
Thermal Properties of Chitosan-roselle Films
Irwana Nainggolan
1,2*
, Tulus Ikhsan Nasution
3,2
and Khairel Rafezi Ahmad
4
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan 20155,
Sumatera Utara, Indonesia
2
Pusat Unggulan Green Chitosan dan Material Maju, Universitas Sumatera Utara, Medan 20155, Sumatera Utara,
Indonesia
3
Department of Physic, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan 20155,
Sumatera Utara, Indonesia
4
School of Materials Engineering, Universiti Malaysia Perlis, Jejawi 02600, Arau, Perlis, Malaysia
Keywords: Chitosan-roselle, Thermal Properties, Casting Method, Cure Temperature.
Abstract: The chitosan-roselle films were fabricated by casting method. The roselle ratio and cure temperature of films
were varied to study the thermal properties of the chitosan-roselle films. Thermal properties of chitosan-
roselle films showed higher heat resistance when blend roselle ratio and cure temperature were increased. The
temperature at maximum degradation rate of chitosan-roselle films was determined by DTG. The chitosan-
roselle films were degrade faster when the roselle ratio increased at ambient temperature. The cure
temperature was varied to 50, 60 and 70
o
C, the most optimum cure temperature was 60
0
C which showed the
lowest weight loss and exhibited the highest heat resistance compared to the other cure temperature. SEM
results showed the surface structure of different ratio of chitosan-roselle films which were cure at 50, 60 and
70
o
C. The surface structure become smoother when the ratio of chitosan-roselle was increased. FTIR result
showed that the intensity percentage of functional group exist in pure chitosan films were improved by adding
roselle to become chitosan-roselle films. The results showed the most optimum cure temperature was 60
o
C
which showed high intensity percentage compared to the other cure temperature.
1 INTRODUCTION
The potential of biopolymers, especially the polymers
obtained from renewable resources has long been
recognized. In the last decade there has been
increasing interest in developing thermoplastic
biopolymers, especially those are derived from
renewable sources. However, these biopolymers are
mostly used in food industry but very less to replace
synthetic materials. Packaging based on conventional
synthetic materials has brought the threat to ecology.
These concerns lead to a need for more effective
safety regulations and better system to maintain the
quality of the food (Portes, 2009). The biopolymers
that are used as coating in food packaging have the
advantages to be available from biocompatible,
biodegradable and better quality of fresh foods as
well as of environmentally friendly packaging.
Biomass is a naturally abundant source and has long
been recognized as sustainable biopolymers, and in
the recent years, growing environmental awareness
has led to studies of biopolymer as alternative
packaging films to be used as edible coatings in food
packaging (Tharanathan, 2003). This approach will
be very interesting from an academic point of view
and large-scale usage in the food industry.
Biopolymers such as polysaccharides, proteins, lipids
and combination of those components are potential to
be made as films. Biopolymer films are known as
edible films. Edible films have been particularly
considered in food preservation because of their
capability in improving global food quality
(Tharanathan, 2003 and Norashikin, 2010).
Nowadays, the materials most used for packaging
are petrochemical based polymers because of their
availability in large quantities with low cost and many
functional features, such as good tensile strength and
tear strength, good barrier properties and heat
stability of oxygen. However, these materials are non-
biodegradable which lead to very serious ecological
and environment pollution problem. As a solution,
there is a need to shift to eco-friendly biodegradable
materials, especially from renewable agriculture by
266
Nainggolan, I., Ikhsan Nasution, T. and Rafezi Ahmad, K.
Thermal Properties of Chitosan-roselle Films.
DOI: 10.5220/0008921402660273
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 266-273
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

products such as chitosan, food processing industry
wastes and low- cost natural resources such as starch
(Alves, 2006).
In this context, chitin is representing as a second
most abundant biopolymer after cellulose (Ou, 2005).
Chitosan is a linear amino polysaccharide obtained
from the deacetylation of chitin (Portes, 2009) (a
process of remove the acetyl function group at chitin),
the major structural component of the exoskeleton of
invertebrates and the cell walls of some plant like
fungi. In addition, in view of the most biopolymers
are either biodegradable or compostable, it can also
be argued that chitosan could be fit with the ‘cradle-
to-cradle’ concept, which means that on disposal, it
could become ‘food’ for the future generation of
materials (Ou, 2005).
Chitosan has biodegradability and
biocompatibility properties, and compared with
chitin, it shows better solubility in common solvents.
This is why chitosan is commonly used in several
applications instead of chitin. Chitin and chitosan
have applications from cosmetic to pharmaceutical
industries and its total annual production estimated at
least 1000 tons per year. Due to this variety of
applications, there is a necessary to fully understand
their molecular nature to increase their uses (Leceta,
2013). Chitosan has been studied as natural
antibacterial, bio-based active films and excellent
compatibility with other substances by the presence
of the high density of amino groups and hydroxyl
groups in chitosan polymer structure (Betzabe, 2009
and Ngah, 2011).
Chitosan film can be easily prepared, but it is
difficult to handle due to the curving during drying
phase, if no assisting means and weight are used to
keep it straight (Kammoun, 2013 and Annala, 2007).
In general, the properties of chitosan solutions depend
on several parameters including ionic strength,
concentration, temperature, acid concentration and
type of acid. Acetic acid was used in this study since
chitosan is soluble in acetic acid (ZhenXing, 2007;
Esam, 2010 and Jayakumar, 2007). In this study,
roselle was used as admixture to produce chitosan-
roselle films. Roselle has unique properties such as
ionic liquid forming because it contains of high
minerals concentration especially ferrum and
minerals enable the blend films are thermally stable.
Blends of chitosan and roselle were produced by
using acetic acid as a solvent media. Different
temperatures which are 50, 60 and 70
o
C were applied
to cure chitosan-roselle films.
Throughout the years, some researches have done
some improvement on the performance of
biodegradable film in the food packaging area. From
all the points above, an attempt was done to
investigate the effect of different chitosan
concentration in a composite biodegradable film
(Cissé, 2013 and Kumirska, 2010). Chitosan is a
linear β-1,4-D-glucosamine is a biocompatible,
nontoxic compound mainly obtained by deacetylation
of chitin, a natural structural component present for
instance in crustaceans. This biopolymer presents
interesting properties by excellent film forming
capacity and gas and aroma barrier properties at dry
conditions, which makes it a suitable material for
designing food packaging structure. However,
biodegradable films are often have limited
temperature resistance (Kammoun, 2013). A simple
blend process of the chitosan solution with roselle
extract was used to produce the chitosan-roselle
which may able to be used to improve the thermal
properties of chitosan films.
2 MATERIALS AND METHOD
Chitosan powder (medium grade, 99.9% purity)
synthesized from crab shell was purchased from Sigma
Aldrich Ltd, medium molecular weight). 2% of acetic
acid with 99.9% purity (Merck) and methanol (GMBH)
were used as solvents in this research. Roselle was
extracted by using a mixture of acetic acid, methanol
and deionized water with ratio 1:4:5 and it was used as
a biomaterial to be blended with chitosan. Chitosan
solution gel was prepared by dissolving weighted
chitosan powder into 50 ml of acetic acid 2%. The
chitosan powder was weighted 1.75 g and pours into
100 ml of acetic acid 2% and then stirred by using a
magnetic stirrer with 300 rpm for 12 hours at room
temperature to fully dissolve the chitosan powder into
the acetic acid. Acetic acid 2% was the most suitable
solvent to dissolve chitosan. This is due to chitosan
which is soluble in weak acid. Chitosan-roselle solution
was prepared by mixing roselle extract with the chitosan
solution. First, to prepare roselle extract, the seed of
roselle flower was removed to obtain roselle calyxes.
Then the calyxes were washed with tab water to remove
unwanted particle and cut into small pieces to be
mashed. Then, it was blended into paste follow by
extract squeezing into a breaker. A mixture of acetic
acid, methanol and deionized water were prepared with
ratio 1:4:5. The 50 ml blended roselle juice was added
into 50 ml mixture of methanol, acetic acid and
deionized water. Then, the mixture was stirred by using
a magnetic stirrer with 300 rpm for 30 minutes at room
temperature. The well mixed mixture was then filtered
by using 125 mm size of filter paper. After the chitosan-
roselle solution was prepared, it was poured into a mold
Thermal Properties of Chitosan-roselle Films
267
and left it to dry in an oven with different curing
temperature which were 50, 60 and 70
o
C. The film
thickness was measured with a 0-25 mm micrometer
with an accuracy of ± 0.01 mm in four random locations
for each film. After the solution completely dry into
film, several characterizations using Differential
Scanning Calorimeter (DSC), Thermogravimetry
Analyzer (TGA), Scanning Electron Microscope (SEM)
and Fourier Transform Infrared Spectrocopy (FTIR)
were performed.
The difference in heat flow of the composite was
measured as a function temperature by using
Differential Scanning Calorimetry (DSC) which
recorded by a NETZSCH DSC 204 F1 (Germany)
instrument with standard ASTM D34218 DSC. The
onset temperature and degree of crystallinity data was
obtained from the DSC thermograms. In this study,
DSC was used to determine the possible transition of the
chitosan-roselle composite film. The thermogram was
run by placing the samples (3-10 mg) in an aluminium
pan and a heating scan was conducted from range of 30
to 480
o
C at a heat rate 10
o
C/min. Purge gas flow was
adjusted to 50 ml/min using nitrogen gas. The mass or
changes in mass of the sample as a function of
temperature or time both was measured by using
Thermogravimetric Analysis (TGA). TGA analysis was
conducted according to ASTM D258 standards. The
specimens of 10 mg were put in the platinum pan. TGA
measures the amount and rate of change in the weight of
material as a function of temperature or time in a
controlled atmosphere. This technique can characterize
materials that exhibit weight loss or gain due to
decomposition, oxidation, or dehydration (Dev Raj,
2013). TGA was used to determine the estimated
lifetime and weight change in the samples.
Purge gas flow need to be adjusted to 50 ml/min
using nitrogen gas. The heating rate was set at 30 to
900
o
C at 10
o
C/min. In this study, the surface
morphology of chitosan-roselle films were examined by
using a Jeol JSM- 6460LA scanning electron
microscopy (SEM) at an accelerating voltage of 5 kV.
FT-IR spectra measurements were conducted by a
Perkin Elmer RX1 FT-IR spectrometer.
3 RESULTS AND DISCUSSION
3.1 Making of Charcoal
All films were easily peeled from the film-casting
mold. Thin film formation was easy due to the low
surface tension on the casting surface. Peeled films
were then conditioned at testing temperature to obtain
soft, flexible, and easy to handle films. Chitosan-
roselle films were cured at temperature 50, 60 and
70
o
C.
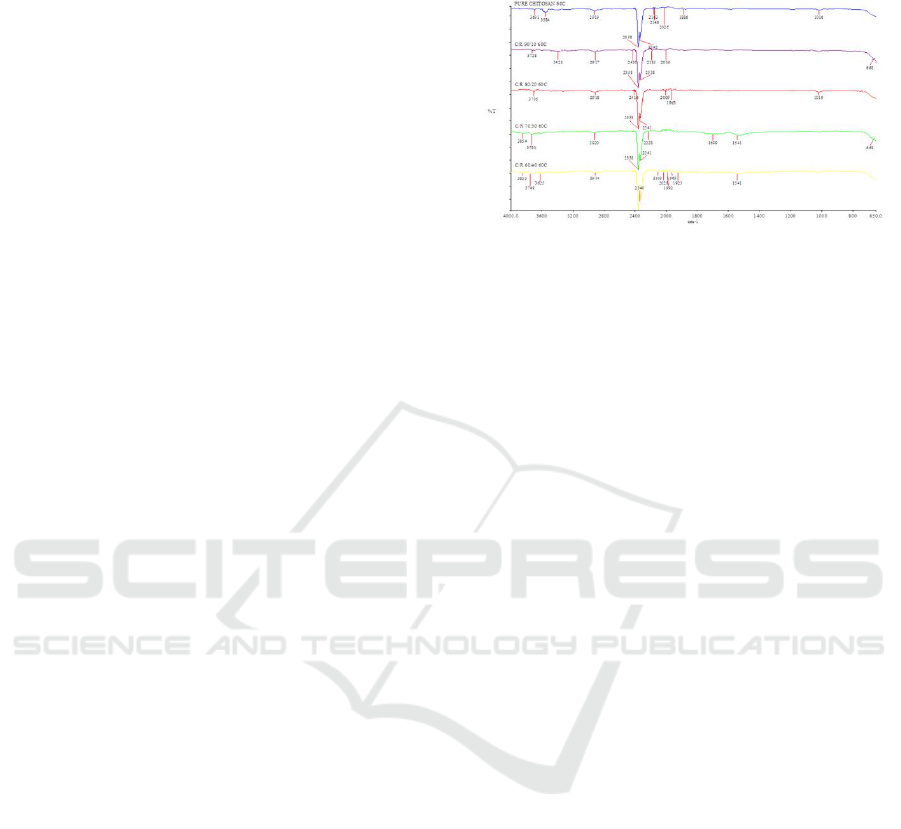
Figure 1: The FTIR spectra of chitosan-roselle films which
are cured at 60
o
C.
FT-IR spectrum was used to identify the chemical
structure of chitosan-roselle films by scanning the
chitosan-roselle films using FT-IR. The results of FT-
IR spectra which were cured at 60
o
C are reported in
Figure 1. The O–H stretching mode of chitosan was
observed in the region of 3200– 3650 cm
-1
where in
these spectra pure chitosan falls at 3618 cm
-1
. This
peak due to overlapping of extension of C-H and O-
H in chitosan. The peak at 2395 cm
–1
is due to
extension of C–H group. The peaks at 2362 and 2339
cm
-1
are due to C-N bond. Since the absorption peaks
ranges is available until 3650 cm
-1
, so the peaks over
3650 cm
-1
were assumed as impurities. The peak at
2060 to 1886 cm
-1
indicating the existence of Fe-S
bond. The presence of the ferrodoxin in the structure
can improve the thermal properties of the film. The
peaks at 1623 cm
–1
were the C=O stretching (amide
I) and NH bending (amide II). The peak near 1780
cm
–1
suggested the presence of a carbonyl group in
the chitosan and roselle films. The absorption peaks
at 1016 and 1019 cm
-1
were probably due to the C–
O–H bending mode. Clear differences can be detected
in the infrared spectra of chitosan and roselle, both in
the different absorbance values and shapes of the
bands and in their location. A decrease in the intensity
of the O–H absorption band at 3323 cm
-1
was
observed, indicating that the hydroxyl group contents
in roselle. The lower xylan content in roselle was
proved by a weak carbonyl band at 1699 cm
-1
. The
enhanced carbonyl absorption peak at 1779 cm
-1
(C=O ester), C–H absorption at 1381 cm
-1
(C–CH3),
and C–O stretching band at 1242 cm
-1
confirmed the
formation of ester bonds. Also, it was proved by an
increase in the intensity of OH in plane bending
vibration at 1366 cm
-1
band specific to the component
cellulose and hemicelluloses. A small band at 16243
cm
-1
were assigned to the absorbed water and ß-
glucosidic linkages between the sugar units,
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
268

respectively. Weak absorptions between 1500 and
1400 cm
-1
arose from the aromatic ring vibrations and
ring breathing with C–O stretching in lignin.
Figure 2 shows curve of weight loss (%) as a
function of temperature for chitosan-roselle films
which are cured at 50 0C with heating rate of 10
o
C
/min in the temperature range from room temperature
to 980
o
C. All ratio of chitosan-roselle films almost
have similarity in the shape of the curves, but
magnitudes of weight loss is varied. The initial weight
loss for all the samples starts near 100
o
C, the loss of
weight is about 10%, as a result of removing the
adsorbed water on the composite and loss of bound
water and acetic acid from the composites. The
weight increase in the range of 33 to 70
o
C is because
of the reaction between chitosan and roselle at ratio
60:40. It was, however, much smaller than the weight
loss in the range of 70 to 120
o
C and then the weight
increases almost disappeared. The weight loss in the
second starts in range 82.13-86.05
o
C and continues
up to 280
o
C of melting temperature, Tm. There was
about 40% weight loss was due to the decomposition
of blend. The third weight loss was observed in the
range 66.35- 67.69
o
C which probably due to the
structural decomposition of the blend caused by the
loss of water. There was about 70% weight loss and
it may be referred to a complex process including the
dehydration of the saccharide rings and
depolymerization. As would be expected, the higher
the gas temperature, the faster the heating rate and the
more rapid and weight loss is increase. The TGA
curves for the chitosan-roselle films with ratio 90:10
and 60:40 illustrated similar patterns of weight loss,
however, chitosan-roselle films with ratio 50:50
exhibited a greater weight loss than the other samples
at temperature around 290
o
C. It was observed that the
weight loss of chitosan-roselle films with ratio 60:40
was the highest at first until Tm. It is probably due to
the easy of chitosan-roselle to react with each other.
After passed the Tm, the weight loss of other ratio
was higher than it. The chitosan-roselle films of ratio
90:10 and 50:50 were more resistance to heat before
reach Tm and rapidly degrade after reach Tm. At this
cure temperature, chitosan-roselle film with ratio
90:10 showed great thermal resistance properties than
other ratios. The peaks of chitosan-roselle blend films
were very weak around temperature 600
o
C may be
due to the desorption of the adsorbed water on the
sample when it was kept overnight. It can be seen
that the DTGA curves and the maximum peaks
temperature shifted as the heating rate increases. An
increase of the heating rate tended to delay thermal
decomposition process towards higher temperatures,
most probably due to heating rate implies that the
material reaches that temperature in a shorter time.
According to the results, the yield of chitosan-roselle
films at about 20% and the behavior of TGA curves
were almost similar in the composites films (Figure
2-Figure 7).
Figure 2: TGA curves for thermal decomposition of
chitosan-roselle films at 50
o
C.
Figure 3: DTGA curves for thermal decomposition of
chitosan-roselle films at 50
o
C.
Figure 3 shows curve of weight loss (%) as a
function of temperature for chitosan-roselle films
which cured at 60
o
C with heating rate of 10
o
C/min in
the temperature range from room temperature to
980
o
C. From the Figure 2-7, all ratio of chitosan-
roselle films almost have similarity in the shape of the
curves, but magnitudes of weight loss were varied.
The initial weight loss for all samples at
approximately 100
o
C is around 10%. This may due to
the evaporation of water and due to the loss of water
content and acetic acid from the composite. The
weight loss in the second range started around 180
o
C
and continued up to 253
o
C melting temperature, Tm.
refer to combustion of cellulose from roselle. The
weight loss third stage observed in the range 255
o
C
corresponds to structural decomposition of the blend
which there was about 66% weight loss of the original
sample. It was observed that the weight loss of
chitosan-roselle films with ratio 60:40 was highest
than others ratio. Combination of chitosan-roselle
improved the thermal properties of chitosan film at
cure temperature 60
o
C. This ratio was more resistance
to heat before reach Tm and rapidly degraded after
reach Tm. At this cure temperature, chitosan-roselle
Thermal Properties of Chitosan-roselle Films
269

with ratio 60:40 showed great thermal resistance
properties than other ratios. According to the results,
the char yield of chitosan-roselle films at about 14%
and the behavior of TGA curves were almost similar
in the composites films (Figure 2-Figure 7). The
results showed that, the char yield of chitosan:roselle
at all ratio were less which were between 15% and
17% respectively. Therefore, the addition of roselle
can improve the thermal stability of chitosan-roselle
films. It was found that the char yield of chitosan-
roselle film was enhanced as addition of roselle in
chitosan-roselle composite. These results means high
thermal stability of chitosan-roselle films.
Figure 4: TGA curves for thermal decomposition of
chitosan-roselle films at 60
o
C.
Figure 5: DTGA curves for thermal decomposition of
chitosan-roselle films at 60
o
C.
Figure 6 shows curve of weight loss (%) as a
function of temperature for chitosan-roselle films
which cured at 70
o
C with heating rate of 10
o
C/min in
the temperature range room temperature to 980
o
C.
From the Figure 7, the initial weight loss for all
samples at approximately 100
o
C was around 10%.
This may due to the evaporation of water and due to
the loss of water content and acetic acid from the
composite. The weight loss in the second range
started around 202.5
o
C which was referred to
combustion of cellulose from roselle. The weight loss
third stage found in range 255-463.1
o
C corresponds
to a complex process including the dehydration of the
saccharide rings and depolymerization. The TGA
curves showed that chitosan-roselle composite films
slowly decline above 350
o
C with a maximum rate of
decomposition occurring at about 300-340
o
C.
Figure 6: TGA curves for thermal decomposition of
chitosan-roselle films at 70
o
C.
Figure 7: DTGA curves for thermal decomposition of
chitosan-roselle films at 70
o
C.
Table 1: The thermal decomposition of chitosan-roselle
films.
Differential scanning calorimeter (DSC)
measurements were performed to estimate the
thermal transition of chitosan-roselle films by ratio
90:10 and 60:40 of chitosan-roselle at different
testing temperature. All the samples were heated from
room temperature to 480°C and rate of 10
o
C/min.
The DSC glass transitions are shown in Figure 8-10.
Figure 8 shows thermal transition of the chitosan-
Sample
Temperature
Decomposition (ºC)
Weight loss (%)
2
nd
stage at
3
rd
stage
2
nd
stage at
3
rd
stage
C:R 90:10 50
◦
C
132.4
193.8
17.87
35.65
C:R 60:40 50
◦
C
203
259
16.85
35.97
C:R 90:10 60
◦
C
180
233.1
32.55
34.55
C:R 60:40 60
◦
C
197
274
17.63
32.96
C:R 90:10 70
◦
C
202.5
-
27.06
-
C:R 60:40 70
◦
C
191
255
15.62
33.27
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
270
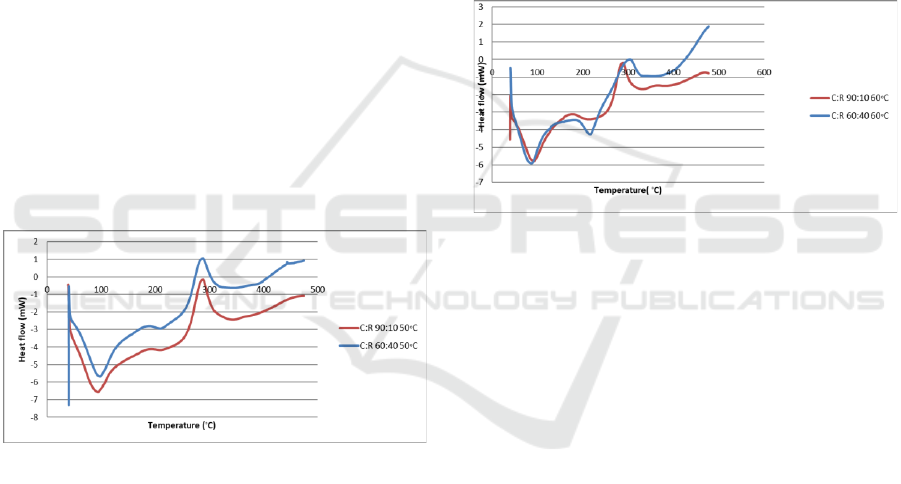
roselle films in cure temperature 50
o
C. Figure 8 does
not show any peak for glass transition temperature. It
shows crystallization and melting point chitosan-
roselle films as stated in the Table 1. Chitosan-roselle
films showed an endothermic peak at around 94.48 to
98.33°C associated with the dehydration (loss of
water associated with the hydrophilic groups of the
polymer) in film matrix. The exothermic peak which
appears in the temperature ranges about 279.45 and
281.89°C corresponds to the decomposition of the
polymer. Based on the previous research, the thermal
degradation of chitosan begins at about 250°C. The
exothermic can be explained through the
crystallization of chitosan. The melting point of
chitosan-roselle films were 212.85 and 209.96 °C
respectively following the ratio. These peak
temperatures tend to shift to lower temperatures with
increased roselle concentration. In crystalline
molecules of original chitosan was packed in a way
that their interactions are strongest. When the
chitosan was blended, it stretches the crystal lattice,
molecules are not in the optimal positions and their
interactions are weaker, thus less energy is required
to break them. At cure temperature 50
o
C, chitosan-
roselle films with ratio 60:40 showed the highest
melting temperature while 90:10 showed the lowest
melting temperature.
Figure 8: The DSC glass transitions of chitosan-roselle
films at 50◦C.
Thermal transition of the chitosan: roselle films
were affected by different ratio of chitosan and roselle
in cure temperatures 60◦C as shown in Figure 9. The
sample were heated from room temperature to 480
o
C
at a rate of 10
o
C/min. Chitosan-roselle films exhibited
a broad endothermic peak at 91.59
o
C and 85.82
o
C
respectively following the ratio because of the
dehydration (loss of water associated with the
hydrophilic groups of the polymer) in the films. The
exothermic peak which appeared in the temperature
ranges at 287.91 and 302.34°C corresponds to the
decomposition of the polymer. Based on the previous
research, the thermal degradation of chitosan begins
at about 250°C. The exothermic can be explained
through the crystallization of chitosan. The melting
point of chitosan-roselle films were 214.77 and
216.69°C respectively following the ratio. In
crystalline molecules of original chitosan was packed
in a way that their interactions are strongest. When
the chitosan is blended, it stretches the crystal lattice,
molecules are not in the optimal positions and their
interactions are weaker, thus less energy is required
to break them. At cure temperature 60
o
C, chitosan-
roselle films with ratio 60:40 showed the highest
melting temperature while 90:10 showed the lowest
melting temperature. When compare the result of cure
temperature of 60
o
C with other two cure temperature,
it showed the highest melting temperature. In other
meaning, 60
o
C is the most optimum temperature to
produce high melting temperature (heat resistance
films).
Figure 9: The DSC glass transitions of chitosan-roselle
films at 60◦C.
Thermal transition of the chitosan-roselle films
were affected by different ratio of chitosan and roselle
in cure temperatures 70
o
C as shown in Figure 10. The
sample were heated from room temperature to 480
o
C
at a rate of 10
o
C/min. Chitosan-roselle films exhibited
a broad endothermic peak at 84.85 and 88.7
o
C
respectively following the ratio because of the
dehydration (loss of water associated with the
hydrophilic groups of the polymer) in the films. The
exothermic peak which appeared in the temperature
ranges at 303.31 and 307.99
o
C corresponded to the
decomposition of the polymer. Based on the previous
research, the thermal degradation of chitosan begins
at about 250°C. The exothermic can be explained
through the crystallization of chitosan. The melting
point of chitosan-roselle films were 215.73 and
208.03°C respectively following the ratio. In
crystalline molecules of original chitosan was packed
in a way that their interactions are strongest. When
the chitosan was blended, it was stretched the crystal
lattice, molecules are not in the optimal positions and
their interactions are weaker, thus less energy is
required to break them. At cure temperature 70
o
C,
chitosan-roselle films with ratio 60:40 showed the
Thermal Properties of Chitosan-roselle Films
271

highest melting temperature while 90:10 showed the
lowest melting temperature. When compare the result
of cure temperature of 70◦C with other two cure
temperature, it showed the lowest melting
temperature.
Figure 10: The DSC glass transitions of chitosan-roselle
films at 70◦C.
Table 2: The melting temperature of chitosan-roselle films.
Sample name
Crystallization
temperature
(
o
C)
Melting
temperature
(
o
C)
C:R 90:10 50
o
C
94.48
212.85
C:R 60:40 50
o
C
98.33
209.96
C:R 90:10 60
o
C
91.59
216.77
C:R 60:40 60
o
C
85.82
214.69
C:R 90:10 70
o
C
84.85
215.73
C:R 60:40 70
o
C
88.70
208.03
Scanning Electron Microscopy (SEM) was used to
study the surface morphology of the chitosan-roselle
films. There are 4 magnification used which are x100,
x250, x500 and x2000. The morphology of chitosan-
roselle films which cured at 60◦C are reported in
Figure 11. SEM images for chitosan-roselle films
with ratio 100:0 and 90:10 showed the largest rough
surface. The surface of chitosan films was smooth and
not homogenous due to the present of phase
separation. Roselle did not disperse well within the
chitosan matrix in the blend films. Chitosan-roselle
films with the ratio of 80:20, 70:30 and 60:40 had
smoother surface than chitosan-roselle films with
ratio 100:0 and 90:10. From the micrograph under
magnification, the surface of chitosan-roselle films
became smoother as the ratio of chitosan-roselle films
decreased. This may be due to chitosan-roselle films
particles have failed to crystallize.
Figure 11: SEM images of chitosan-roselle films which
cured at 60◦C.
4 CONCLUSIONS
FTIR result showed that the intensity percentage of
functional group exist in pure chitosan films were
improved by adding roselle to become chitosan-
roselle films. Besides, it showed the most optimum
cure temperature was 60
o
C which showed high
intensity percentage compared to other cure
temperature (50 and 70
o
C). From the thermal testing
(TGA, DTG, and DSC), the melting temperature of
chitosan-roselle films showed higher melting
temperature when increase the roselle ratio in the
blend films at cure temperature 50, 60 and 70
o
C.
This means that chitosan-roselle films showed
higher heat resistance when ratio of roselle
increased. The temperature at maximum
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
272

degradation rate of chitosan-roselle films determined
by DTG were lower when increased the roselle ratio
in the blend films at cure temperature of 50, 60 and
70
o
C. Thus, the chitosan-roselle films were degrade
faster when increased the roselle ratio in blend film
at ambient temperature. From the cure temperature
50, 60 and 70
o
C, the most optimum cure temperature
was 60
o
C which showed the lowest weight loss and
exhibited the highest heat resistance compared to
other cure temperature. SEM results showed the
surface structure of different ratio of chitosan-roselle
films which were cure at 60◦C. The higher ratio of
chitosan-roselle, the surface structure become
smoother. As conclusion, chitosan-roselle films have
higher heat resistance, degraded faster and have
smoother surface at most optimum cure temperature
(60
o
C). The smoother surface of chitosan-roselle
films made the higher thermal resistance of these
blend films.
ACKNOWLEDGEMENTS
This work was supported by Faculty of Mathematic
and Natural Sciences, Universitas Sumatera Utara
and School of Materials Engineering, Universiti
Malaysia Perlis.
REFERENCES
Alves, V., Costa, N., Hilliou, L., Larotonda, F., Gonçalves,
M., Sereno, A., & Coelhoso, I., 2006. Design of
biodegradable composite films for food packaging.
Desalination, 199(1-3), 331-333.
Betzabe Gonza Lez-Campos, Evgen Prokhorov, Gabriel
Luna-Bárcenas, Abril Fonseca-García, & Isaac
C.Sanchez., 2009. Dielectric relaxations of chitosan:
The effect of water on the α-relaxation and the glass
transition temperature. Journal of Polymer Science Part
B Polymer Physic, 47(22), 2259-2271.
Cissé M., Kouakou A.C., Montet D., Loiseau G., Ducamp-
Collin M.N., 2013. Food Hydrocolloids, 30 (2), 576-
580.
Dev Raj. and J. Yamini, 2013. Preparation and
Characterization of Pharmaceutical Grade Chitosan and
Hydrated Chitosan Gums for Topical Preparations.
Chem. Phys Journal. 1(9), 858-860.
Esam A. El-hefian and Abdul H. Yahaya., 2010.
Rheological study of chitosan and its blends: An
overview of chitosan, International Journal of
Carbohydrate Chemistry 4(02), 210-220
Portes, E., Gardrat, C., Alain, C., & Véronique, C., 2009.
Environmentally friendly films based on chitosan and
tetrahydrocurcuminoid derivatives exhibiting
antibacterial and antioxidative properties. Carbohydrate
polymers. 76(4), 578-584.
Jayakumar, N. Nwe, S. Tokura, H. Tamura, 2007. Sulfated
chitin and chitosan as novel biomaterials. International
Journal of Biological Macromolecules, 40(3), 175-181
Kammoun , M., Haddar , M., Kallel , T. K., Dammak, M.,
& Sayari, A., 2013. Biological properties and
biodegradation studies of chitosan biofilms plasticized
with PEG and glycerol. International Journal of
Biological Macromolecules.
Kumirska, Jolanta., Czerwicka, M., KaczyÅski, Z.,
Bychowska, A., Brzozowski, K., Thoming, J., &
Stepnowski, P., 2010. Application of Spectroscopic
Methods for Structural Analysis of Chitin and Chitosan.
Marine Drugs. 8(5), 1567-636.
Leceta, I., Guerrero, P., Ibarburu, I., Dueñas, M.T., de la
Caba, K., 2013. Characterization and antimicrobial
analysis of chitosan-based films. Journal of Food
Engineering, 116,(2013), 889–899.
Ngah, W.S.W., Fatinathan, S. and Yosop, N.A., 2011.
Isotherm and kinetic studies on the adsorption of humic
acid onto chitosan-H2SO4 beads. Desalination. 272,
293–300.
Norashikin, M. I., 2010. Fabrication and Characterization
of Sawdust. World Academy of Science, Engineering
and Technology, 716-719.
Ou, Y. Wang, S. Tang, C. Huang, and M. G. Jackson, 2005.
"Role of ferulic acid in preparing edible films from soy
protein isolate," Journal Food Eng, vol. 70, pp. 205-
210.
Tharanathan, R. N., 2003. Biodegradable films and
composite coatings: past, present and future. Trends in
Food Science and Technology, 14, 71-78.
Tuija Annala, 2007. Chitosan Film Preparation, Instruction
for laboratory experiments, Rev 0.
ZhenXing Tang, and JunQinQin, 2007. Use of chitosan gel
for the purification of protein, Engineering AND
Technology, College of Chemical Engineering and
Materials Science, Zheijiang University of Technology,
Hangzhou, Zhejiang, China.
Thermal Properties of Chitosan-roselle Films
273
