
Preparation and Characterization of Chitosan with Activated Carbon
as Adsorbent to Reduce Level Metal Cadmium (Cd) and Nickel (Ni)
Fitri Purnama Sari
1
, Harry Agusnar
2*
and Muhammad Taufik
2
1
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1, Medan 20155, Indonesia
Keywords: Activated Carbon, Cadmium, Chitosan, Coffee Ground, Nickel.
Abstract: Preparation and characterization of chitosan with activated carbon have been made with the aim of reducing
the metal content of Cadmium (Cd) and Nickel (Ni) in standard solutions. Characterization chitosan with
activated carbon by FT-IR, SEM, and test adsorption by using AAS. Characterization of chitosan and
chitosan - activated carbon by FT-IR shows that there is no difference in wavelength: as for the emerging
groups, NH groups (3448.72 cm
-1
), CH groups (2924.09 cm
-1
), C = C groups (1635.64 cm
-1
), C-N group
(1381 cm
-1
), and NH group (3441.01 cm
-1
). SEM characterization on chitosan - activated carbon shows a
rude surface. Absorptions of Cd and Ni in chitosan that best with addition carbon of 0.6 g that is 74.54%
and 73.43%.
1 INTRODUCTION
Industrial activities which are rapidly developing
have made the contamination of heavy metal ion in
water increase. This condition has caused serious
environmental problem throughout the world (Li et
al., 2016). Heavy metal from industrial waste such
as lead, copper, and cadmium can pollute water, sea
level, and soil. The International institution has
confirmed that cadmium is latent metabolic poison
since it is very dangerous for the life of organism
and can affect human health.
Electroplating industry has significant risk for
environment and human beings because wastewater
contains heavy metal ion which is not biodegradable
and tends to be accumulated in living organism
which causes toxic effect or carcinogenic. One of the
contents found in electroplating waste is nickel
metal; this metal is usually used in electroplating
industry due to its anti-corrosion (Raja Sulaiman et
al., 2018). Nickel is a silver white, hard and ductile
metal. Ni normally forms cubic crystal lattice
(Coman et al., 2013). Nickel is used for production
of stainless steel, nonferrous alloys and anticorrosion
and temperatures resistance properties.(Reck et al.,
2008)
According to the International Board for cancer
study, cadmium is known as carcinogenic (Jeon,
2017) . It is accumulated in human kidneys and
liver, and it can cause various diseases such as
kidney dysfunction, hypertension, diarrhea,
stomach-ache, and bone disorder (Pal and Pal,
2017). Waste which contains cadmium metal comes
from electroplating industry, the making of batteries,
pesticides, and mining which bring about water, air,
and soil pollution (Al-Malack and Dauda, 2017).
There are several methods used to remove heavy
metals from wastewater is precipitation, membrane
filtration, ion exchange (Hegazi, 2013). The
adsorption method is the most commonly used
because it is more efficient, more economical and
uses cheap natural adsorbents (Li et al., 2016).
Chitosan is poly-(2-amino-2-deoxy-β-(1-4)-D-
glucopyranose) with the molecule formula of
(C
6
H
11
NO
4
)n which can be obtained from chitin
deacetylation (Rahate, 2013).
Its molecular weight between 300 – 1000 kDa.
Chitosan produced from crustacean shell such as
crab and shrimp. These shells contain 30 – 40
proteins, 30 – 50% calcium carbonate and 20-30%
chitin. Chitosan is natural chelating make chitosan
useful in wastewater treatment by allowing for the
binding and removal of metal ions such as copper,
280
Purnama Sari, F., Agusnar, H. and Taufik, M.
Preparation and Characterization of Chitosan with Activated Carbon as Adsorbent to Reduce Level Metal Cadmium (Cd) and Nickel (Ni).
DOI: 10.5220/0008921802800286
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 280-286
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
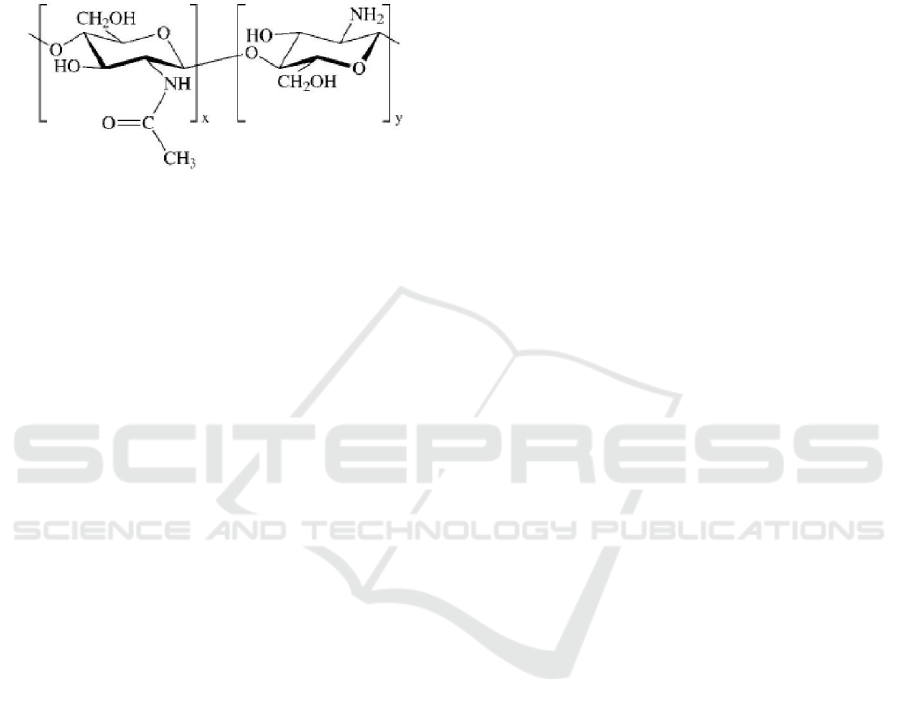
lead, mercury, and uranium from wastewater.
Chitosan (Figure 1) has advantages such as
biodegradability, natural origin, abundance,
reactivity. It has many application include medical,
agricultural, food processing, nutritional
enhancement, cosmetics and waste and water
treatment. (Paridah et al., 2016).
Figure 1: Chemical structure chitosan.
Chitosan is absorbent which is very effective to
remove heavy metal ion because chitosan structure
has abundant hydrophilic hydroxyl group and
polycationic amine group which can bind metal
(Ahmad et al., 2017). Among various natural
polymers, chitosan is the second largest biopolymer
in nature after cellulose (Choi et al., 2016). It is a
very effective natural polymer which can be used as
absorbent because it is biodegradable (Ahmad et al.,
2017) and not poisonous (Choi et al., 2016); it also
has bio-compatibility (Liu and Bai, 2014) and
bioactivity (Vakili et al., 2014).
Chitosan has several weakness is mechanical
properties and low thermal stability, porosity and
small surface area. Vakili et al., 2014 modified the
structure of chitosan into chitosan beads, membranes
and films, to improve adsorption ability, physical
and mechanical properties. Liu and Bai, 2014
explained that modifying chitosan into semi-IPN
(Interpenetrating Network) hydrogel, nano-magnetic
particles, chitosan grafting polymers, and chitosan
composites can improve chitosan adsorption ability.
Coffee is the most common drink which is
consumed throughout the world. It reaches 400
billion glasses per year and produces around 8,000
tons of coffee grounds per year. Coffee ground is
still considered as waste since it takes a very long
time to be decomposed, compared with the other
types of waste (Zein et al., 2017).
One of the advantages of coffee grounds is that
they can be used as absorbent, tannin compounds
which contain polyhydroxy and polyphenol groups
which can be bound with metal cation which forms
chelate (Utomo and Hunter, 2006). Besides that,
coffee grounds also contain carbon, nitrogen,
lipophilic compound, ethanol, lignin, alkaloid,
polysaccharide, and chlorogenic acid (Pujol et al.,
2013).
The ingredients which can be used for carbon are
tea, coffee grounds, and rice husk. Carbon from active
charcoal can absorb inorganic contaminants (Djati
Utomo, 2015). Carbon from active charcoal is usually
used for absorbent since it is flexible and more
effective; besides that, it has wider surface and good
capacity to decrease metal and the other poisonous
compounds (Salehi et al., 2016) (Zhang et al., 2016).
(Hernández Rodiguez et al., 2018) reported that
adsorption of Nickel from aqueous solutions of
activated carbon from spent coffee. Activated carbon
is effective adsorbent for decrease concentrations of
metal ions in aqueous solutions. These adsorbents
have high amount of micropores and mesopores,
large surface area.
2 MATERIALS AND METHODS
2.1 Material
All materials used in this research were of chitosan,
acetate, aquadest, NaOH, activated carbon, standard
solution of Cd
2+
1000 mg/L, and standard solution of
Ni
2+
1000 mg/L.
2.2 Methods
2.2.1 The Making of Chitosan
Chitosan 1.2 grams of, dissolved with 3% of acetate,
about 60 mL, and stirred until it became
homogenous. It was then poured into acrylic glass
and dried up in a oven at the temperature of 60
0
C
within 24 hours. The result was immersed with
NaOH 1M within 24 hours. It was then removed
from the acrylic glass and washed with aquadest
until it was neutral. Finally, it was dried up in room
temperature and stored in desiccators. yielded
absorbent was analyzed by FT IR, SEM, and tensile
strength testing.
2.2.2 The Making of Chitosan – Activated
Carbon
Chitosan 1,2 g, dissolved with 3% of acetate about
60 mL, added by 0.3 gram of activated carbon,
stirred until it was homogenous, poured into acrylic
glass, dried up in an oven at the temperature of 60
0
C
within 24 hours. The result was immersed with
NaOH 1M within 24 hours. It was then removed
from acrylic glass and washed with aquadest until it
Preparation and Characterization of Chitosan with Activated Carbon as Adsorbent to Reduce Level Metal Cadmium (Cd) and Nickel (Ni)
281

was neutral. Finally, it was dried up in room
temperature and stored in desiccators. The yielded
absorbent was analyzed by FT IR, SEM and tensile
strength testing. The same treatment was done with
the variation of weight of additional carbon of 0.4,
0.5, and 0.6 g.
2.2.3 The Use of Chitosan and Chitosan –
Activated Carbon as Adsorbent to
Decrease the Concentration of
Cadmium (Cd) and Nickel (Ni)
Chitosan adsorbent and chitosan-activated carbon
were used to decrease the content of Cd and Ni
metal in standard solution. Chitosan and chitosan-
activated carbon were put into column. About 50 mL
of Cd and Ni standard solution was sucked by a
straw, and it was then skipped from the column with
vacuum pump, and the solution was collected to be
analyzed by using AAS (Atom Absorption
Spectrophotometer). The same treatment was done
for chitosan-activated carbon of coffee grounds with
the variation of weight 0.4, 0.5, and 0.6 g.
3 RESULTS AND DISCUSSIONS
3.1 Characterization of Chitosan and
Chitosan – Activated Carbon
The peaks which appeared in the FT-IR spectrum
was showed in the Figure 2 and Table 1.
Figure 2: FT-IR Spectrum of Chitosan and Chitosan-
Activated Carbon.
Based on FT-IR spectrum in chitosan, there was
OH functional group in the wavelength of 3749.62
cm
-1
and strain vibration of N-H primary amine in
wavelength of 3448.72 cm
-1
(Boggione et al., 2017).
In the wavelength of 1381.03 cm
-1
there was C-N
functional group (Omidi and Kakanejadifard, 2019) .
There was C-H bound in –CH
2
in the wavelength of
2924.09 cm
-1
. In the wavelength of 1635.64 cm
-1
there was vibration peaks of C=O of secondary
amide group. In the wavelength of 1084.14, 1033.85
cm
-1
there was asymmetrical vibration from C-O
functional group (Paluszkiewicz et al., 2011).
The result of the analysis on FI-IR spectrum in
chitosan and chitosan-active carbon of coffee
grounds showed that there was the peaks of
absorption in the wavelength of 3749.62 cm
-1
which
indicated the existence of OH strain vibration, in the
wavelength of 3448.72 cm
-1
which indicated the
existence of N-H strain vibration, in the wavelength
of 2924.09 cm
-1
which indicated that there was C-H
group of aliphatic chain, in the wavelength of
1635.64 cm
-1
which indicated that there was C=O
group from secondary amide, and in the wavelength
of 1381.03, 1084.14, 1033.85 cm
-1
which indicated
that there were
C-N and C-O groups.
Table 1: Functional group of chitosan and chitosan –
activated carbon.
Sample
Wavenumber (cm
-1
)
Functional
Groups
Chitosan
3749.62
OH
3448.72
N-H
2924.09
C-H
1635.64
C=O
1381.03
C-N
1084.14
1033.85
C-O
Chit-Activated
Carbon
386.35
OH
3448.72
N-H
2924.09
C-H
1635.64
C=O
1381.03
C-N
10722.42
1033.85
C-O
In the spectrum of chitosan and chitosan added
by active carbon adsorbent of coffee grounds, there
was no difference in wavelength which indicated
that physical interaction occurred between carbon
and chitosan.
3.2 Characterization of Chitosan and
Chitosan – Activated Carbon with
SEM
Chitosan adsorbent (Figure 3) characterized by SEM
was aimed to find out its morphology.
4000 3500 3000 2500 2000 1500 1000 500
0
5
10
15
20
25
30
35
40
45
50
% T
Wavelengths (cm
-1
)
Chit
Chit-Activated Carbon
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
282
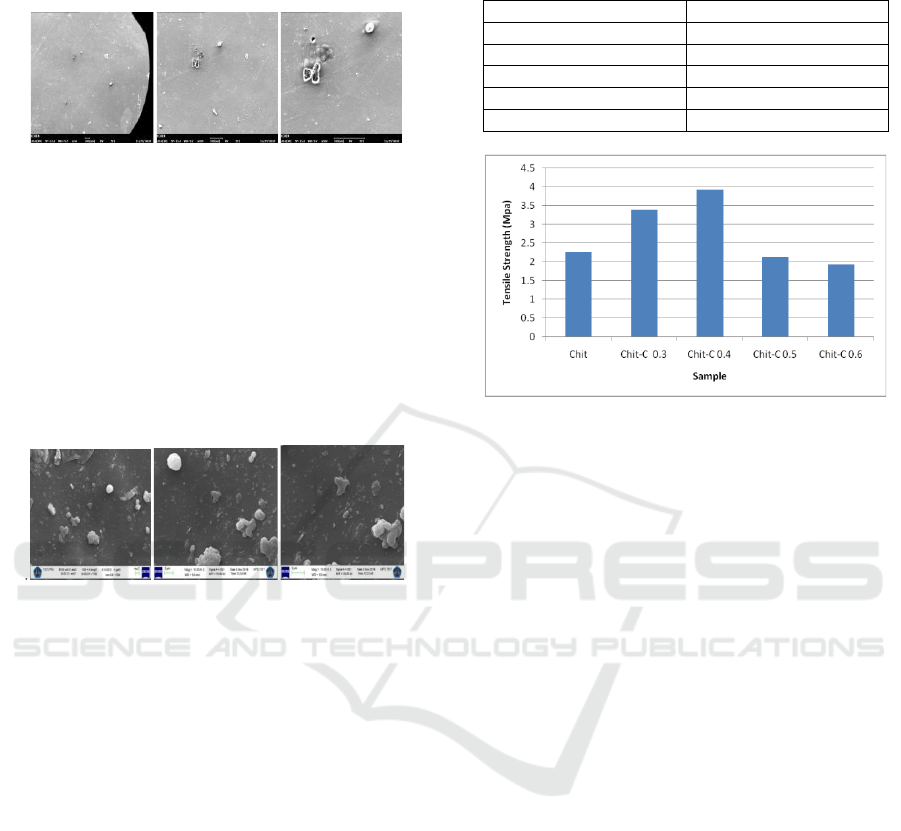
3.2.1 Morphological Analysis of Chitosan
with SEM
Figure 3: Image of SEM on Chitosan adsorbent surface
with enlargement (A) 64x, (B) 200x and (C) 500x.
Chitosan morphological adsorbent was not fine
enough and homogenous because chitosan was not
distributed equally with acetate solvent. This
condition caused the establishment of clump, but it
could also be caused by air bubble which was
trapped during the mould of adsorbent.
3.2.2 Morphological Analysis of Chitosan
with SEM
Figure 4: Image of SEM on Chitosan – activated carbon
Surface with Enlargement (A) 5.000 times, (B) 10.000
times and (C) 15.000 times.
The result of chitosan morphology with the
addition of active carbon could be seen in Figure 4.
Rough surface and some undistributed carbon
particles were caused by the process of making
adsorbent was not homogenous. The addition of
active carbon to chitosan adsorbent influenced the
smoothness of the surface and the compatibility of
the arranging materials (Lessa et al., 2018).
3.3 Characterization of Chitosan and
Chitosan – Activated Carbon
Tensile strength testing in this research aims to
determine the tensile strength of chitosan and
chitosan - activated carbon. The result of the tensile
test from the processing of chitosan and chitosan -
activated carbon samples with a variation of the
weight of carbon addition of 0.3, 0.4, 0.5 and 0.6 g.
Table 2: Tensile strength of chitosan and chitosan –
activated carbon.
Sample
Tensile Strength (MPa)
Chitosan
2.266
Chit – C 0.3 g
3.392
Chit – C 0.4 g
3.924
Chit – C 0.5 g
2.123
Chit – C 0.6 g
1.927
Figure 5: Tensile Strength.
As in Table 2 and Figure 5, chitosan without the
addition of carbon have tensile test strength of 2.266
MPa, and chitosan with the addition of variations in
the weight of activated carbon have different tensile
strength tests. Chitosan with 0.3 g of carbon addition
has a tensile strength of 3.392 MPa, addition 0.4
carbon is 3.924 MPa, addition 0.5 g carbon is 2.123
MPa, addition carbon 0.6 is 1.927 MPa.
Optimum tensile strength results in chitosan with
the addition 0.4 g carbon. Tensile strength test
results have increased with the addition of activated
carbon. activated carbon from coffee ground acts as
an reinforcing agent due to the non-electrostatic
interactions that are bound between activated carbon
and chitosan (Lessa et al., 2018). Addition activated
carbon more than 0.4 g, the tensile strength has
decreases. that with increasing carbon concentration
in chitosan composites it will reduce the value of
tensile strength.
3.4 Analysis Samples with Atomic
Absorption Spectrophotometer
(AAS)
In this research, before the sample analysis was
carried out, we examined the sensitivity and linearity
of atomic absorption spectrophotometer (AAS)
instruments with equipment operating conditions
such as Table 3 and Table 4 below:
Preparation and Characterization of Chitosan with Activated Carbon as Adsorbent to Reduce Level Metal Cadmium (Cd) and Nickel (Ni)
283
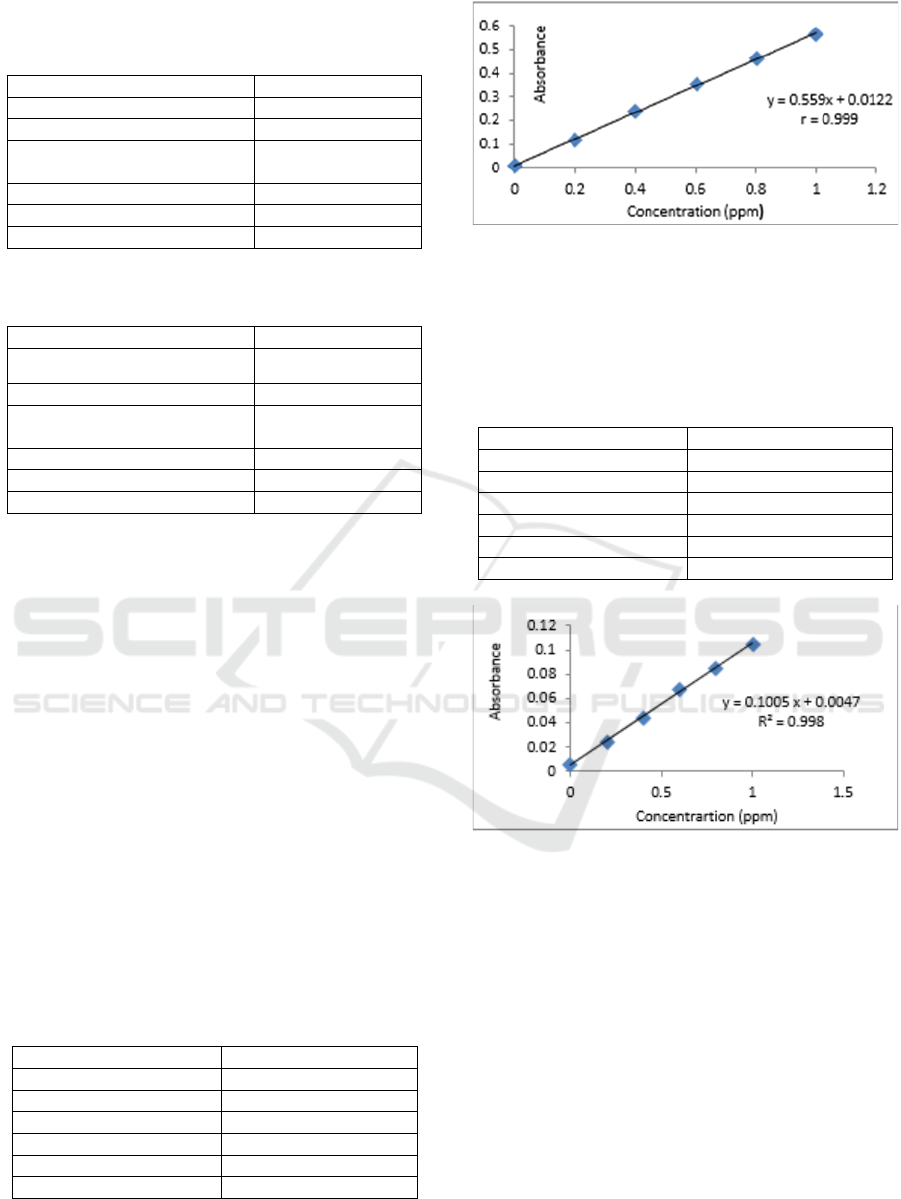
Table 3: Conditions for AAS instruments Shimadzu type
AA-7000 on measurement of concentration Cadmium
(Cd).
Parameter
Cadmium (Cd)
Wavelength (nm)
228,8
Flame
Air – C
2
H
2
Burning gas flow rate
(L / min)
1,8
Air flow rate (L / min)
15,0
Gap Width (nm)
0,7
Furnace Heigth (nm)
7
Table 4: Conditions for AAS instruments Shimadzu type
AA-7000 on measurement of concentration Nickel (Ni).
Parameter
Nickel (Ni)
Wavelength (nm)
232
Flame
Air – C
2
H
2
Burning gas flow rate
(L / min)
1,8
Air flow rate (L / min)
15,0
Gap Width (nm)
0,7
Furnace Heigth (nm)
7
Based on Tables 3 and 4 above the wavelength
for the measurement of nickel and cadmium is
different, the use of cathode lamps that are suitable
for the metal to be analyzed. The cathode lamp will
emit radiation energy that corresponds to the energy
needed for the transition of atomic electrons. With
giving a voltage to a certain current the metal begins
to glow and the cathode metal atom will be
evaporated by sprinkling. The atom will be excited
then emit radiation at a certain wavelength.
3.4.1 Linearity Test
Linearity test aims to determine the correlation
between the concentration of standard solutions with
the response/signal from the absorbance instrument.
In this research evaluation is done by making a
calibration curve (concentration of standard solution
versus absorbance solution) can be seen in the Table
5:
Table 5: Absorbance Linearity Test of Cadmium (Cd).
Concentration (ppm)
Absorbance
0
0,0100
0,2
0,1203
0,4
0,2366
0,6
0,3509
0,8
0,4587
1
0,6773
Figure 6: Calibration Curve of Standard solution
Cadmium.
Based on the calibration curve above (Figure 6),
the correlation coefficient (r) is 0.999, indicating that
the instrument used has a good response.
Table 6: Absorbance Linearity Test of Nickel (Ni).
Concentration (ppm)
Absorbance
0
0,056
0,2
0,0234
0,4
0,0441
0,6
0,0672
0,8
0,0857
1
0.1044
Figure 7: Calibration Curve of Standard solution Nickel.
Based on the calibration curve above (Figure 7),
the correlation coefficient (r) is 0.999, indicating that
the instrument used has a good response.
3.5 Analysis on Measuring Cadmium
Metal
The result of measuring Cd concentration in the
samples with AAS could be seen in the following
Table.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
284
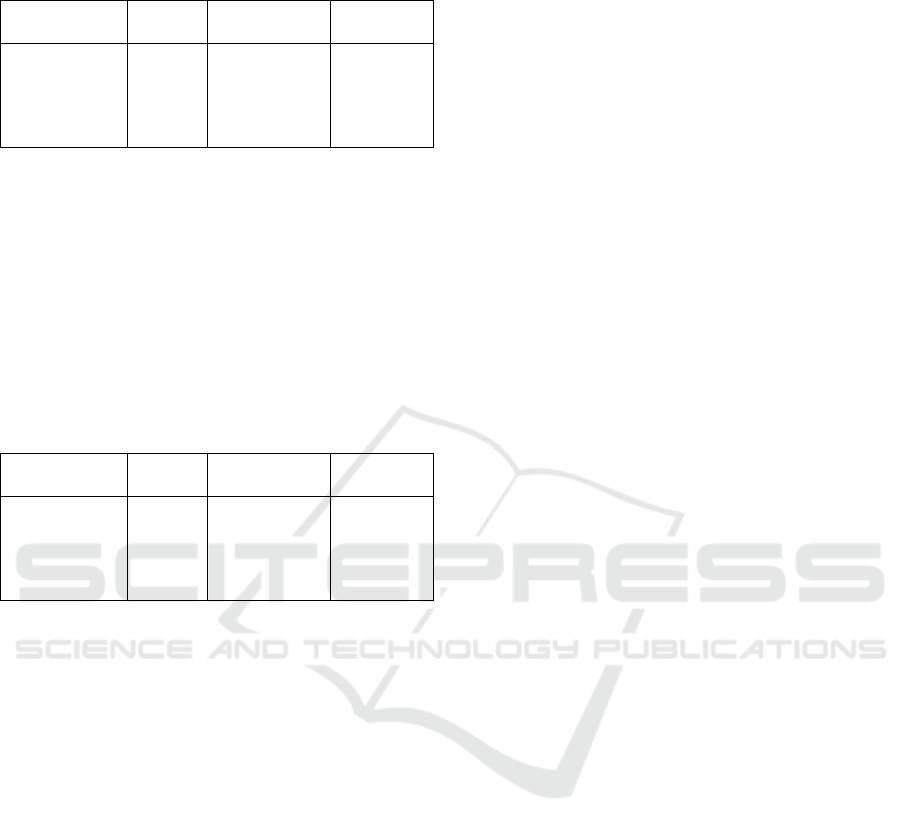
Table 7: Result of Measuring Cd Concentration in the
Samples with AAS.
Sample
Abs
Concentration
(ppm)
%
Adsorption
Chit
Chit – C 0,3 g
Chit – C 0,4 g
Chit – C 0,5 g
Chit – C 0,6 g
0,5571
0,4414
0,3734
0,3089
0,2957
0,9764
0,7769
0,6480
0,5327
0,5091
51,17 %
61,52 %
67,59 %
73,36 %
74,54 %
Based on the data in Table 7, it was found that
the largest amount of Cd metal absorption was in the
chitosan with the addition of 0.6 gram carbon
(74.54%), while the least amount of Cd metal
absorption was in the chitosan without the addition
of carbon (51.1%).
The result of measuring Ni concentration in the
samples with AAS could be seen in the following
Table.
Table 8: Result of Measuring Ni Concentration in the
Samples with AAS.
Sample
Abs
Concentration
(ppm)
%
Adsorption
Chit
Chit – C 0,3 g
Chit – C 0,4 g
Chit – C 0,5 g
Chit – C 0,6 g
0,0897
0,0721
0,0682
0,0609
0,0581
0,8450
0,6706
0,6318
0,5590
0,5313
57,71 %
66,46 %
68,40 %
72,03 %
73,43 %
Based on the data in Table 8, it was found that
the largest amount of absorption of Ni metal was in
the chitosan adsorbent with the addition of 0.6 gram
of active carbon (57.71%). The amount of active
carbon added to chitosan adsorbent (73.43%) while
the least amount of chitosan adsorbent without the
addition of active carbon was 57.71%. The amount
of active carbon added to chitosan adsorbent
influenced the increase in percentage (%) of metal
absorption.
Chitosan has active amine and hydroxyl group
and the capacity to stick on some types of metal. It
can be used as adsorbent of heavy metal such as Zn,
Cd, Cu, Pb, Mg, and Fe. Chitosan active site, either
in the form of NH
2
or in the protonated
NH
3
condition, is able to adsorb heavy metal through
the mechanism of establishing chelate or ion
exchanging. Chitosan have good complexing ability,
-NH
2
groups on chitosan interactions with metals
(Obregón-Valencia and Sun-Kou, 2014).
Amine and hydroxyl group of chitosan was able
to bind metal through some mechanism, including
chemical interaction (like the establishment of
chelate) and electrostatic interaction (like ion
exchanging or the establishment of ion pair). In the
result of their research, it was reported that chitosan
which has been coated with active carbon increased
its percentage (%) of absorptive power on cadmium
metal (Hydari et al., 2012).
Activated carbon is commonly used as adsorbent
to absorb metal because it has high capacity to
absorb and has good endurance against abrasion.
Active carbon has porous structure and wide surface.
(Obregón-Valencia and Sun-Kou, 2014) reported
that active carbon was able to absorb cadmium metal
because it had high chemical reactivity. The use of
commercial active carbon is limited since its price is
relatively high. Active carbon is very effective
adsorbent in absorbing metal in waste water because
of its large number of micropores and mesopores, its
wide surface, and its big pores, and the functional
group on its surface interacts with heavy metal ion
(Hernández Rodiguez et al., 2018). Carbon has the
capacity to absorb metal because it has large pores.
The more the in its pores so that metal content in
chitosan decrease by adding more carbon.
4 CONCLUSIONS
Chitosan and chitosan-active carbon can be used as
adsorbent to decrease Cd and Ni metal content. The
best decrease in Cd metal content is found in
chitosan by adding 0.6 g carbon (75.45%) and the
best decrease in Ni metal content is found in
chitosan by adding 0.6 g carbon (73.43%).
REFERENCES
Ahmad, M., Manzoor, K., Ikram, S., 2017. Versatile
nature of hetero-chitosan based derivatives as
biodegradable adsorbent for heavy metal ions; a
review. Int. J. Biol. Macromol. 105, 190–203.
Al-Malack, M.H., Dauda, M., 2017. Competitive
adsorption of cadmium and phenol on activated carbon
produced from municipal sludge. J. Environ. Chem.
Eng. 5, 2718–2729.
Boggione, M.J., Mahl, C.R.A., Beppu, M.M., Farruggia,
B., 2017. Synthesis and characterization of chitosan
membranes functionalized with amino acids and
copper for adsorption of endoglucanase. Powder
Technol. 315, 250–257.
Choi, C., Nam, J.P., Nah, J.W., 2016. Application of
chitosan and chitosan derivatives as biomaterials. J.
Ind. Eng. Chem. 33, 1–10.
Coman, V., Robotin, B., Ilea, P., 2013. Nickel
recovery/removal from industrial wastes: A review.
Resour. Conserv. Recycl. 73, 229–238.
Preparation and Characterization of Chitosan with Activated Carbon as Adsorbent to Reduce Level Metal Cadmium (Cd) and Nickel (Ni)
285

Djati Utomo, H., 2015. Lead Adsorption onto Various
Solid Surfaces. Nat. Resour. 6, 152–158.
Hegazi, H.A., 2013. Removal of heavy metals from
wastewater using agricultural and industrial wastes as
adsorbents. HBRC J. 9, 276–282.
Hernández Rodiguez, M., Yperman, J., Carleer, R.,
Maggen, J., Daddi, D., Gryglewicz, G., Van der
Bruggen, B., Falcón Hernández, J., Otero Calvis, A.,
2018. Adsorption of Ni(II) on spent coffee and coffee
husk based activated carbon. J. Environ. Chem. Eng.
6, 1161–1170.
Hydari, S., Sharififard, H., Nabavinia, M., Parvizi, M.
reza, 2012. A comparative investigation on removal
performances of commercial activated carbon,
chitosan biosorbent and chitosan/activated carbon
composite for cadmium. Chem. Eng. J. 193–194, 276–
282.
Jeon, C., 2017. Adsorption and recovery of immobilized
coffee ground beads for silver ions from industrial
wastewater. J. Ind. Eng. Chem. 53, 261–267.
Lessa, E.F., Nunes, M.L., Fajardo, A.R., 2018.
Chitosan/waste coffee-grounds composite: An
efficient and eco-friendly adsorbent for removal of
pharmaceutical contaminants from water. Carbohyd.
Polym. 189, 257–266.
Li, M., Zhang, Z., Li, R., Wang, J.J., Ali, A., 2016.
Removal of Pb(II) and Cd(II) ions from aqueous
solution by thiosemicarbazide modified chitosan. Int.
J. Biol. Macromol. 86, 876–884.
Liu, C., Bai, R., 2014. Recent advances in chitosan and its
derivatives as adsorbents for removal of pollutants
from water and wastewater. Curr. Opin. Chem. Eng. 4,
62–70.
Obregón-Valencia, D., Sun-Kou, M.D.R., 2014.
Comparative cadmium adsorption study on activated
carbon prepared from aguaje (Mauritia flexuosa) and
olive fruit stones (Olea europaea L.). J. Environ.
Chem. Eng. 2, 2280–2288.
Omidi, S., Kakanejadifard, A., 2019. Modification of
chitosan and chitosan nanoparticle by long chain
pyridinium compounds: Synthesis, characterization,
antibacterial, and antioxidant activities. Carbohyd.
Polym. 208, 477–485.
Pal, P., Pal, A., 2017. Surfactant-modified chitosan beads
for cadmium ion adsorption. Int. J. Biol. Macromol.
104, 1548–1555.
Paluszkiewicz, C., Stodolak, E., Hasik, M., Blazewicz, M.,
2011. FT-IR study of montmorillonite-chitosan
nanocomposite materials. Spectrochim. Acta - Part A
Mol. Biomol. Spectrosc. 79, 784–788.
Paridah, M.., Moradbak, A., Mohamed, A.., Owolabi, F.
abdulwahab taiwo, Asniza, M., Abdul Khalid, S.H..,
2016. We are IntechOpen , the world ’ s leading
publisher of Open Access books Built by scientists ,
for scientists TOP 1 %. Intech i, 13.
Pujol, D., Liu, C., Gominho, J., Olivella, M.À., Fiol, N.,
Villaescusa, I., Pereira, H., 2013. The chemical
composition of exhausted coffee waste. Ind. Crop.
Prod. 50, 423–429.
Rahate, K.P., 2013. AN OV ERVIEW ON VARIOUS
MODIFICATIONS OF CHITOSAN AND IT’S
APPLICATIONS R. Rajasree and K.P. Rahate*
Amrita School of Pharmacy, Amrita Viswa
Vidyapeetham University, AIMS Ponekkara P. O.,
Kochi – 682041, Kerala, India 4, 4175–4193.
Raja Sulaiman, R.N., Othman, N., Mohamed Noah, N.F.,
Jusoh, N., 2018. Removal of nickel from industrial
effluent using a synergistic mixtures of acidic and
solvating carriers in palm oil-based diluent via
supported liquid membrane process. Chem. Eng. Res.
Des. 137, 360–375.
Reck, B.K., Müller, D.B., Rostkowski, K., Graedel, T.E.,
n.d. Supporting Information 67, 1–26.
Salehi, E., Daraei, P., Arabi Shamsabadi, A., 2016. A
review on chitosan-based adsorptive membranes.
Carbohyd. Polym. 152, 419–432.
Utomo, H.D., Hunter, K.A., 2006. Adsorption of heavy
metals by exhausted coffee grounds as a potential
treatment method for waste waters. e-Journal Surf. Sci.
Nanotechnol. 4, 504–506.
Vakili, M., Rafatullah, M., Salamatinia, B., Abdullah,
A.Z., Ibrahim, M.H., Tan, K.B., Gholami, Z.,
Amouzgar, P., 2014. Application of chitosan and its
derivatives as adsorbents for dye removal from water
and wastewater: A review. Carbohyd. Polym. 113,
115–130.
Zein, S.H., Gyamera, B.A., Skoulou, V.K., 2017.
Nanocarbons from acid pretreated Waste Coffee
Grounds using microwave radiation. Mater. Lett. 193,
46–49.
Zhang, L., Zeng, Y., Cheng, Z., 2016. Removal of heavy
metal ions using chitosan and modified chitosan: A
review. J. Mol. Liq. 214, 175–191.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
286
