
316

Figure 7: SEM micrograph of Hydrogel 4 using 0.6 g
MCC.
Figure 8: SEM micrograph of Hydrogel 4 using 0.8 g
MCC.
Figure 8 the surface of hydrogel 5 with the
addition of 0.8 g MCC shows the surface of the
hydrogel is not homogenous, coarse, and it looks a
lot porous. Lots of porous because the shape of the
surface looks a lot in the inner and dark areas.
4 CONCLUSIONS
From the research that has been done on the thermal
and morphological properties of polyvinyl alcohol-
based hydrogels containing microcrystalline
cellulose, it is undeniably about making optimal
mixtures in the manufacture of hydrogels from PVA,
MCC and acrylic acid using the ammonium
persulfate initiator and crosslinking, N'N-Methylene
bisacrylamide by involving (0,4: 0,6: 4: 0,06: 0,2) g.
As much as 0.6 g MCC has the highest swelling
ratio of 3245% in Hydrogel 4. The higher the
swelling ratio, the more optimal absorption.
Hydrogels that have absorbency are characterized
optimally using FTIR, SEM, and DSC. The results
of FTIR data obtained can be concluded that there
was not a significant change in functional groups on
hydrogel 1 and hydrogel 4. This is because
hydrogels only occur physical interactions on
hydrogen bonds between O-H functional groups of
MCC, carboxylates, and polyvinyl alcohol. Thermal
stability decreased with the addition of MCC 0.6 g
from a temperature of 482.28
o
C to a temperature of
473.79
o
C. It means the addition of 0.6 g MCC can
increase absorption and decrease the thermal
stability. The Surface morphology of hydrogel 1
looks homogeneous, smooth and has no pore. When
the addition with 0.6 g MCC the surface of the
hydrogel slightly coarse, and has a slight porous.
The porous on the surface of the hydrogel increases
when additing of 0.8 g MCC and decreases
absorption
ACKNOWLEDGEMENTS
The authors would like to thank to The Higher
Education Directorate, Ministry of Research,
Technology and Higher Education for granting the
research fund to carry out this work through:
“Penelitian Tesis Mahasiswa, DRPM RISTEKDIKTI
2019 0f Universitas Sumatera Utara”.
REFERENCES
Ahmed, E. M. (2015). Hydrogel: Preparation,
characterization, and applications: A review. Journal
of advanced research, 6(2), 105–121.
https://doi.org/10.1016/j.jare.2013.07.006
Ambrosio, L., Demitri, C., & Sannino, A. (2011).
Superabsorbent cellulose-based hydrogels for
biomedical applications. In Biomedical Hydrogels:
Biochemistry, Manufacture and Medical Applications.
https://doi.org/10.1016/B978-1-84569-590-3.50002-3
Anah, L., & Astrini, N. (2015). Sintesa Dan Karakterisasi
Hidrogel Super Absorben Polimer (Sap) Berbasis
Selulosa Menggunakan Crosslinking Agent Water-
Soluble Carbodiimide (Wsc). Jurnal Selulosa, 5(01),
1–6. https://doi.org/10.25269/jsel.v5i01.73
Anamica, & P. P. Pande. (2017). Polymer Hydrogels and
Their Applications. International Journal of Materials
Science. https://doi.org/10.1007/978-3-319-46458-9_7
Thermal and Morphological Properties of Polyvinyl Alcohol-based Hydrogel Containing Microcrystal Cellulose
317

Bajpai, S. K., & Swarnkar, M. P. (2014). New Semi-IPN
Hydrogels Based on Cellulose for Biomedical
Application. Journal of Polymers, 2014, 1–12.
https://doi.org/10.1155/2014/376754
Bhattacharya, S. S., Mishra, A., Pal, D., Ghosh, A. K.,
Ghosh, A., Banerjee, S., & Sen, K. K. (2012).
Synthesis and Characterization of Poly (acrylic
acid)/Poly (vinyl alcohol)-xanthan Gum
Interpenetrating Network (IPN) Superabsorbent
Polymeric Composites. Polymer - Plastics Technology
and Engineering. https://doi.org/10.1080/
03602559.2012.671421
Brinchi, L., Cotana, F., Fortunati, E., & Kenny, J. M.
(2013). Production of nanocrystalline cellulose from
lignocellulosic biomass: Technology and applications.
Carbohydrate Polymers.
https://doi.org/10.1016/j.carbpol.2013.01.033
Burdick, J. A., & Stevens, M. M. (2005). Biomedical
hydrogels. Biomaterials, Artificial Organs and Tissue
Engineering, 107–115. https://doi.org/10.1533/
9781845690861.2.107
Chiellini, E., Corti, A., D’Antone, S., & Solaro, R. (2003).
Biodegradation of poly (vinyl alcohol) based
materials. Progress in Polymer Science (Oxford).
https://doi.org/10.1016/S0079-6700(02)00149-1
Ha, J., Kim, M., Lee, W., Lee, H., Han, C., Koh, W. G., &
Ryu, D. Y. (2018). Direct measurement of crosslinked
surface layer in superabsorbent poly (acrylic acid).
Materials Letters. https://doi.org/10.1016/j.
matlet.2018.05.079
Ioelovich, M., & Leykin, A. (2006). Microcrystalline
cellulose: Nano-structure formation. Cellulose
Chemistry and Technology.
Kiatkamjornwong, S. (2007). ScienceAsia, 33(s1), 039.
https://doi.org/10.2306/scienceasia1513-
1874.2007.33(s1).039
Mahdavinia, G. R., Zohuriaan-Mehr, M. J., & Pourjavadi,
A. (2004). Modified chitosan III, superabsorbency,
salt- and pH-sensitivity of smart ampholytic hydrogels
from chitosan-g-PAN. Polymers for Advanced
Technologies. https://doi.org/10.1002/pat.408
Maitra, J., & Shukla, V. K. (2014). Cross-linking in
Hydrogel - A Review. American Journal of Polymer
Science. https://doi.org/10.5923/j.ajps.20140402.01
Ni, J., Wen, Y., & Liu, H. (2015). Preparation of micro-
crystalline cellulose-reinforced polyacrylic acid
hydrogel and its application in paper/polyacrylic acid
composites. BioResources. https://doi.org/10.15376/
biores.10.3.4843-4853
Peresin, M. S., Habibi, Y., Vesterinen, A. H., Rojas, O. J.,
Pawlak, J. J., & Seppälä, J. V. (2010). Effect of
moisture on electrospun nanofiber composites of poly
(vinyl alcohol) and cellulose nanocrystals.
Biomacromolecules.
https://doi.org/10.1021/bm1006689
Saragih, G., Tamrin, T., Marpongahtun, Nasution, D. Y.,
& Abdillah. (2018). Preparation of semi-IPN hydrogel
from starch nanoparticles of magrove fruit and
monomer acrylic acid using crosslinker N,N’
methylene bisacrylamide. In AIP Conference
Proceedings. https://doi.org/10.1063/1.5082454
Sinha, S. (2018). Biodegradable superabsorbents: Methods
of preparation and application—A review.
Fundamental Biomaterials: Polymers. Elsevier Ltd.
https://doi.org/10.1016/B978-0-08-102194-1.00014-1
Spagnol, C., Rodrigues, F. H. A., Pereira, A. G. B.,
Fajardo, A. R., Rubira, A. F., & Muniz, E. C. (2012).
Superabsorbent hydrogel composite made of cellulose
nanofibrils and chitosan-graft-poly (acrylic acid).
Carbohydrate Polymers. https://doi.org/10.1016/j.
carbpol.2011.10.017
Sunardi, Irwan, A., Nurjannah, & Istikowati, W. T.
(2013). Pengaruh Penambahan Jumlah Inisiator
Amonium Persulfat (APS) Terhadap Karakteristik
Polimer Superabsorben Asam Akrilat dan Selulosa
Batang Alang-Alang (Imperata cylindrica). In
Posiding Seminar Nasional Penelitian, Pendidikan dan
Penerapan MIPA Fakultas MIPA, Universitas Negeri
Yogyakarta, ISBN: 978 – 979 -96880 – 7 - 1.
https://doi.org/10.13140/2.1.5170.2561
Swantomo, D., Megasari, K., & Saptaaji, R. (2008).
Pembuatan Komposit Polimer Superabsorben dengan
Mesin Berkas Elektron.
Teodorescu, M., Bercea, M., & Morariu, S. (2018).
Biomaterials of Poly (vinyl alcohol) and Natural
Polymers. Polymer Reviews. https://doi.org/10.1080/
15583724.2017.1403928
Vieira, R. P., Fernandes, A. R., Kaneko, T. M.,
Consiglieri, V. O., Pinto, C. A. S. D. O., Pereira, C. S.
C., … Velasco, M. V. R. (2009). Physical and
physicochemical stability evaluation of cosmetic
formulations containing soybean extract fermented by
Bifidobacterium animalis. Brazilian Journal of
Pharmaceutical Sciences.
https://doi.org/10.1590/S1984-82502009000300018
Yang, T. (2012). Mechanical and Swelling properties of
Hydrogels. Wiley.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
318
